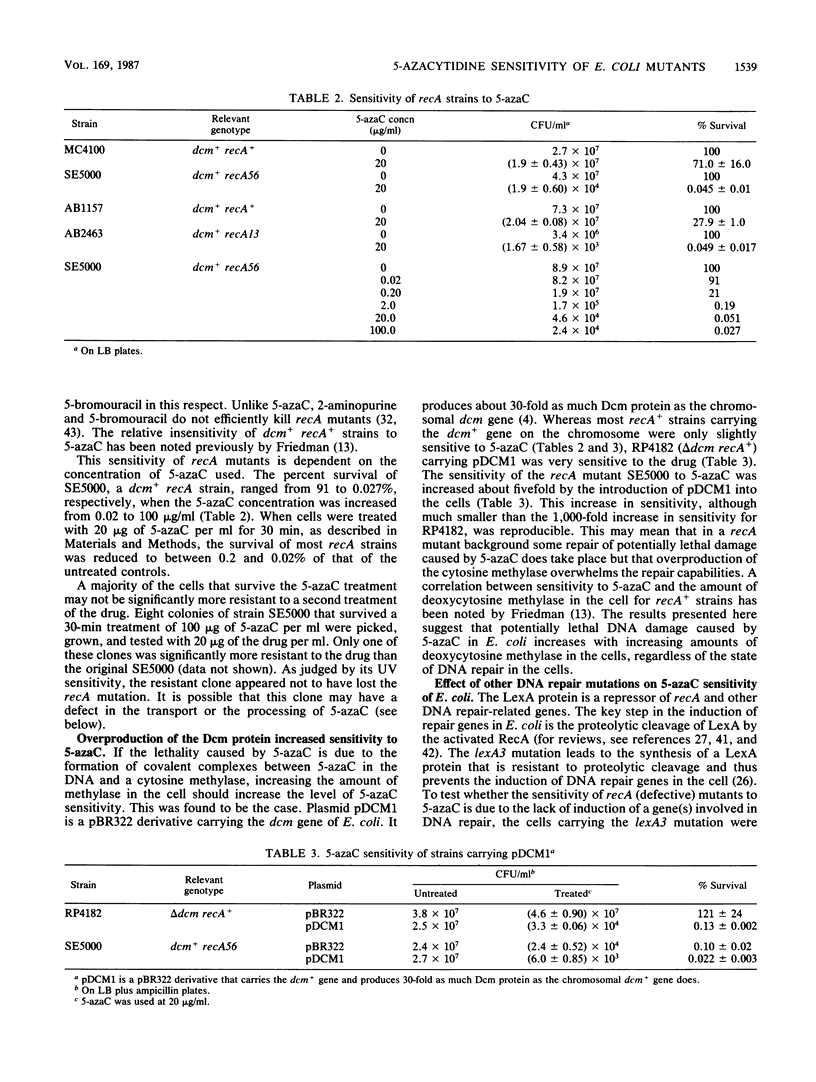
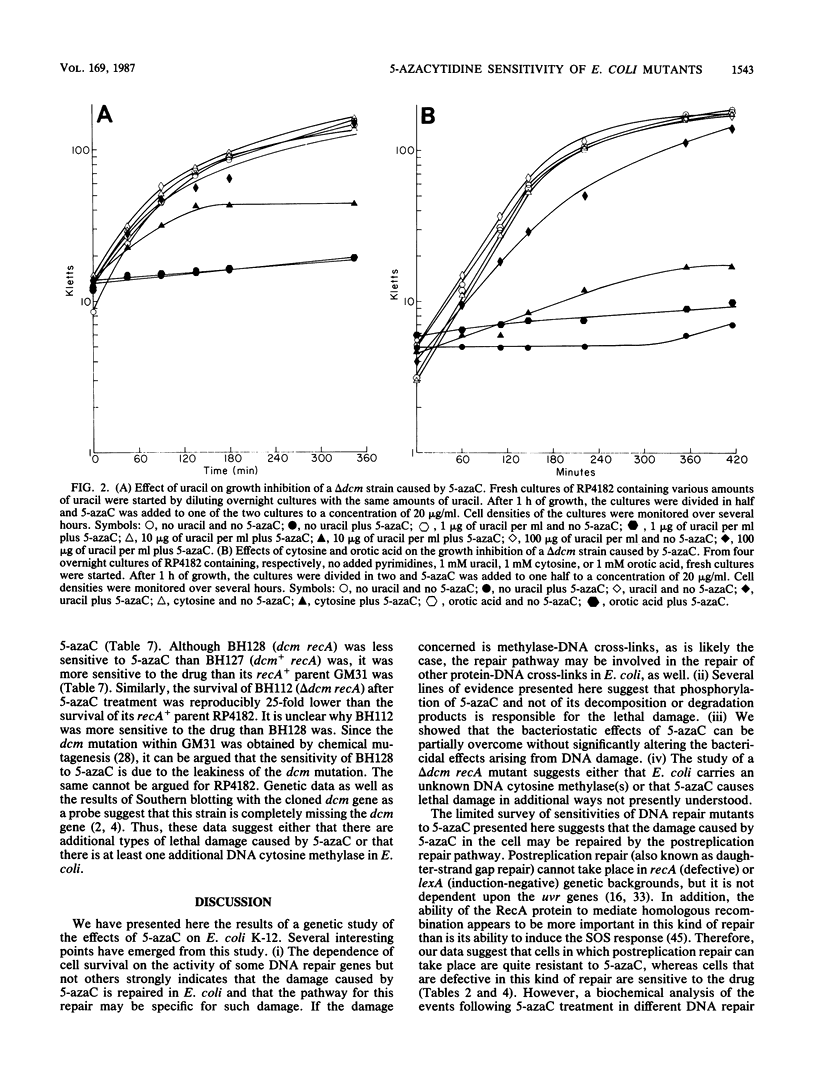
Abstract
DNA containing 5-azacytidine (5-azaC) has been shown to form stable detergent-resistant complexes with cytosine methylases. We reasoned that if 5-azaC treatment causes protein-DNA cross-links in vivo, then mutations in DNA repair and recombination genes may increase the sensitivity of a cell to 5-azaC. We found that although recA (defective) and lexA (induction-negative) mutants of Escherichia coli were very sensitive to the drug, mutations in uvrA and ung genes had little effect on cell lethality. The sensitivity of recA strains to 5-azaC was dose dependent and was enhanced by the overproduction of a DNA cytosine methylase in the cell. Unexpectedly, a strain of E. coli carrying a recA mutation and a deletion of the DNA cytosine methylase gene (dcm) was found to be significantly sensitive to 5-azaC. Study of mutations in the pyrimidine salvage pathway of E. coli suggests that direct phosphorylation of 5-azaC, rather than phosphorylation of its degradation products, is largely responsible for the lethal effects of the drug. The addition of uracil to the growth medium has little effect on cell lethality of recA mutants, but it partially reversed the inhibition of cell growth caused by 5-azaC. This reversal of the bacteriostatic effects of the drug could not be achieved by adding cytosine or orotic acid to the growth medium and required the presence of functional UMP-pyrophosphorylase (gene upp) in the cell.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEXANDER P., MOROSON H. Cross-linking of deoxyribonucleic acid to protein following ultra-violet irradiation different cells. Nature. 1962 Jun 2;194:882–883. doi: 10.1038/194882a0. [DOI] [PubMed] [Google Scholar]
- Bale A., d'Alarcao M., Marinus M. G. Characterization of DNA adenine methylation mutants of Escherichia coli K12. Mutat Res. 1979 Feb;59(2):157–165. doi: 10.1016/0027-5107(79)90153-2. [DOI] [PubMed] [Google Scholar]
- Barbé J., Gibert I., Guerrero R. 5-Azacytidine: survival and induction of the SOS response in Escherichia coli K-12. Mutat Res. 1986 Jul;166(1):9–16. doi: 10.1016/0167-8817(86)90035-0. [DOI] [PubMed] [Google Scholar]
- Bhagwat A. S., Sohail A., Roberts R. J. Cloning and characterization of the dcm locus of Escherichia coli K-12. J Bacteriol. 1986 Jun;166(3):751–755. doi: 10.1128/jb.166.3.751-755.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christman J. K., Schneiderman N., Acs G. Interaction of DNA methyltransferase and other non-histone proteins isolated from Friend erythroleukemia cell nuclei with 5-azacytosine residues in DNA. Prog Clin Biol Res. 1985;198:105–118. [PubMed] [Google Scholar]
- Doskocil J., Sorm F. The determination of 5-azapyrimidines and their derivatives in bacterial RNA. FEBS Lett. 1971 Nov 15;19(1):30–32. doi: 10.1016/0014-5793(71)80598-7. [DOI] [PubMed] [Google Scholar]
- Elledge S. J., Walker G. C. The muc genes of pKM101 are induced by DNA damage. J Bacteriol. 1983 Sep;155(3):1306–1315. doi: 10.1128/jb.155.3.1306-1315.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis D. G., Fisher B., Edmiston S., Mount D. W. Dual role for Escherichia coli RecA protein in SOS mutagenesis. Proc Natl Acad Sci U S A. 1985 May;82(10):3325–3329. doi: 10.1073/pnas.82.10.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornace A. J., Jr, Little J. B. DNA-protein cross-linking by chemical carcinogens in mammalian cells. Cancer Res. 1979 Mar;39(3):704–710. [PubMed] [Google Scholar]
- Friedman S. Bactericidal effect of 5-azacytidine on Escherichia coli carrying EcoRII restriction-modification enzymes. J Bacteriol. 1982 Jul;151(1):262–268. doi: 10.1128/jb.151.1.262-268.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S. Binding of the EcoRII methylase to azacytosine-containing DNA. Nucleic Acids Res. 1986 Jun 11;14(11):4543–4556. doi: 10.1093/nar/14.11.4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S. The inhibition of DNA(cytosine-5)methylases by 5-azacytidine. The effect of azacytosine-containing DNA. Mol Pharmacol. 1981 Mar;19(2):314–320. [PubMed] [Google Scholar]
- Friedman S. The irreversible binding of azacytosine-containing DNA fragments to bacterial DNA(cytosine-5)methyltransferases. J Biol Chem. 1985 May 10;260(9):5698–5705. [PubMed] [Google Scholar]
- Ganesan A. K., Seawell P. C. The effect of lexA and recF mutations on post-replication repair and DNA synthesis in Escherichia coli K-12. Mol Gen Genet. 1975 Dec 1;141(3):189–205. doi: 10.1007/BF00341799. [DOI] [PubMed] [Google Scholar]
- Hammer-Jespersen K., Munch-Petersen A. Mutants of Escherichia coli unable to metabolize cytidine: isolation and characterization. Mol Gen Genet. 1973 Nov 2;126(2):177–186. doi: 10.1007/BF00330992. [DOI] [PubMed] [Google Scholar]
- Hanawalt P. C., Cooper P. K., Ganesan A. K., Smith C. A. DNA repair in bacteria and mammalian cells. Annu Rev Biochem. 1979;48:783–836. doi: 10.1146/annurev.bi.48.070179.004031. [DOI] [PubMed] [Google Scholar]
- Jones P. A. Altering gene expression with 5-azacytidine. Cell. 1985 Mar;40(3):485–486. doi: 10.1016/0092-8674(85)90192-8. [DOI] [PubMed] [Google Scholar]
- Jones P. A. Effects of 5-azacytidine and its 2'-deoxyderivative on cell differentiation and DNA methylation. Pharmacol Ther. 1985;28(1):17–27. doi: 10.1016/0163-7258(85)90080-4. [DOI] [PubMed] [Google Scholar]
- Keener S. L., McNamee K. P., McEntee K. Cloning and characterization of recA genes froM Proteus vulgaris, Erwinia carotovora, Shigella flexneri, and Escherichia coli B/r. J Bacteriol. 1984 Oct;160(1):153–160. doi: 10.1128/jb.160.1.153-160.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C. J., Walker G. C. Expression of the E. coli uvrA gene is inducible. Nature. 1981 Feb 26;289(5800):808–810. doi: 10.1038/289808a0. [DOI] [PubMed] [Google Scholar]
- Lindahl T. An N-glycosidase from Escherichia coli that releases free uracil from DNA containing deaminated cytosine residues. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3649–3653. doi: 10.1073/pnas.71.9.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Edmiston S. H., Pacelli L. Z., Mount D. W. Cleavage of the Escherichia coli lexA protein by the recA protease. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3225–3229. doi: 10.1073/pnas.77.6.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Marinus M. G., Morris N. R. Isolation of deoxyribonucleic acid methylase mutants of Escherichia coli K-12. J Bacteriol. 1973 Jun;114(3):1143–1150. doi: 10.1128/jb.114.3.1143-1150.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May M. S., Hattaman S. Deoxyribonucleic acid-cytosine methylation by host- and plasmid-controlled enzymes. J Bacteriol. 1975 Apr;122(1):129–138. doi: 10.1128/jb.122.1.129-138.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morand P., Blanco M., Devoret R. Characterization of lexB mutations in Escherichia coli K-12. J Bacteriol. 1977 Aug;131(2):572–582. doi: 10.1128/jb.131.2.572-582.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronen A. 2-Aminopurine. Mutat Res. 1980 Jan;75(1):1–47. doi: 10.1016/0165-1110(80)90026-3. [DOI] [PubMed] [Google Scholar]
- Rupp W. D., Howard-Flanders P. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J Mol Biol. 1968 Jan 28;31(2):291–304. doi: 10.1016/0022-2836(68)90445-2. [DOI] [PubMed] [Google Scholar]
- SMITH K. C. Dose dependent decrease in extractability of DNA from bacteria following irradiation with ultraviolet light or with visible light plus dye. Biochem Biophys Res Commun. 1962 Jul 3;8:157–163. doi: 10.1016/0006-291x(62)90255-3. [DOI] [PubMed] [Google Scholar]
- Santi D. V., Garrett C. E., Barr P. J. On the mechanism of inhibition of DNA-cytosine methyltransferases by cytosine analogs. Cell. 1983 May;33(1):9–10. doi: 10.1016/0092-8674(83)90327-6. [DOI] [PubMed] [Google Scholar]
- Santi D. V., Norment A., Garrett C. E. Covalent bond formation between a DNA-cytosine methyltransferase and DNA containing 5-azacytosine. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6993–6997. doi: 10.1073/pnas.81.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. M., Constantinides P. A., Jones P. A. 5-Azacytidine, DNA methylation, and differentiation. Curr Top Microbiol Immunol. 1984;108:115–127. doi: 10.1007/978-3-642-69370-0_8. [DOI] [PubMed] [Google Scholar]
- Veselý J. Mode of action and effects of 5-azacytidine and of its derivatives in eukaryotic cells. Pharmacol Ther. 1985;28(2):227–235. doi: 10.1016/0163-7258(85)90012-9. [DOI] [PubMed] [Google Scholar]
- Walker G. C. Inducible DNA repair systems. Annu Rev Biochem. 1985;54:425–457. doi: 10.1146/annurev.bi.54.070185.002233. [DOI] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M., Parisi E. C. Bromouracil mutagenesis: mispairing or misrepair? Mutat Res. 1974 Dec;25(3):407–409. doi: 10.1016/0027-5107(74)90071-2. [DOI] [PubMed] [Google Scholar]
- Wu J. C., Santi D. V. On the mechanism and inhibition of DNA cytosine methyltransferases. Prog Clin Biol Res. 1985;198:119–129. [PubMed] [Google Scholar]
- Yarranton G. T., Sedgwick S. G. Cloned truncated recA genes in E. coli II. Effects of truncated gene products on in vivo recA+ protein activity. Mol Gen Genet. 1982;185(1):99–104. doi: 10.1007/BF00333797. [DOI] [PubMed] [Google Scholar]


