Abstract
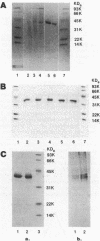
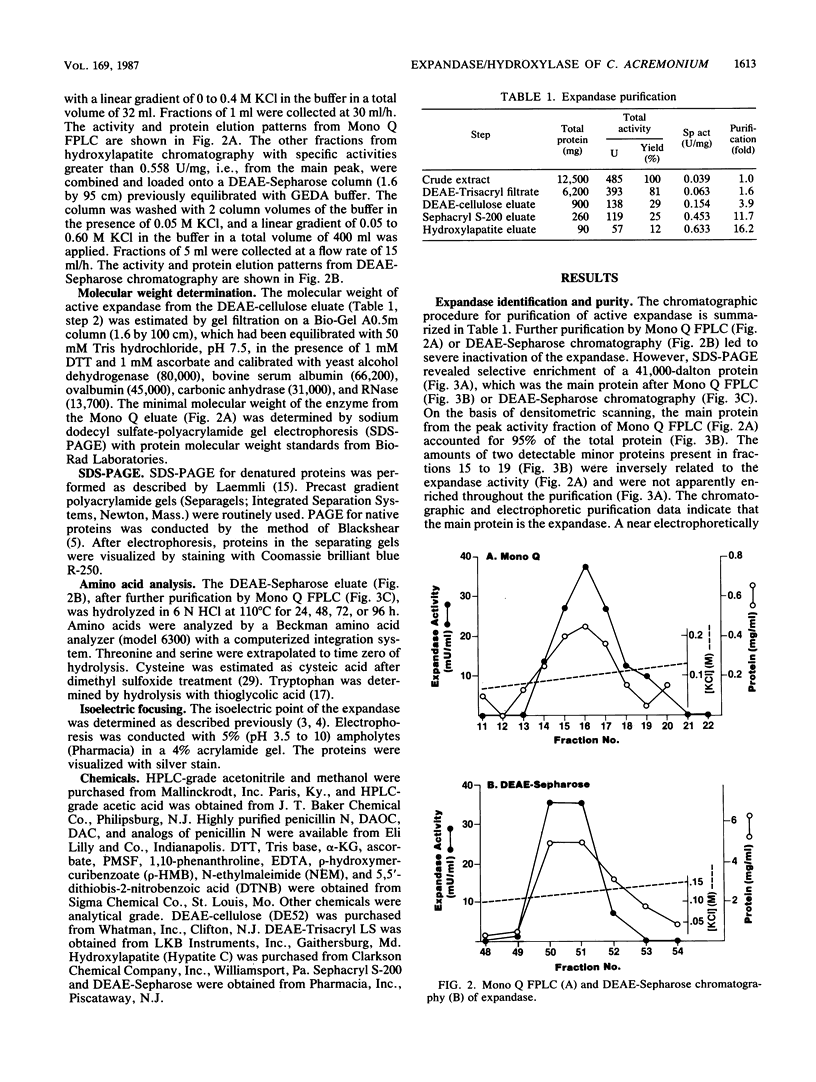
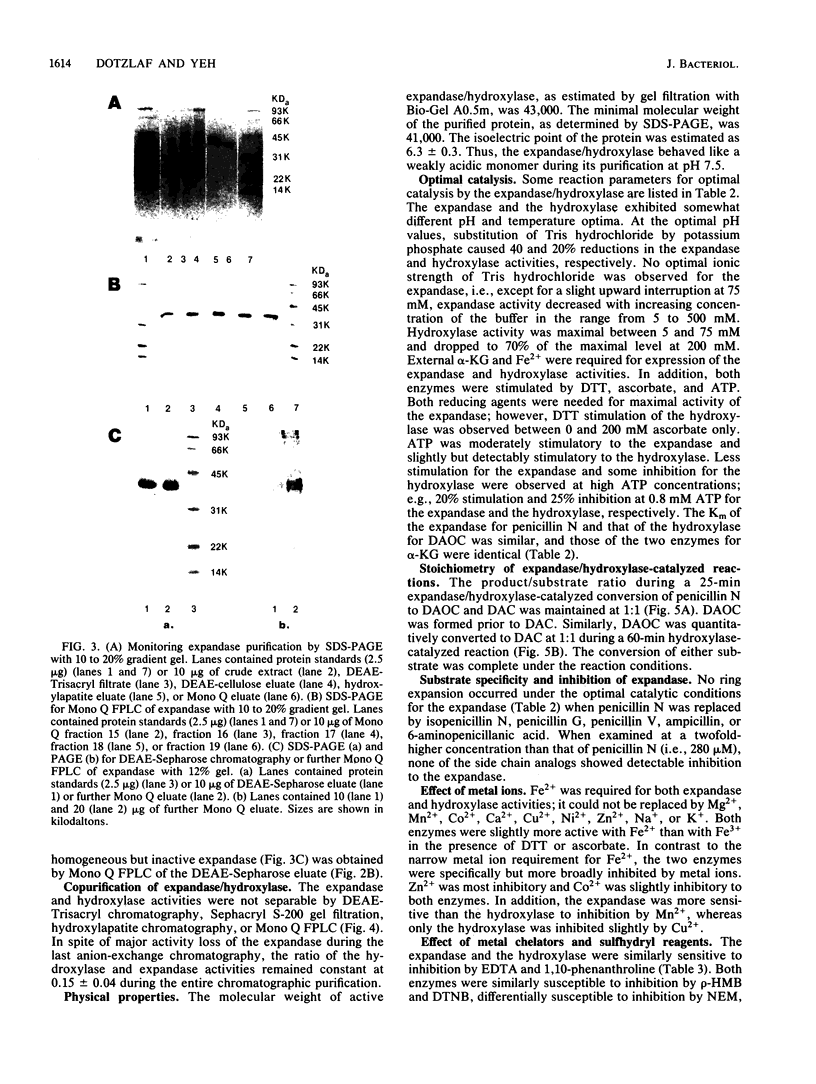
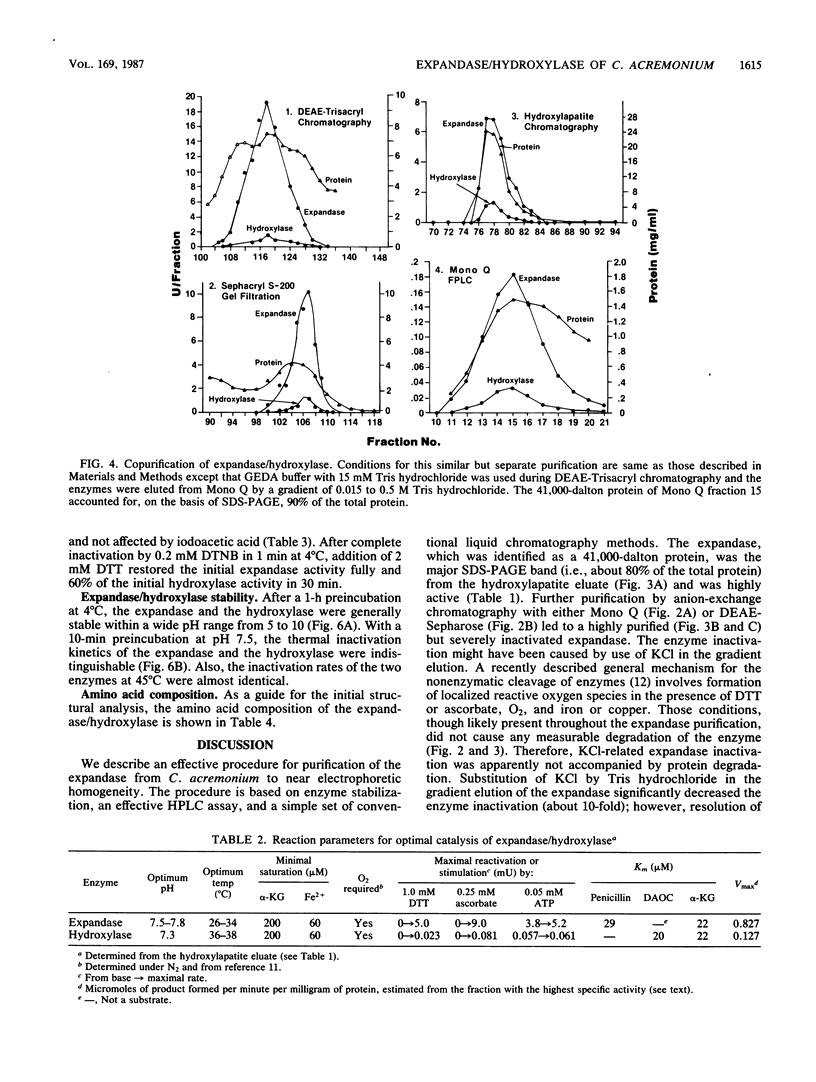
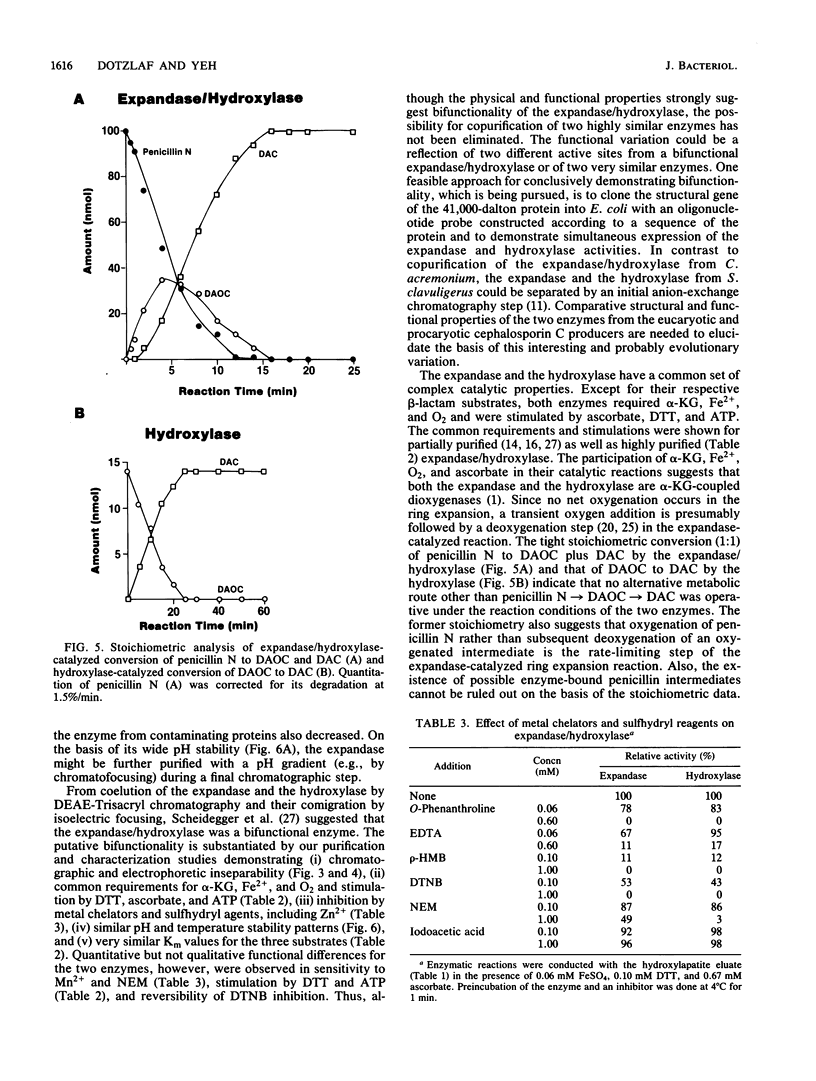
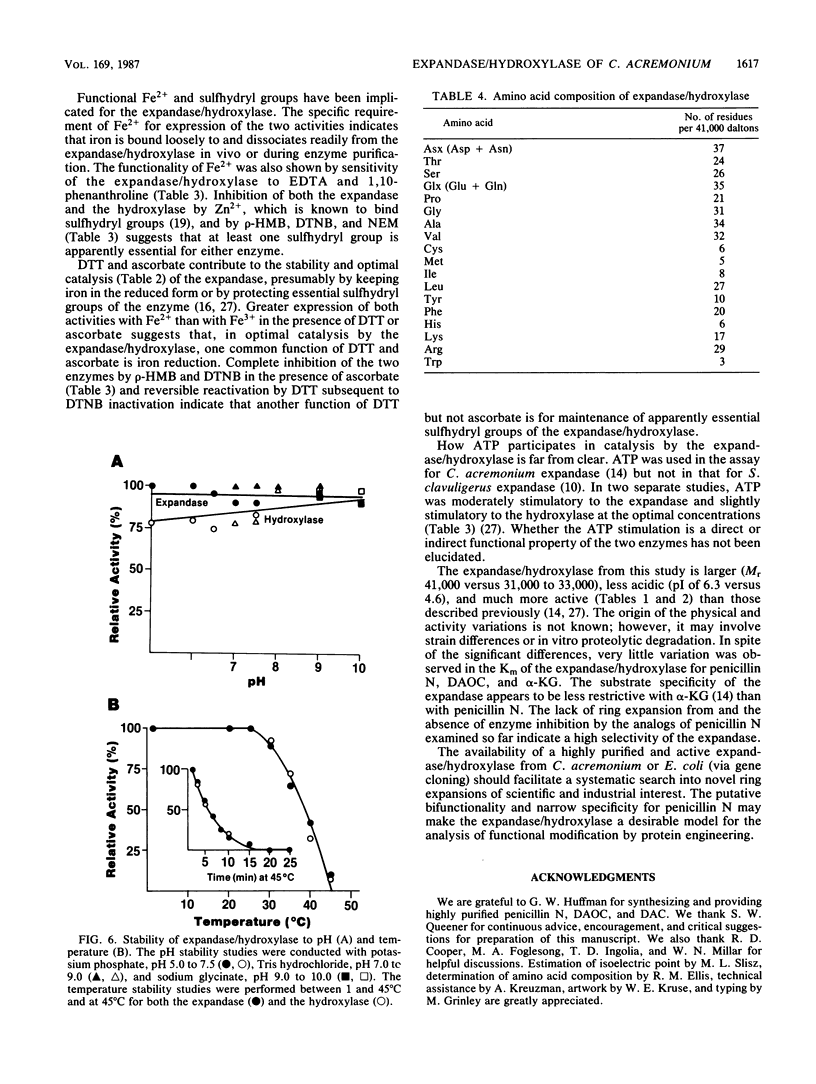
Deacetoxycephalosporin C synthetase (expandase), which catalyzes ring expansion of penicillin N to deacetoxycephalosporin C (DAOC), has been stabilized in vitro and purified to near homogeneity from the industrially important fungus Cephalosporium acremonium. Throughout the purification, the expandase activity remained physically associated with and in a constant ratio of 7:1 to DAOC hydroxylase activity. The latter activity mediates hydroxylation of DAOC to deacetylcephalosporin C (DAC). The copurified expandase/hydroxylase appeared to be monomeric, with a molecular weight of 41,000 +/- 2,000 and an isoelectric point of 6.3 +/- 0.3. Both catalytic activities required alpha-ketoglutarate, Fe2+, and O2 and were stimulated by ascorbate, dithiothreitol, and ATP. The Fe2+ requirement was specific, and sulfhydryl groups in the purified protein were apparently essential for both ring expansion and hydroxylation. The kinetics and stoichiometry of DAOC/DAC formation from the expandase/hydroxylase-catalyzed reactions suggested that ring expansion of penicillin N preceded hydroxylation of DAOC.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson N. G., Anderson N. L. Analytical techniques for cell fractions. XXI. Two-dimensional analysis of serum and tissue proteins: multiple isoelectric focusing. Anal Biochem. 1978 Apr;85(2):331–340. doi: 10.1016/0003-2697(78)90229-4. [DOI] [PubMed] [Google Scholar]
- Anderson N. L., Anderson N. G. Analytical techniques for cell fractions. XXII. Two-dimensional analysis of serum and tissue proteins: multiple gradient-slab gel electrophoresis. Anal Biochem. 1978 Apr;85(2):341–354. doi: 10.1016/0003-2697(78)90230-0. [DOI] [PubMed] [Google Scholar]
- Blackshear P. J. Systems for polyacrylamide gel electrophoresis. Methods Enzymol. 1984;104:237–255. doi: 10.1016/s0076-6879(84)04093-3. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carr L. G., Skatrud P. L., Scheetz M. E., 2nd, Queener S. W., Ingolia T. D. Cloning and expression of the isopenicillin N synthetase gene from Penicillium chrysogenum. Gene. 1986;48(2-3):257–266. doi: 10.1016/0378-1119(86)90084-3. [DOI] [PubMed] [Google Scholar]
- Hollander I. J., Shen Y. Q., Heim J., Demain A. L., Wolfe S. A pure enzyme catalyzing penicillin biosynthesis. Science. 1984 May 11;224(4649):610–612. doi: 10.1126/science.6546810. [DOI] [PubMed] [Google Scholar]
- Jensen S. E., Westlake D. W., Wolfe S. Analysis of penicillin N ring expansion activity from Streptomyces clavuligerus by ion-pair high-pressure liquid chromatography. Antimicrob Agents Chemother. 1983 Sep;24(3):307–312. doi: 10.1128/aac.24.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen S. E., Westlake D. W., Wolfe S. Deacetoxycephalosporin C synthetase and deacetoxycephalosporin C hydroxylase are two separate enzymes in Streptomyces clavuligerus. J Antibiot (Tokyo) 1985 Feb;38(2):263–265. doi: 10.7164/antibiotics.38.263. [DOI] [PubMed] [Google Scholar]
- Kim K., Rhee S. G., Stadtman E. R. Nonenzymatic cleavage of proteins by reactive oxygen species generated by dithiothreitol and iron. J Biol Chem. 1985 Dec 15;260(29):15394–15397. [PubMed] [Google Scholar]
- Kohsaka M., Demain A. L. Conversion of penicillin N to cephalosporin(s) by cell-free extracts of Cephalosporium acremonium. Biochem Biophys Res Commun. 1976 May 17;70(2):465–473. doi: 10.1016/0006-291x(76)91069-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Martin-Zanca D. M., Martín J. F. Carbon catabolite regulation of the conversion of penicillin N into cephalosporin C. J Antibiot (Tokyo) 1983 Jun;36(6):700–708. doi: 10.7164/antibiotics.36.700. [DOI] [PubMed] [Google Scholar]
- Matsubara H., Sasaki R. M. High recovery of tryptophan from acid hydrolysates of proteins. Biochem Biophys Res Commun. 1969 Apr 29;35(2):175–181. doi: 10.1016/0006-291x(69)90263-0. [DOI] [PubMed] [Google Scholar]
- Miller R. D., Huckstep L. L., McDermott J. P., Queener S. W., Kukolja S., Spry D. O., Elzey T. K., Lawrence S. M., Neuss N. High performance liquid chromatography (HPLC) of natural products. IV. The use of HPLC in biosynthetic studies of cephalosporin C in the cell-free system. J Antibiot (Tokyo) 1981 Aug;34(8):984–993. doi: 10.7164/antibiotics.34.984. [DOI] [PubMed] [Google Scholar]
- Mukerji S. K., Pimstone N. R. In vitro studies of the mechanism of inhibition of rat liver uroporphyrinogen decarboxylase activity by ferrous iron under anaerobic conditions. Arch Biochem Biophys. 1986 Feb 1;244(2):619–629. doi: 10.1016/0003-9861(86)90630-2. [DOI] [PubMed] [Google Scholar]
- NEWTON G. G., ABRAHAM E. P. Cephalosporin C, a new antibiotic containing sulphur and D-alpha-aminoadipic acid. Nature. 1955 Mar 26;175(4456):548–548. doi: 10.1038/175548a0. [DOI] [PubMed] [Google Scholar]
- Neuss N., Berry D. M., Kupka J., Demain A. L., Queener S. W., Duckworth D. C., Huckstep L. L. High performance liquid chromatography (HPLC) of natural products V. The use of HPLC in the cell-free biosynthetic conversion of alpha-aminoadipyl-cysteinyl-valine (LLD) into isopenicillin N. J Antibiot (Tokyo) 1982 May;35(5):580–584. doi: 10.7164/antibiotics.35.580. [DOI] [PubMed] [Google Scholar]
- Pang C. P., Chakravarti B., Adlington R. M., Ting H. H., White R. L., Jayatilake G. S., Baldwin J. E., Abraham E. P. Purification of isopenicillin N synthetase. Biochem J. 1984 Sep 15;222(3):789–795. doi: 10.1042/bj2220789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos F. R., López-Nieto M. J., Martín J. F. Isopenicillin N synthetase of Penicillium chrysogenum, an enzyme that converts delta-(L-alpha-aminoadipyl)-L-cysteinyl-D-valine to isopenicillin N. Antimicrob Agents Chemother. 1985 Mar;27(3):380–387. doi: 10.1128/aac.27.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson S. M., Belagaje R., Blankenship D. T., Chapman J. L., Perry D., Skatrud P. L., VanFrank R. M., Abraham E. P., Baldwin J. E., Queener S. W. Isolation, sequence determination and expression in Escherichia coli of the isopenicillin N synthetase gene from Cephalosporium acremonium. Nature. 1985 Nov 14;318(6042):191–194. doi: 10.1038/318191a0. [DOI] [PubMed] [Google Scholar]
- Scheidegger A., Küenzi M. T., Nüesch J. Partial purification and catalytic properties of a bifunctional enzyme in the biosynthetic pathway of beta-lactams in Cephalosporium acremonium. J Antibiot (Tokyo) 1984 May;37(5):522–531. doi: 10.7164/antibiotics.37.522. [DOI] [PubMed] [Google Scholar]
- Shen Y. Q., Heim J., Solomon N. A., Wolfe S., Demain A. L. Repression of beta-lactam production in Cephalosporium acremonium by nitrogen sources. J Antibiot (Tokyo) 1984 May;37(5):503–511. doi: 10.7164/antibiotics.37.503. [DOI] [PubMed] [Google Scholar]
- Spencer R. L., Wold F. A new convenient method for estimation of total cystine-cysteine in proteins. Anal Biochem. 1969 Oct 15;32(1):185–190. doi: 10.1016/0003-2697(69)90123-7. [DOI] [PubMed] [Google Scholar]