Abstract
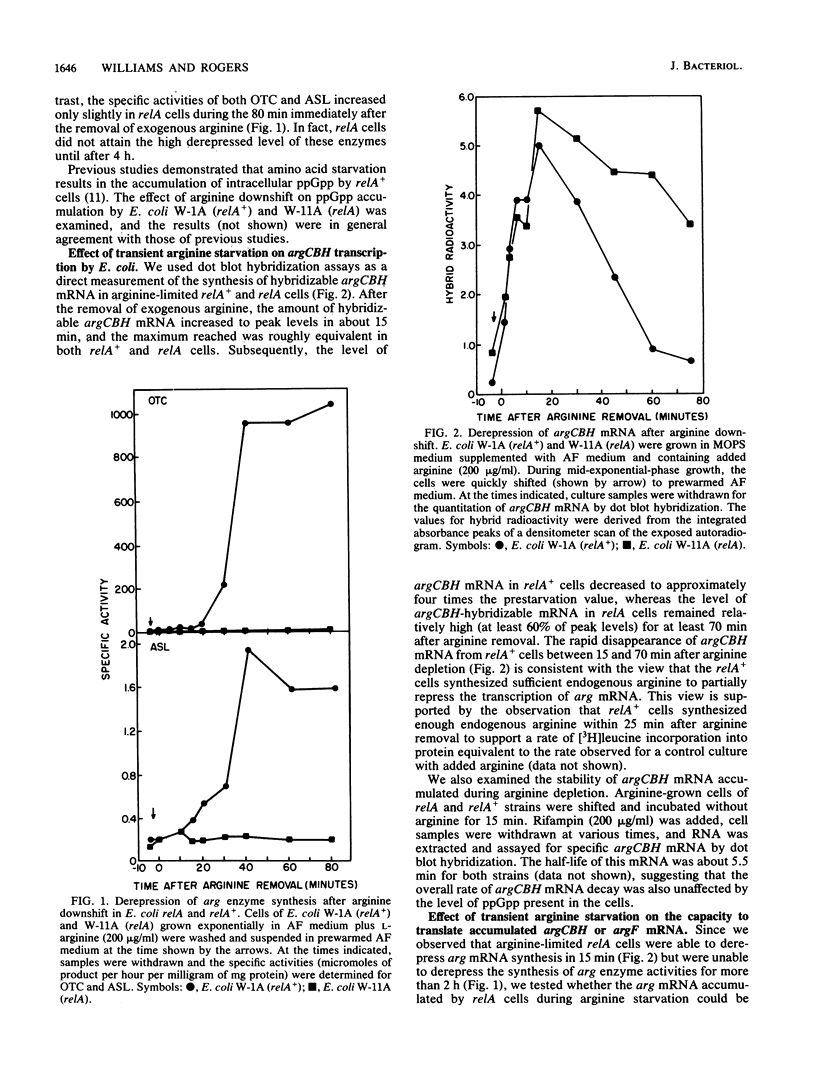
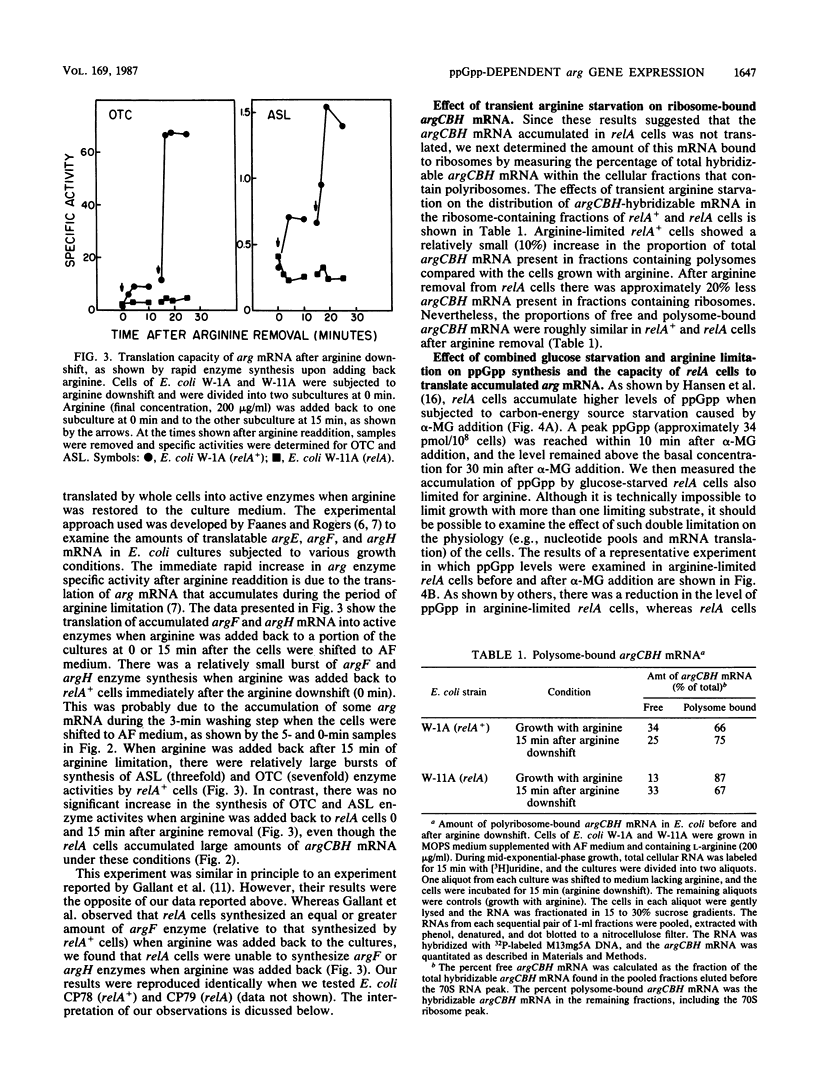
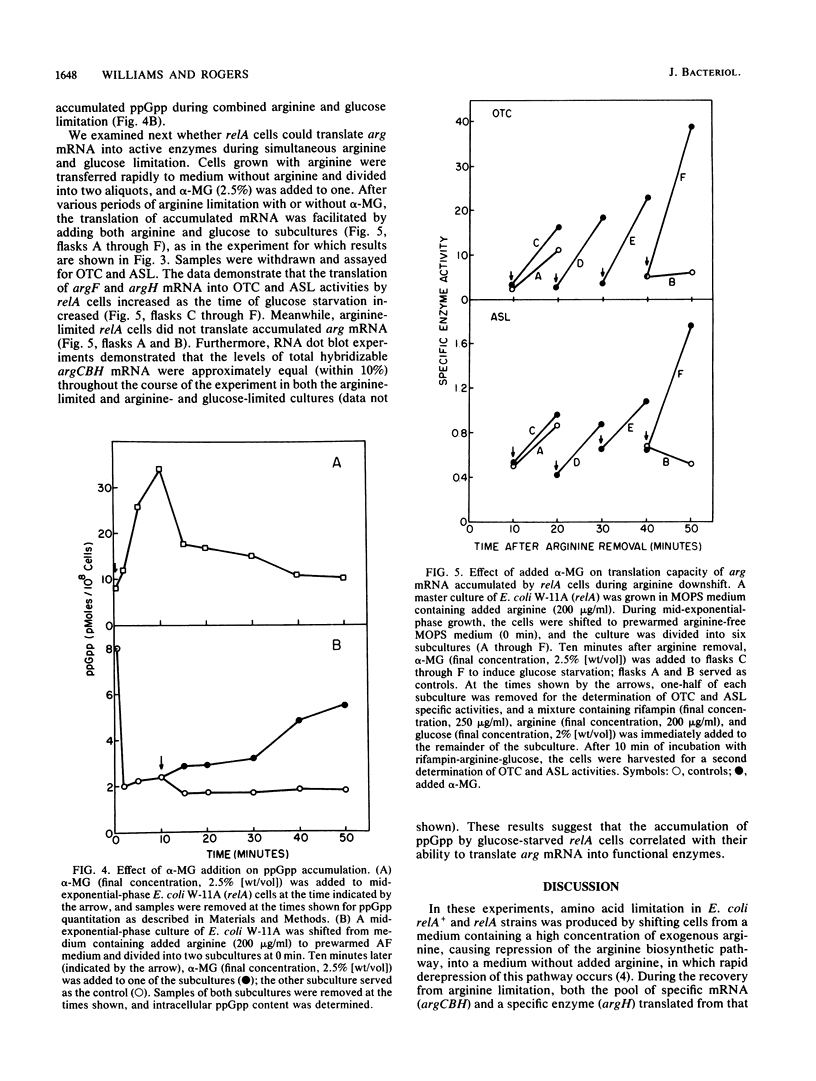
The transcription and translation of operons for arginine biosynthetic enzymes after arginine removal (arginine down shift) were studied in relA and relA+ strains of Escherichia coli. After arginine down shift, derepression of synthesis of the arginine biosynthetic enzymes ornithine carbamoyltransferase (argF) and argininosuccinate lyase (argH) began at about 15 min in relA+ cells but was delayed in relA cells for more than 2 h. However, both relA+ and relA cells accumulated high levels of argCBH mRNA, as shown by dot blot hybridization, after arginine down shift. After 15 min of arginine limitation, the proportion of ribosome-bound argCBH mRNA was equivalent in both relA+ and relA cells. During the 15 min after the arginine down shift, relA+ cells produced a significant burst of argF and argH enzyme synthesis when arginine was added back to the culture, whereas relA cells did not produce this burst of enzyme synthesis. The relA cells regained the ability to produce a burst of argF and argH enzyme synthesis when alpha-methylglucose-induced glucose starvation was combined with arginine limitation. Significant guanosine 5'-diphosphate 3'-diphosphate accumulated in relA cells under this condition. Our results support the view that during periods of severe amino acid limitation guanosine 5'-diphosphate 3'-diphosphate acts in some way to ensure the translation of argCBH mRNA.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bochner B. R., Ames B. N. Complete analysis of cellular nucleotides by two-dimensional thin layer chromatography. J Biol Chem. 1982 Aug 25;257(16):9759–9769. [PubMed] [Google Scholar]
- Bochner B. R., Ames B. N. Selective precipitation orthophosphate from mixtures containing labile phosphorylated metabolites. Anal Biochem. 1982 May 1;122(1):100–107. doi: 10.1016/0003-2697(82)90257-3. [DOI] [PubMed] [Google Scholar]
- Cenatiempo Y., Sung M. T., Cozzone A. J. Polysome level and stability in stringent E. coli strains under aminoacyl-tRNA deprivation. Biochem Biophys Res Commun. 1975 Jan 2;64(3):939–946. doi: 10.1016/0006-291x(75)90138-2. [DOI] [PubMed] [Google Scholar]
- Cunin R., Glansdorff N., Piérard A., Stalon V. Biosynthesis and metabolism of arginine in bacteria. Microbiol Rev. 1986 Sep;50(3):314–352. doi: 10.1128/mr.50.3.314-352.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix D. B., Thompson R. C. Elongation factor Tu.guanosine 3'-diphosphate 5'-diphosphate complex increases the fidelity of proofreading in protein biosynthesis: mechanism for reducing translational errors introduced by amino acid starvation. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2027–2031. doi: 10.1073/pnas.83.7.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faanes R., Rogers P. Repression of enzymes of arginine biosynthesis by L-canavanine in arginyl-transfer ribonucleic acid synthetase mutants of Escherichia coli. J Bacteriol. 1972 Oct;112(1):102–113. doi: 10.1128/jb.112.1.102-113.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faanes R., Rogers P. Roles of arginine and canavanine in the synthesis and repression of ornithine transcarbamylase by Escherichia coli. J Bacteriol. 1968 Aug;96(2):409–420. doi: 10.1128/jb.96.2.409-420.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley D., Dennis P., Gallant J. Mechanism of the rel defect in beta-galactosidase synthesis. J Bacteriol. 1981 Jan;145(1):641–643. doi: 10.1128/jb.145.1.641-643.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen J. D. A study of the relationship between polyribosomes and messenger RNA in Escherichia coli. J Mol Biol. 1968 Mar 14;32(2):183–200. doi: 10.1016/0022-2836(68)90003-x. [DOI] [PubMed] [Google Scholar]
- Gallant J. A. Stringent control in E. coli. Annu Rev Genet. 1979;13:393–415. doi: 10.1146/annurev.ge.13.120179.002141. [DOI] [PubMed] [Google Scholar]
- Glass R. E., Jones S. T., Ishihama A. Genetic studies on the beta subunit of Escherichia coli RNA polymerase. VII. RNA polymerase is a target for ppGpp. Mol Gen Genet. 1986 May;203(2):265–268. doi: 10.1007/BF00333964. [DOI] [PubMed] [Google Scholar]
- Godson G. N., Sinsheimer R. L. Use of Brij lysis as a general method to prepare polyribosomes from Escherichia coli. Biochim Biophys Acta. 1967 Dec 19;149(2):489–495. doi: 10.1016/0005-2787(67)90176-1. [DOI] [PubMed] [Google Scholar]
- Hall B., Gallant J. Defective translation in RC - cells. Nat New Biol. 1972 May 31;237(74):131–135. doi: 10.1038/newbio237131a0. [DOI] [PubMed] [Google Scholar]
- Kajitani M., Ishihama A. Promoter selectivity of Escherichia coli RNA polymerase. Differential stringent control of the multiple promoters from ribosomal RNA and protein operons. J Biol Chem. 1984 Feb 10;259(3):1951–1957. [PubMed] [Google Scholar]
- Kryzek R. A., Rogers P. Dual regulation by arginine of the expression of the Escherichia coli argECBH operon. J Bacteriol. 1976 Apr;126(1):348–364. doi: 10.1128/jb.126.1.348-364.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyzek R. A., Rogers P. Effect of arginine on the stability and size of argECBH messenger ribonucleic acid in Escherichia coli. J Bacteriol. 1976 Apr;126(1):365–376. doi: 10.1128/jb.126.1.365-376.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyzek R., Rogers P. Arginine control of transcription of argECBH messenger ribonucleic acid in Escherichia coli. J Bacteriol. 1972 Jun;110(3):945–954. doi: 10.1128/jb.110.3.945-954.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamond A. I., Travers A. A. Genetically separable functional elements mediate the optimal expression and stringent regulation of a bacterial tRNA gene. Cell. 1985 Feb;40(2):319–326. doi: 10.1016/0092-8674(85)90146-1. [DOI] [PubMed] [Google Scholar]
- Lamond A. I., Travers A. A. Stringent control of bacterial transcription. Cell. 1985 May;41(1):6–8. doi: 10.1016/0092-8674(85)90050-9. [DOI] [PubMed] [Google Scholar]
- Morris D. W., DeMoss J. A. Polysome transitions and the regulation of ribonucleic acid synthesis in Escherichia coli. Proc Natl Acad Sci U S A. 1966 Jul;56(1):262–268. doi: 10.1073/pnas.56.1.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVICK R. P., MAAS W. K. Control by endogenously synthesized arginine of the formation of ornithine transcarbamylase in Escherichia coli. J Bacteriol. 1961 Feb;81:236–240. doi: 10.1128/jb.81.2.236-240.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierlich D. P. Amino acid control over RNA synthesis: a re-evaluation. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1345–1352. doi: 10.1073/pnas.60.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M., Gourse R., Baughman G. Regulation of the synthesis of ribosomes and ribosomal components. Annu Rev Biochem. 1984;53:75–117. doi: 10.1146/annurev.bi.53.070184.000451. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. The suppression of defective translation by ppGpp and its role in the stringent response. Cell. 1978 Jul;14(3):545–557. doi: 10.1016/0092-8674(78)90241-6. [DOI] [PubMed] [Google Scholar]
- OKAMOTO K., SUGINO Y., NOMURA M. Synthesis and turnover of phage messenger RNA in E. coli infected with bacteriophage T4 in the presence of chloromycetin. J Mol Biol. 1962 Nov;5:527–534. doi: 10.1016/s0022-2836(62)80126-0. [DOI] [PubMed] [Google Scholar]
- Parker J., Pollard J. W., Friesen J. D., Stanners C. P. Stuttering: high-level mistranslation in animal and bacterial cells. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1091–1095. doi: 10.1073/pnas.75.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott L. M., Jones M. E. Modified methods for the determination of carbamyl aspartate. Anal Biochem. 1969 Dec;32(3):408–419. doi: 10.1016/s0003-2697(69)80008-4. [DOI] [PubMed] [Google Scholar]
- Primakoff P., Artz S. W. Positive control of lac operon expression in vitro by guanosine 5'-diphosphate 3'-diphosphate. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1726–1730. doi: 10.1073/pnas.76.4.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primakoff P. In vivo role of the relA+ gene in regulation of the lac operon. J Bacteriol. 1981 Jan;145(1):410–416. doi: 10.1128/jb.145.1.410-416.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGERS P., NOVELLI G. D. Formation of ornithine transcarbamylase in cells and protoplasts of Escherichia coli. Biochim Biophys Acta. 1959 Jun;33(2):423–436. doi: 10.1016/0006-3002(59)90132-5. [DOI] [PubMed] [Google Scholar]
- Ron E. Z. Polysome turnover during amino acid starvation in Escherichia coli. J Bacteriol. 1971 Oct;108(1):263–268. doi: 10.1128/jb.108.1.263-268.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sells B. H., Ennis H. L. Polysome stability in relaxed and stringent strain of Escherichia coli during amino acid starvation. J Bacteriol. 1970 Jun;102(3):666–671. doi: 10.1128/jb.102.3.666-671.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens J. C., Artz S. W., Ames B. N. Guanosine 5'-diphosphate 3'-diphosphate (ppGpp): positive effector for histidine operon transcription and general signal for amino-acid deficiency. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4389–4393. doi: 10.1073/pnas.72.11.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs J. D., Hall B. D. Level of tryptophan messenger RNA in Escherichia coli. J Mol Biol. 1968 Oct 28;37(2):289–302. doi: 10.1016/0022-2836(68)90268-4. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E. G., Ehrenberg M., Kurland C. G. Kinetic suppression of translational errors by (p)ppGpp. Mol Gen Genet. 1982;185(2):269–274. doi: 10.1007/BF00330797. [DOI] [PubMed] [Google Scholar]
- Wagner E. G., Kurland C. G. Translational accuracy enhanced in vitro by (p)ppGpp. Mol Gen Genet. 1980;180(1):139–145. doi: 10.1007/BF00267363. [DOI] [PubMed] [Google Scholar]
- Yang H. L., Zubay G., Urm E., Heiness G., Cashel M. Effects of guanosine tetraphosphate, guanosine pentaphosphate, and beta-gamma methylenyl-guanosine pentaphosphate on gene expression of Escherichia coli in vitro. Proc Natl Acad Sci U S A. 1974 Jan;71(1):63–67. doi: 10.1073/pnas.71.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zidwick M. J., Keller G., Rogers P. Regulation and coupling of argECBH mRNA and enzyme synthesis in cell extracts of Escherichia coli. J Bacteriol. 1984 Aug;159(2):640–646. doi: 10.1128/jb.159.2.640-646.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zidwick M. J., Korshus J., Rogers P. Positive control of expression of the argECBH gene cluster in vitro by guanosine 5'-diphosphate 3'-diphosphate. J Bacteriol. 1984 Aug;159(2):647–651. doi: 10.1128/jb.159.2.647-651.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


