Abstract
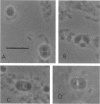
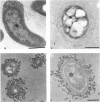
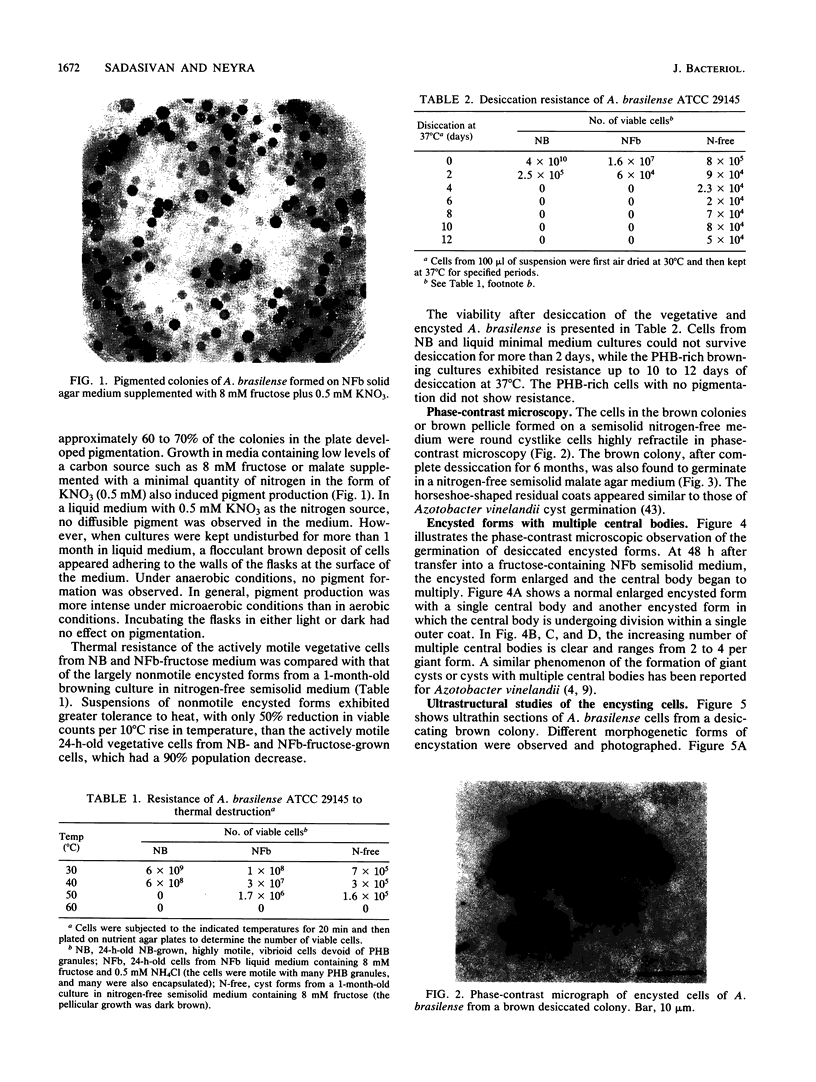
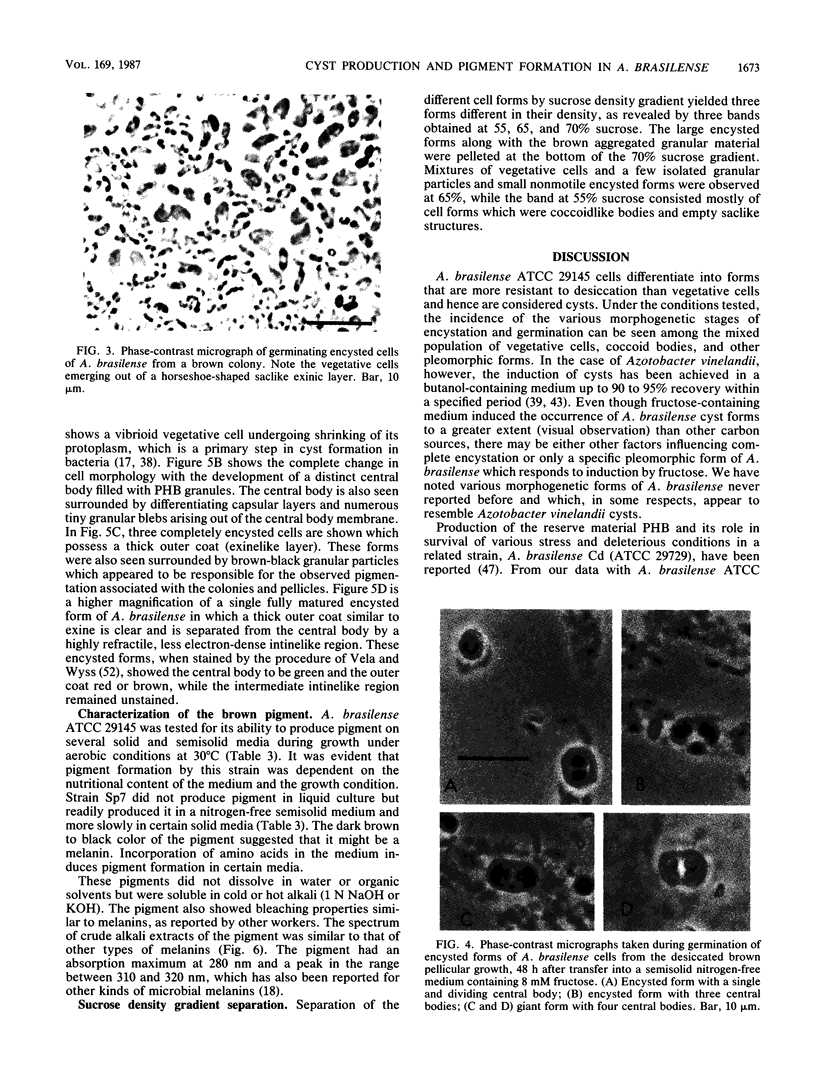
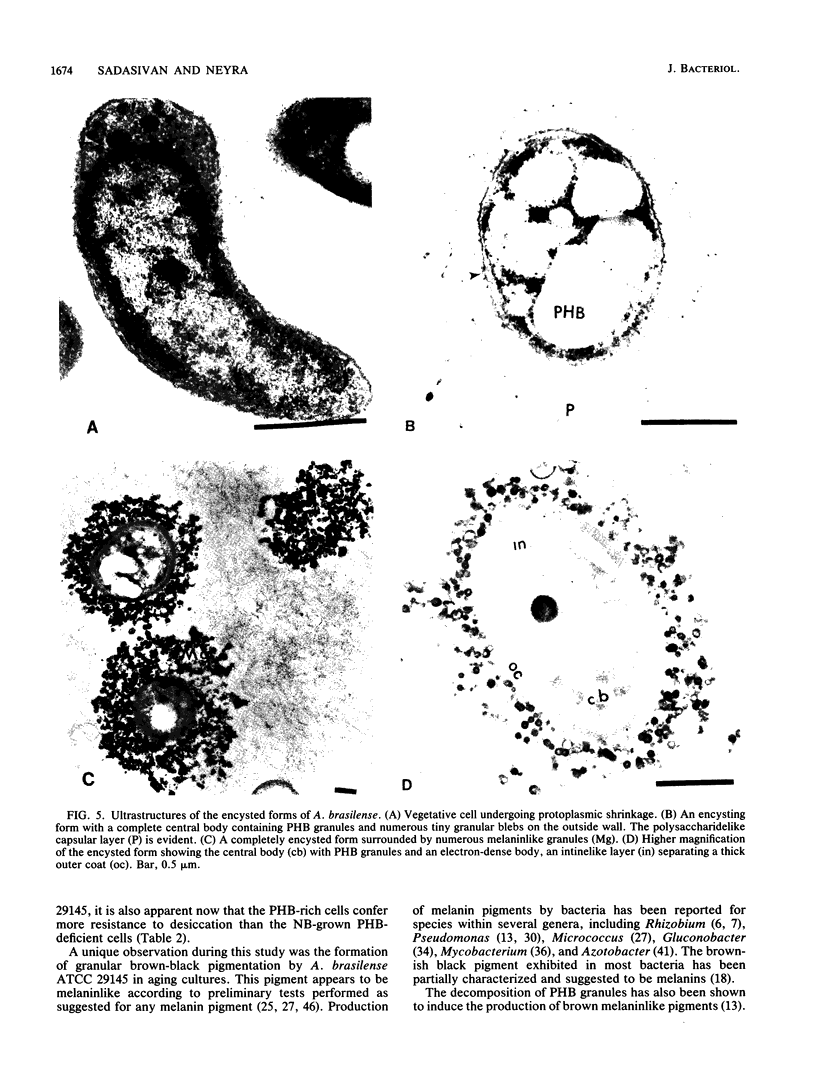
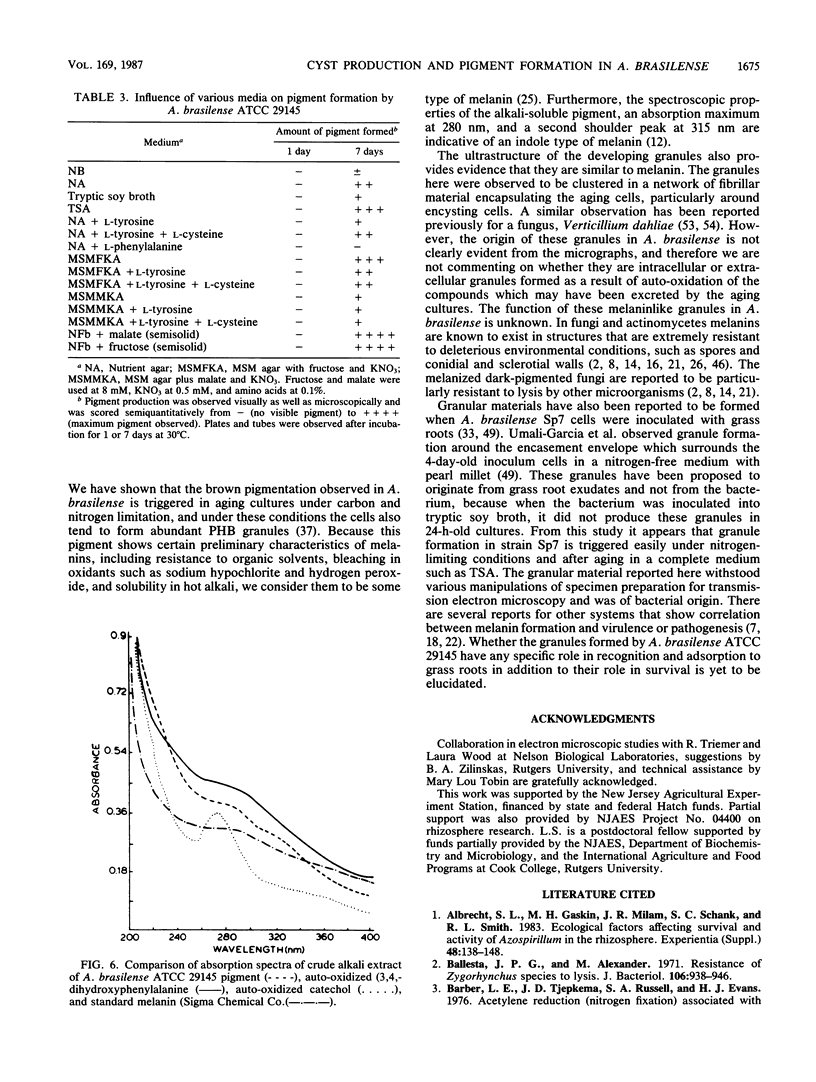
Encystation in Azospirillum brasilense ATCC 29145 was observed by using routine laboratory staining and phase-contrast and electron microscopy. Encystment occurred in liquid and in solid or semisolid media containing fructose (8 mM) and KNO3 (0.5 mM). The encysted forms consisted of a central body filled with poly-beta-hydroxybutyric acid granules, an electron-transparent intinelike region, and a thick outer layer. Enlarged giant encysted forms with multiple central bodies were also observed during the germination of a desiccated brown colony. Morphogenetically different forms in an aging culture could be resolved by sucrose density gradient centrifugation. The dense encysted forms along with numerous granules in a fibrillar network pelleted at 70% sucrose, while empty saclike envelopes along with vegetative cells and coccoid bodies pelleted at 55% sucrose. Different media induced various degrees of pigmentation in A. brasilense ATCC 29145 after aging. The pigment possessed several of the properties reported for microbial melanins, including insolubility in water and organic solvents, solubility in cold and hot alkali, and bleaching in hydrogen peroxide. The UV absorption maxima of the alkali extract were at 280 and 310 nm. Electron micrographs of the brown pigment showed that it occurred as aggregated granules surrounding the encysting cells as well as being excreted into the medium in an aging culture. It is concluded that A. brasilense ATCC 29145 produces compounds that form a brown pigment similar to melanin and are expressed under the influence of certain cultural conditions conducive for encystment.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballesta J. P., Alexander M. Resistance of Zygorhynchus species to lysis. J Bacteriol. 1971 Jun;106(3):938–945. doi: 10.1128/jb.106.3.938-945.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L., Jackson L. E., Shankel D. M. Formation of multiple central bodies in giant cysts of Azotobacter vinelandii. J Bacteriol. 1968 Jul;96(1):266–269. doi: 10.1128/jb.96.1.266-269.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg R. H., Tyler M. E., Novick N. J., Vasil V., Vasil I. K. Biology of azospirillum-sugarcane association: enhancement of nitrogenase activity. Appl Environ Microbiol. 1980 Mar;39(3):642–649. doi: 10.1128/aem.39.3.642-649.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield B. J., Alexander M. Melanins and resistance of fungi to lysis. J Bacteriol. 1967 Apr;93(4):1276–1280. doi: 10.1128/jb.93.4.1276-1280.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagle G. D., Vela G. R. Giant cysts and cysts with multiple central bodies in Azotobacter vinelandii. J Bacteriol. 1971 Jul;107(1):315–319. doi: 10.1128/jb.107.1.315-319.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E., Okon Y., Kigel J., Nur I., Henis Y. Increase in Dry Weight and Total Nitrogen Content in Zea mays and Setaria italica Associated with Nitrogen-fixing Azospirillum spp. Plant Physiol. 1980 Oct;66(4):746–749. doi: 10.1104/pp.66.4.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das K. C., Abramson M. B., Katzman R. Neuronal pigments: spectroscopic characterization of human brain melanin. J Neurochem. 1978 Mar;30(3):601–605. doi: 10.1111/j.1471-4159.1978.tb07814.x. [DOI] [PubMed] [Google Scholar]
- Delafield F. P., Doudoroff M., Palleroni N. J., Lusty C. J., Contopoulos R. Decomposition of poly-beta-hydroxybutyrate by pseudomonads. J Bacteriol. 1965 Nov;90(5):1455–1466. doi: 10.1128/jb.90.5.1455-1466.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskew D. L., Focht D. D., Ting I. P. Nitrogen fixation, denitrification, and pleomorphic growth in a highly pigmented Spirillum lipoferum. Appl Environ Microbiol. 1977 Nov;34(5):582–585. doi: 10.1128/aem.34.5.582-585.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchins V. M., Sadoff H. L. Morphogenesis of cysts in Azotobacter vinelandii. J Bacteriol. 1970 Oct;104(1):492–498. doi: 10.1128/jb.104.1.492-498.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivins B. E., Holmes R. K. Isolation and characterization of melanin-producing (mel) mutants of Vibrio cholerae. Infect Immun. 1980 Mar;27(3):721–729. doi: 10.1128/iai.27.3.721-729.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo M. J., Alexander M. Inhibition of the lysis of fungi by melanins. J Bacteriol. 1967 Sep;94(3):624–629. doi: 10.1128/jb.94.3.624-629.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon-Chung K. J., Rhodes J. C. Encapsulation and melanin formation as indicators of virulence in Cryptococcus neoformans. Infect Immun. 1986 Jan;51(1):218–223. doi: 10.1128/iai.51.1.218-223.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara G. W., Davey M. R., Lucas J. A. Effect of inoculation of Zea mays with Azospirillum brasilense strains under temperate conditions. Can J Microbiol. 1981 Sep;27(9):871–877. doi: 10.1139/m81-138. [DOI] [PubMed] [Google Scholar]
- Ogunnariwo J., Hamilton-Miller J. M. Brown- and red-pigmented Pseudomonas aeruginosa: differentiation between melanin and pyorubrin. J Med Microbiol. 1975 Feb;8(1):199–203. doi: 10.1099/00222615-8-1-199. [DOI] [PubMed] [Google Scholar]
- Pope L. M., Wyss O. Outer layers of the Azotobacter vinelandii cyst. J Bacteriol. 1970 Apr;102(1):234–239. doi: 10.1128/jb.102.1.234-239.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakaran K., Harris E. B., Kirchheimer W. F. The nature of the phenolase enzyme in Mycobacterium leprae: structure-activity relationships of substrates and comparison with other copper proteins and enzymes. Microbios. 1972;5(20):273–281. [PubMed] [Google Scholar]
- SUSSMAN A. S., LINGAPPA Y., BERNSTEIN I. A. EFFECT OF LIGHT AND MEDIA UPON GROWTH AND MELANIN FORMATION IN CLADOSPORIUM MANSONI. Mycopathol Mycol Appl. 1963 Oct 30;20:307–314. doi: 10.1007/BF02089218. [DOI] [PubMed] [Google Scholar]
- Sadasivan L., Neyra C. A. Flocculation in Azospirillum brasilense and Azospirillum lipoferum: exopolysaccharides and cyst formation. J Bacteriol. 1985 Aug;163(2):716–723. doi: 10.1128/jb.163.2.716-723.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoff H. L. Comparative aspects of morphogenesis in three prokaryotic genera. Annu Rev Microbiol. 1973;27:133–153. doi: 10.1146/annurev.mi.27.100173.001025. [DOI] [PubMed] [Google Scholar]
- Sadoff H. L. Encystment and germination in Azotobacter vinelandii. Bacteriol Rev. 1975 Dec;39(4):516–539. doi: 10.1128/br.39.4.516-539.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen M., Sen S. P. Interspecific transformation in Azotobacter. J Gen Microbiol. 1965 Oct;41(1):1–6. doi: 10.1099/00221287-41-1-1. [DOI] [PubMed] [Google Scholar]
- Smith R. L., Bouton J. H., Schank S. C., Quesenberry K. H., Tyler M. E., Milam J. R., Gaskins M. H., Littell R. C. Nitrogen Fixation in Grasses Inoculated with Spirillum lipoferum. Science. 1976 Sep 10;193(4257):1003–1005. doi: 10.1126/science.193.4257.1003. [DOI] [PubMed] [Google Scholar]
- Socolofsky M. D., Wyss O. CYSTS OF AZOTOBACTER. J Bacteriol. 1961 Jun;81(6):946–954. doi: 10.1128/jb.81.6.946-954.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umali-Garcia M., Hubbell D. H., Gaskins M. H., Dazzo F. B. Association of azospirillum with grass roots. Appl Environ Microbiol. 1980 Jan;39(1):219–226. doi: 10.1128/aem.39.1.219-226.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VELA G. R., WYSS O. IMPROVED STAIN FOR VISUALIZATION OF AZOTOBACTER ENCYSTMENT. J Bacteriol. 1964 Feb;87:476–477. doi: 10.1128/jb.87.2.476-477.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vela G. R. Survival of Azotobacter in dry soil. Appl Microbiol. 1974 Jul;28(1):77–79. doi: 10.1128/am.28.1.77-79.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler M. H., Tolmsoff W. J., Bell A. A., Mollenhauer H. H. Ultrastructural and chemical distinction of melanins formed by Verticillium dahliae from (+)-scytalone, 1,8-dihydroxynaphthalene, catechol, and L-3,4-dihydroxyphenylalanine. Can J Microbiol. 1978 Mar;24(3):289–297. doi: 10.1139/m78-049. [DOI] [PubMed] [Google Scholar]
- Wheeler M. H., Tolmsoff W. J., Meola S. Ultrastructure of melanin formation in Verticillium dahliae with (+)-scytalone as a biosynthetic intermediate. Can J Microbiol. 1976 May;22(5):702–711. doi: 10.1139/m76-103. [DOI] [PubMed] [Google Scholar]
- van Berkum P., Bohlool B. B. Evaluation of nitrogen fixation by bacteria in association with roots of tropical grasses. Microbiol Rev. 1980 Sep;44(3):491–517. doi: 10.1128/mr.44.3.491-517.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]