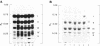Abstract
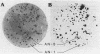
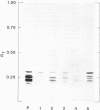
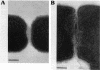
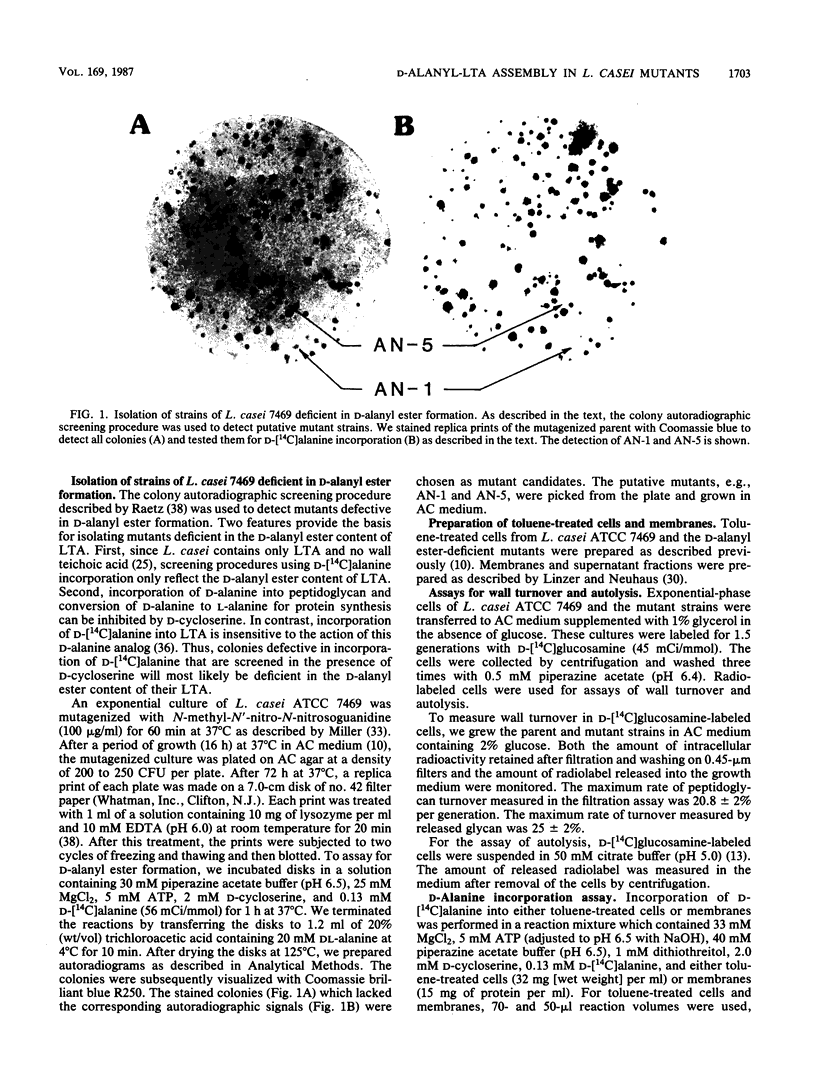
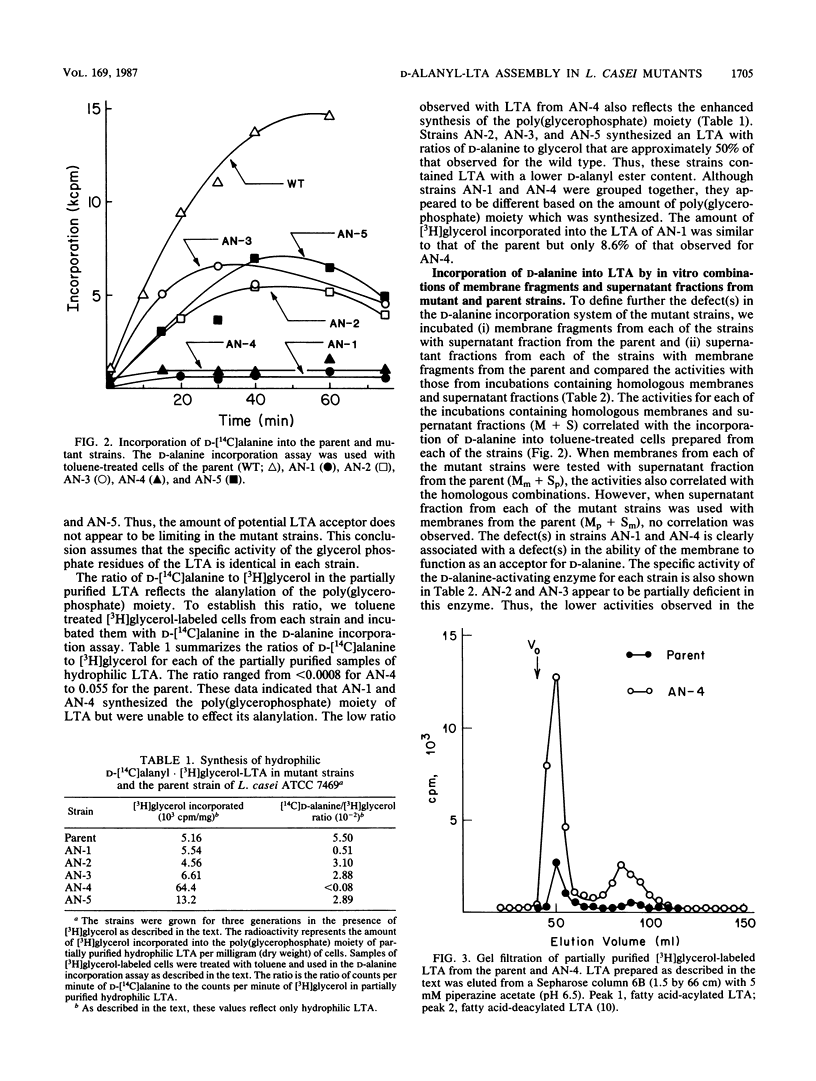
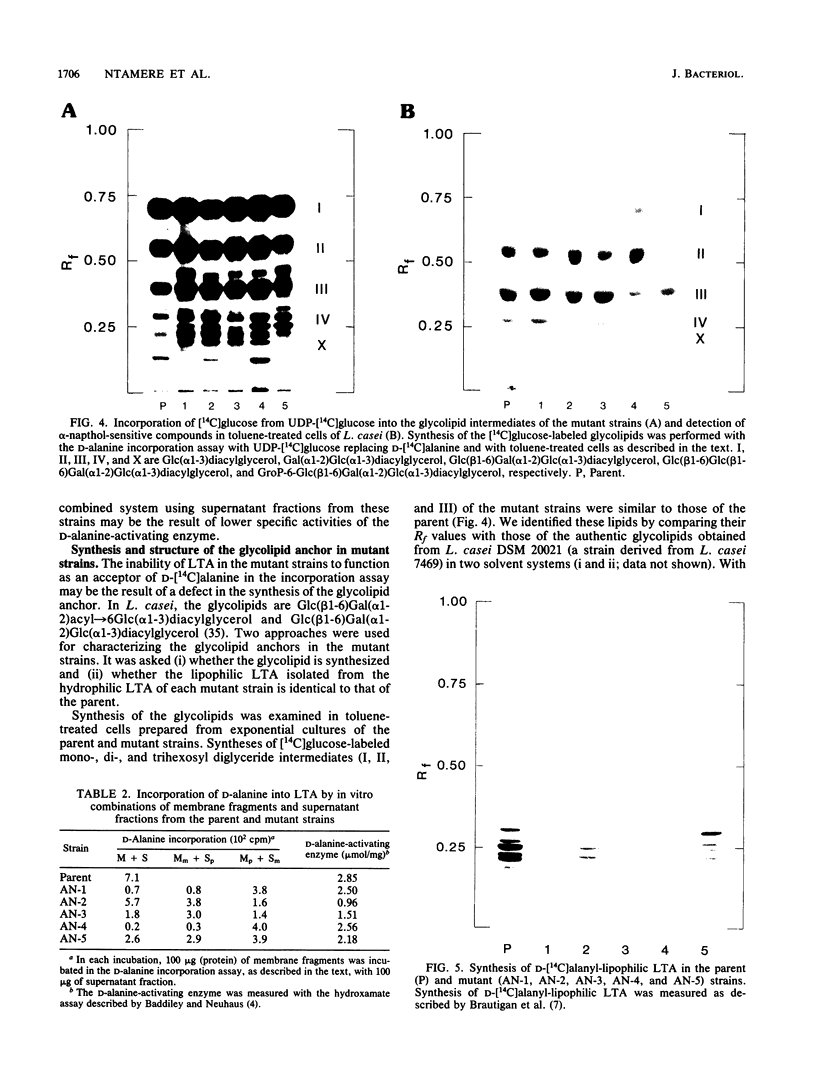
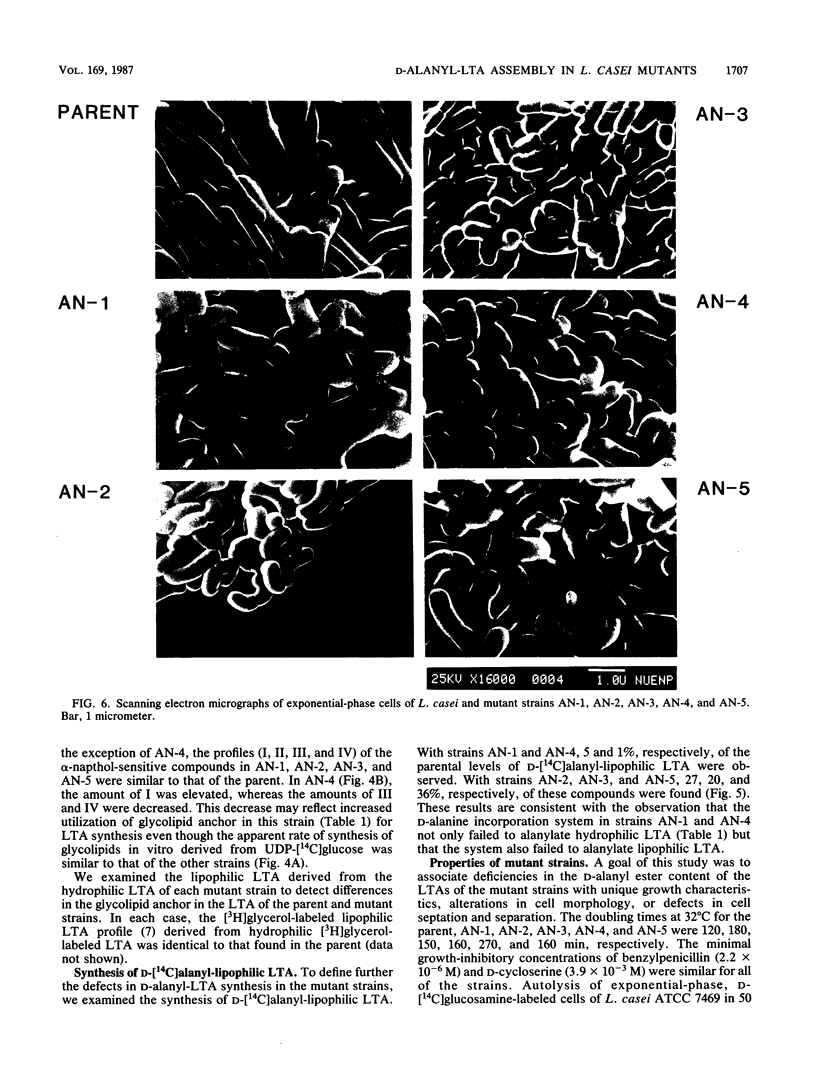
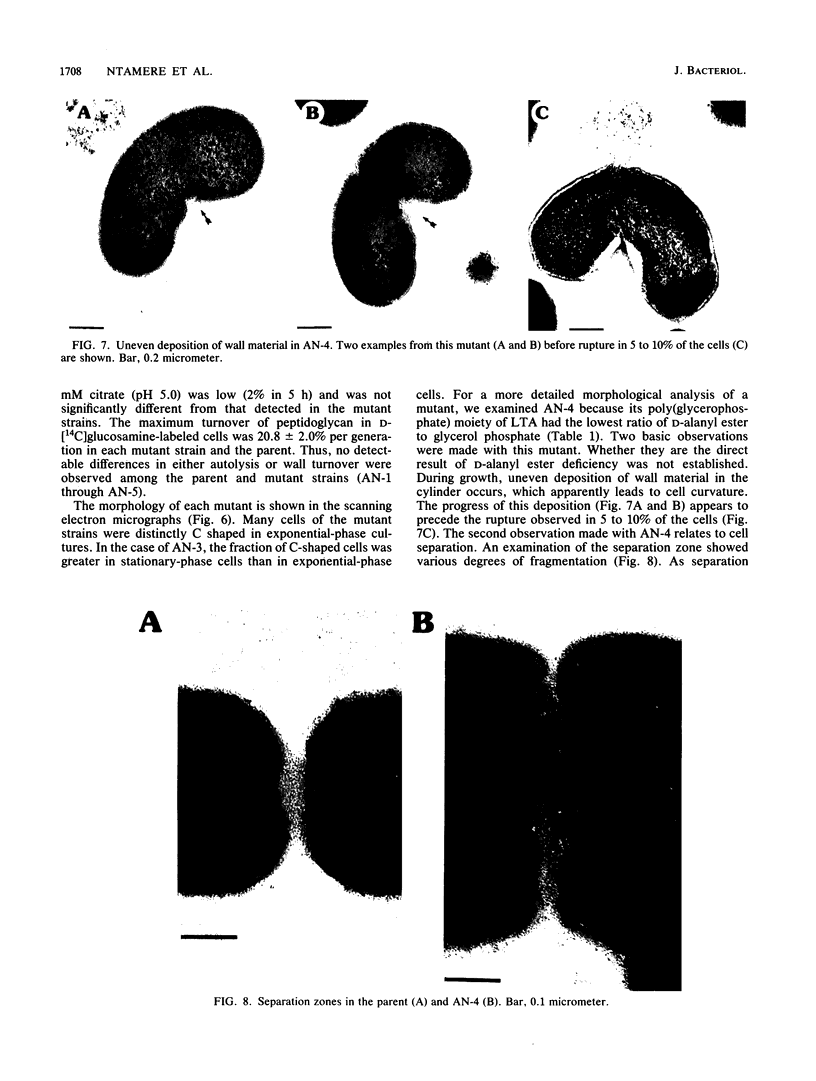
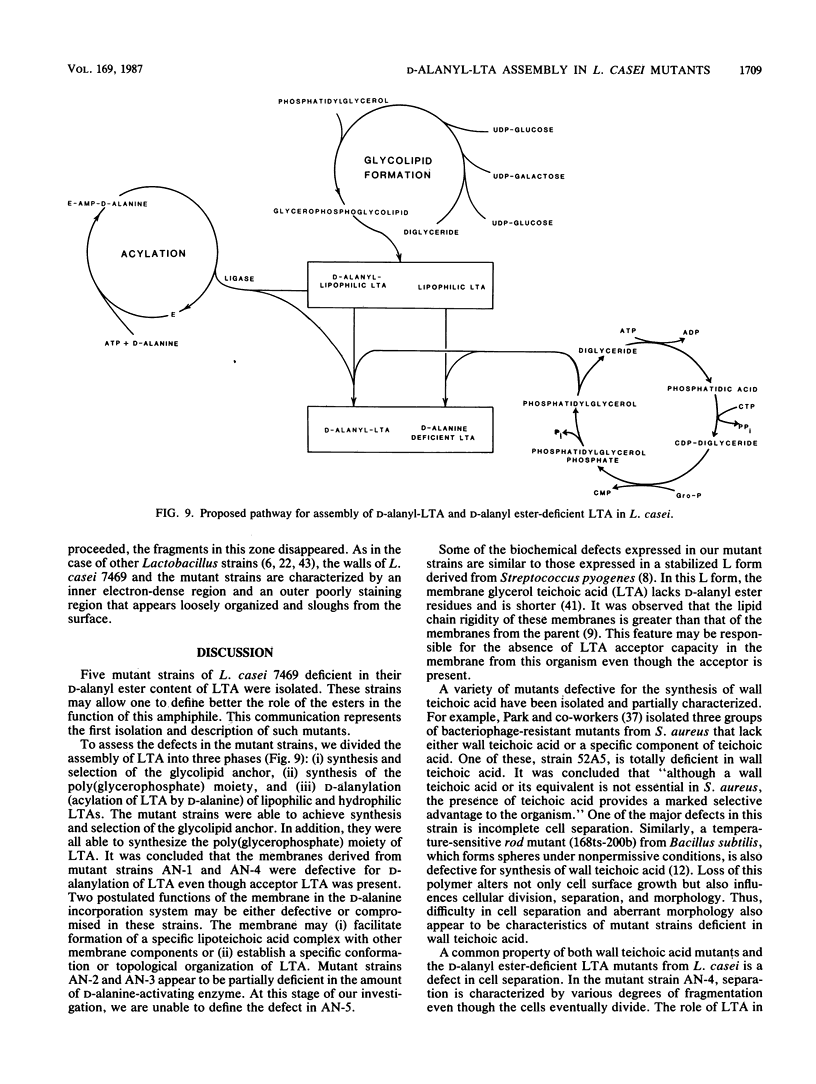
D-Alanyl-lipoteichoic acid (D-alanyl-LTA) from Lactobacillus casei ATCC 7469 contains a poly(glycerophosphate) moiety that is acylated with D-alanyl ester residues. The physiological function of these residues is not well understood. Five mutant strains of this organism that are deficient in the esters of this amphiphile were isolated and characterized. When compared with the parent, strains AN-1 and AN-4 incorporated less than 10% of D-[14C]alanine into LTA, whereas AN-2, AN-3, and AN-5 incorporated 50%. The synthesis of D-[14C]alanyl-lipophilic LTA was virtually absent in the first group and was approximately 30% in the second group. The mutant strains synthesized and selected the glycolipid anchor for LTA assembly. In addition, all of the strains synthesized the poly(glycerophosphate) moiety of LTA to the same extent as did the parent or to a greater extent. It was concluded that the membranes from the mutant strains AN-1 and AN-4 are defective for D-alanylation of LTA even though acceptor LTA is present. Mutant strains AN-2 and AN-3 appear to be partially deficient in the amount of the D-alanine-activating enzyme. Aberrant morphology and defective cell separation appear to result from this deficiency in D-alanyl ester content.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archibald A. R., Baddiley J., Heptinstall S. The alanine ester content and magnesium binding capacity of walls of Staphylococcus aureus H grown at different pH values. Biochim Biophys Acta. 1973 Feb 16;291(3):629–634. doi: 10.1016/0005-2736(73)90468-9. [DOI] [PubMed] [Google Scholar]
- BADDILEY J., NEUHAUS F. C. The enzymic activation of D-alanine. Biochem J. 1960 Jun;75:579–587. doi: 10.1042/bj0750579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Baddiley J. Teichoic acids in cell walls and membranes of bacteria. Essays Biochem. 1972;8:35–77. [PubMed] [Google Scholar]
- Boothby D., Daneo-Moore L., Higgins M. L., Coyette J., Shockman G. D. Turnover of bacterial cell wall peptidoglycans. J Biol Chem. 1973 Mar 25;248(6):2161–2169. [PubMed] [Google Scholar]
- Brautigan V. M., Childs W. C., 3rd, Neuhaus F. C. Biosynthesis of D-alanyl-lipoteichoic acid in Lactobacillus casei: D-alanyl-lipophilic compounds as intermediates. J Bacteriol. 1981 Apr;146(1):239–250. doi: 10.1128/jb.146.1.239-250.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevion M., Panos C., Linzer R., Neuhaus F. C. Incorporation of D-alanine into the membrane of Streptococcus pyogenes and its stabilized L-form. J Bacteriol. 1974 Dec;120(3):1026–1032. doi: 10.1128/jb.120.3.1026-1032.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevion M., Panos C., Paxton J. Membrane studies of Streptococcus pyogenes and its L-form growing in hypertonic and physiologically isotonic media. An electron spin resonance spectroscopy approach. Biochim Biophys Acta. 1976 Mar 5;426(2):288–301. doi: 10.1016/0005-2736(76)90338-2. [DOI] [PubMed] [Google Scholar]
- Childs W. C., 3rd, Neuhaus F. C. Biosynthesis of D-alanyl-lipoteichoic acid: characterization of ester-linked D-alanine in the in vitro-synthesized product. J Bacteriol. 1980 Jul;143(1):293–301. doi: 10.1128/jb.143.1.293-301.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland R. F., Wicken A. J., Daneo-Moore L., Shockman G. D. Inhibition of wall autolysis in Streptococcus faecalis by lipoteichoic acid and lipids. J Bacteriol. 1976 Apr;126(1):192–197. doi: 10.1128/jb.126.1.192-197.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. M., Popkin T. J., Boylan R. J., Mendelson N. H. Ultrastructure of a temperature-sensitive rod- mutant of Bacillus subtilis. J Bacteriol. 1970 Sep;103(3):793–810. doi: 10.1128/jb.103.3.793-810.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyette J., Ghuysen J. M. Wall autolysin of Lactobacillus acidophilus strain 63 AM gasser. Biochemistry. 1970 Jul 21;9(15):2952–2955. doi: 10.1021/bi00817a003. [DOI] [PubMed] [Google Scholar]
- Fan D. P., Beckman M. M., Cunningham W. P. Ultrastructural studies on a mutant of Bacillus subtilis whose growth is inhibited due to insufficient autolysin production. J Bacteriol. 1972 Mar;109(3):1247–1257. doi: 10.1128/jb.109.3.1247-1257.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler F., Glaser L. The synthesis of polyribitol phosphate. II. On the mechanism of polyribitol phosphate polymerase. J Biol Chem. 1974 May 10;249(9):2690–2695. [PubMed] [Google Scholar]
- Fischer W., Koch H. U., Rösel P., Fiedler F. Alanine ester-containing native lipoteichoic acids do not act as lipoteichoic acid carrier. Isolation, structural and functional characterization. J Biol Chem. 1980 May 25;255(10):4557–4562. [PubMed] [Google Scholar]
- Fischer W., Rösel P., Koch H. U. Effect of alanine ester substitution and other structural features of lipoteichoic acids on their inhibitory activity against autolysins of Staphylococcus aureus. J Bacteriol. 1981 May;146(2):467–475. doi: 10.1128/jb.146.2.467-475.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W., Rösel P. The alanine ester substitution of lipoteichoic acid (LTA) in Staphylococcus aureus. FEBS Lett. 1980 Oct 6;119(2):224–226. doi: 10.1016/0014-5793(80)80257-2. [DOI] [PubMed] [Google Scholar]
- Hancock I., Baddiley J. In vitro synthesis of the unit that links teichoic acid to peptidoglycan. J Bacteriol. 1976 Mar;125(3):880–886. doi: 10.1128/jb.125.3.880-886.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heptinstall S., Archibald A. R., Baddiley J. Teichoic acids and membrane function in bacteria. Nature. 1970 Feb 7;225(5232):519–521. doi: 10.1038/225519a0. [DOI] [PubMed] [Google Scholar]
- Higgins M. L., Coyette J., Shockman G. D. Sites of cellular autolysis in Lactobacillus acidophilus. J Bacteriol. 1973 Dec;116(3):1375–1382. doi: 10.1128/jb.116.3.1375-1382.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A. H., Hancock I. C., Baddiley J. The function of teichoic acids in cation control in bacterial membranes. Biochem J. 1973 Jan;132(1):83–93. doi: 10.1042/bj1320083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höltje J. V., Tomasz A. Lipoteichoic acid: a specific inhibitor of autolysin activity in Pneumococcus. Proc Natl Acad Sci U S A. 1975 May;72(5):1690–1694. doi: 10.1073/pnas.72.5.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELEMEN M. V., BADDILEY J. Structure of the intracellular glycerol teichoic acid from Lactobacillus casei A.T.C.C. 7469. Biochem J. 1961 Aug;80:246–254. doi: 10.1042/bj0800246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch H. U., Fischer W., Fiedler F. Influence of alanine ester and glycosyl substitution on the lipoteichoic acid carrier activity of lipoteichoic acids. J Biol Chem. 1982 Aug 25;257(16):9473–9479. [PubMed] [Google Scholar]
- Lambert P. A., Hancock I. C., Baddiley J. Influence of alanyl ester residues on the binding of magnesium ions to teichoic acids. Biochem J. 1975 Dec;151(3):671–676. doi: 10.1042/bj1510671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert P. A., Hancock I. C., Baddiley J. Occurrence and function of membrane teichoic acids. Biochim Biophys Acta. 1977 May 31;472(1):1–12. doi: 10.1016/0304-4157(77)90012-0. [DOI] [PubMed] [Google Scholar]
- Linzer R., Neuhaus F. C. Biosynthesis of membrane teichoic acid. A role of the D-alanine-activating enzyme. J Biol Chem. 1973 May 10;248(9):3196–3201. [PubMed] [Google Scholar]
- MacArthur A. E., Archibald A. R. Effect of culture pH on the D-alanine ester content of lipoteichoic acid in Staphylococcus aureus. J Bacteriol. 1984 Nov;160(2):792–793. doi: 10.1128/jb.160.2.792-793.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M., Fischer W. The glycolipids of Lactobacillus casei DSM 20021. Hoppe Seylers Z Physiol Chem. 1977 Nov;358(11):1439–1453. doi: 10.1515/bchm2.1977.358.2.1439. [DOI] [PubMed] [Google Scholar]
- Nakano M., Fischer W. Trihexosyldiacylglycerol and acyltrihexosyldiacylglycerol as lipid anchors of the lipoteichoic acid of Lactobacillus casei DSM 20021. Hoppe Seylers Z Physiol Chem. 1978 Jan;359(1):1–11. doi: 10.1515/bchm.1978.359.1.1. [DOI] [PubMed] [Google Scholar]
- Neuhaus F. C., Linzer R., Reusch V. M., Jr Biosynthesis of membrane teichoic acid: role of the D-alanine-activating enzyme and D-alanine: membrane acceptor ligase. Ann N Y Acad Sci. 1974 May 10;235(0):502–518. doi: 10.1111/j.1749-6632.1974.tb43287.x. [DOI] [PubMed] [Google Scholar]
- Park J. T., Shaw D. R., Chatterjee A. N., Mirelman D., Wu T. Mutants of staphylococci with altered cell walls. Ann N Y Acad Sci. 1974 Jul 31;236(0):54–62. doi: 10.1111/j.1749-6632.1974.tb41481.x. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R. Isolation of Escherichia coli mutants defective in enzymes of membrane lipid synthesis. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2274–2278. doi: 10.1073/pnas.72.6.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusch V. M., Jr, Neuhaus F. C. D-Alanine: membrane acceptor ligase from Lactobacillus casei. J Biol Chem. 1971 Oct 25;246(20):6136–6143. [PubMed] [Google Scholar]
- Slabyj B. M., Panos C. Teichoic acid of a stabilized L-form of Streptococcus pyogenes. J Bacteriol. 1973 Jun;114(3):934–942. doi: 10.1128/jb.114.3.934-942.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Séchaud J., Kellenberger E. Electron microscopy of DNA-containing plasms. IV. Glutaraldehyde-uranyl acetate fixation of virus-infected bacteria for thin sectioning. J Ultrastruct Res. 1972 Jun;39(5):598–607. doi: 10.1016/s0022-5320(72)90124-4. [DOI] [PubMed] [Google Scholar]
- Thorne K. J., Barker D. C. Bactoprenol, ATPase and acetate activating enzymes of a vesicular fraction from Lactobacillus casei. Eur J Biochem. 1969 Dec;11(3):582–591. doi: 10.1111/j.1432-1033.1969.tb00810.x. [DOI] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicken A. J., Gibbens J. W., Knox K. W. Comparative studies on the isolation of membrane lipoteichoic acid from Lactobacillus fermenti. J Bacteriol. 1973 Jan;113(1):365–372. doi: 10.1128/jb.113.1.365-372.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]