Abstract
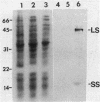
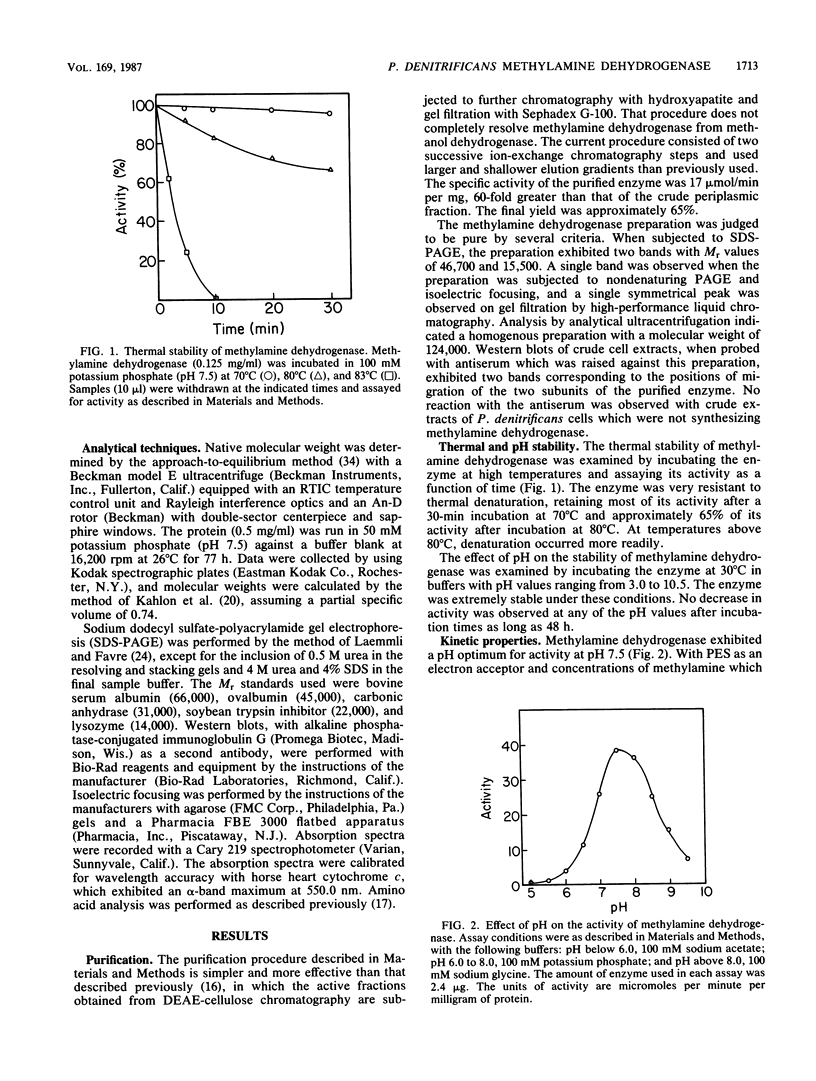
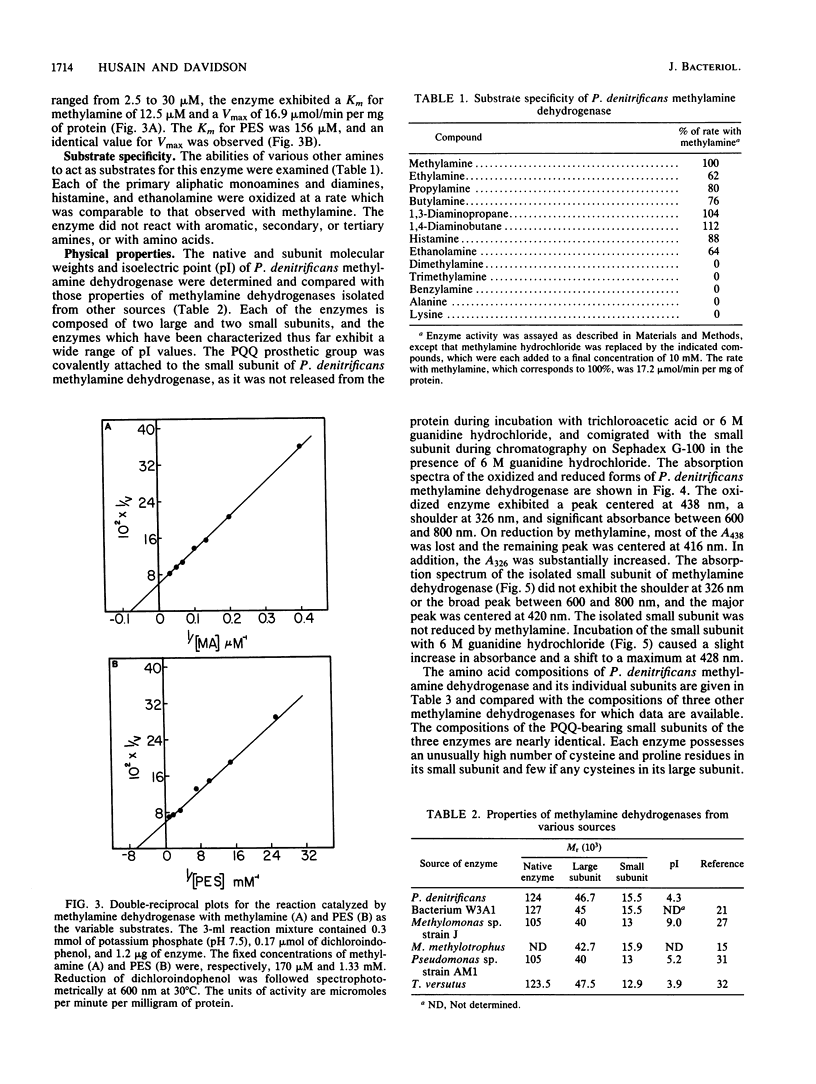
Methylamine dehydrogenase from Paracoccus denitrificans was purified to homogeneity in two steps from the periplasmic fraction of methylamine-grown cells. The enzyme exhibited a pI value of 4.3 and was composed of two 46,700-dalton subunits and two 15,500-dalton subunits. Each small subunit possessed a covalently bound pyrrolo-quinoline quinone prosthetic group. The amino acid compositions of the large and small subunits are very similar to those of other methylamine dehydrogenases which have been isolated from taxonomically different sources. The enzyme was able to catalyze the oxidation of a wide variety of primary aliphatic amines and diamines, but it did not react with secondary, tertiary, or aromatic amines. The enzyme exhibited optimal activity at pH 7.5, with Km values of 12.5 microM for methylamine and 156 microM for phenazine ethosulfate and a Vmax of 16.9 mumol/min per mg of protein. No loss of enzyme activity was observed after incubation for 48 h at pH values ranging from 3.0 to 10.5, and the enzyme was very stable to thermal denaturation. Enzyme activity and immunological detection of each subunit were only observed with cells which had been grown on methylamine as a carbon source.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG J. M. THE MOLAR EXTINCTION COEFFICIENT OF 2,6-DICHLOROPHENOL INDOPHENOL. Biochim Biophys Acta. 1964 Apr 4;86:194–197. doi: 10.1016/0304-4165(64)90180-1. [DOI] [PubMed] [Google Scholar]
- Alefounder P. R., Ferguson S. J. A periplasmic location for methanol dehydrogenase from Paracoccus denitrificans: implications for proton pumping by cytochrome aa3. Biochem Biophys Res Commun. 1981 Feb 12;98(3):778–784. doi: 10.1016/0006-291x(81)91179-7. [DOI] [PubMed] [Google Scholar]
- Ameyama M., Matsushita K., Ohno Y., Shinagawa E., Adachi O. Existence of a novel prosthetic group, PQQ, in membrane-bound, electron transport chain-linked, primary dehydrogenases of oxidative bacteria. FEBS Lett. 1981 Aug 3;130(2):179–183. doi: 10.1016/0014-5793(81)81114-3. [DOI] [PubMed] [Google Scholar]
- Davidson V. L., Husain M., Neher J. W. Electron transfer flavoprotein from Methylophilus methylotrophus: properties, comparison with other electron transfer flavoproteins, and regulation of expression by carbon source. J Bacteriol. 1986 Jun;166(3):812–817. doi: 10.1128/jb.166.3.812-817.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson V. L. Regulation by carbon source of enzyme expression and slime production in bacterium W3A1. J Bacteriol. 1985 Nov;164(2):941–943. doi: 10.1128/jb.164.2.941-943.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duine J. A., Frank J., Jr The prosthetic group of methanol dehydrogenase. Purification and some of its properties. Biochem J. 1980 Apr 1;187(1):221–226. doi: 10.1042/bj1870221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duine J. A., Frank J., van Zeeland J. K. Glucose dehydrogenase from Acinetobacter calcoaceticus: a 'quinoprotein'. FEBS Lett. 1979 Dec 15;108(2):443–446. doi: 10.1016/0014-5793(79)80584-0. [DOI] [PubMed] [Google Scholar]
- Eady R. R., Large P. J. Purification and properties of an amine dehydrogenase from Pseudomonas AM1 and its role in growth on methylamine. Biochem J. 1968 Jan;106(1):245–255. doi: 10.1042/bj1060245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray K. A., Knaff D. B., Husain M., Davidson V. L. Measurement of the oxidation-reduction potentials of amicyanin and c-type cytochromes from Paracoccus denitrificans. FEBS Lett. 1986 Oct 27;207(2):239–242. doi: 10.1016/0014-5793(86)81496-x. [DOI] [PubMed] [Google Scholar]
- Husain M., Davidson V. L. An inducible periplasmic blue copper protein from Paracoccus denitrificans. Purification, properties, and physiological role. J Biol Chem. 1985 Nov 25;260(27):14626–14629. [PubMed] [Google Scholar]
- Husain M., Davidson V. L. Characterization of two inducible periplasmic c-type cytochromes from Paracoccus denitrificans. J Biol Chem. 1986 Jul 5;261(19):8577–8580. [PubMed] [Google Scholar]
- Husain M., Davidson V. L., Smith A. J. Properties of Paracoccus denitrificans amicyanin. Biochemistry. 1986 May 6;25(9):2431–2436. doi: 10.1021/bi00357a020. [DOI] [PubMed] [Google Scholar]
- KORNBERG H. L., MORRIS J. G. THE UTILIZATION OF GLYCOLLATE BY MICROCOCCUS DENITRIFICANS: THE BETA-HYDROXYASPARTATE PATHWAY. Biochem J. 1965 Jun;95:577–586. doi: 10.1042/bj0950577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlon T. S., Glines L. A., Lindgren F. T. Analytic ultracentrifugation of plasma lipoproteins. Methods Enzymol. 1986;129:26–45. doi: 10.1016/0076-6879(86)29060-6. [DOI] [PubMed] [Google Scholar]
- Kenney W. C., McIntire W. Characterization of methylamine dehydrogenase from bacterium W3A1. Interaction with reductants and amino-containing compounds. Biochemistry. 1983 Aug 2;22(16):3858–3868. doi: 10.1021/bi00285a022. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Lim L. W., Mathews F. S., Husain M., Davidson V. L. Preliminary X-ray crystallographic study of amicyanin from Paracoccus denitrificans. J Mol Biol. 1986 May 5;189(1):257–258. doi: 10.1016/0022-2836(86)90398-0. [DOI] [PubMed] [Google Scholar]
- Lobenstein-Verbeek C. L., Jongejan J. A., Frank J., Duine J. A. Bovine serum amine oxidase: a mammalian enzyme having covalently bound PQQ as prosthetic group. FEBS Lett. 1984 May 21;170(2):305–309. doi: 10.1016/0014-5793(84)81333-2. [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Hiraoka B. Y., Tobari J. Methylamine dehydrogenase of Pseudomonase sp. J Isolation and properties of the subunits. Biochim Biophys Acta. 1978 Feb 10;522(2):303–310. doi: 10.1016/0005-2744(78)90064-5. [DOI] [PubMed] [Google Scholar]
- Matsumoto T. Methylamine dehydrogenase of Pseudomonas sp. J. Purification and properties. Biochim Biophys Acta. 1978 Feb 10;522(2):291–302. doi: 10.1016/0005-2744(78)90063-3. [DOI] [PubMed] [Google Scholar]
- McNerney T., O'connor M. L. Regulation of enzymes associated with C-1 metabolism in three facultative methylotrophs. Appl Environ Microbiol. 1980 Aug;40(2):370–375. doi: 10.1128/aem.40.2.370-375.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai S., Matsumoto T., Tobari J. Methylamine dehydrogenase of Pseudomonas AM1. A subunit enzyme. J Biochem. 1978 Jun;83(6):1599–1607. doi: 10.1093/oxfordjournals.jbchem.a132071. [DOI] [PubMed] [Google Scholar]
- Vellieux F. M., Frank J., Swarte M. B., Groendijk H., Duine J. A., Drenth J., Hol W. G. Purification, crystallization and preliminary X-ray investigation of quinoprotein methylamine dehydrogenase from Thiobacillus versutus. Eur J Biochem. 1986 Jan 15;154(2):383–386. doi: 10.1111/j.1432-1033.1986.tb09409.x. [DOI] [PubMed] [Google Scholar]
- Weaver C. A., Lidstrom M. E. Methanol dissimilation in Xanthobacter H4-14: activities, induction and comparison to Pseudomonas AM1 and Paracoccus denitrificans. J Gen Microbiol. 1985 Sep;131(9):2183–2197. doi: 10.1099/00221287-131-9-2183. [DOI] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]
- de Beer R., Duine J. A., Frank J., Large P. J. The prosthetic group of methylamine dehydrogenase from Pseudomonas AM1: evidence for a quinone structure. Biochim Biophys Acta. 1980 Apr 25;622(2):370–374. doi: 10.1016/0005-2795(80)90050-1. [DOI] [PubMed] [Google Scholar]