Abstract
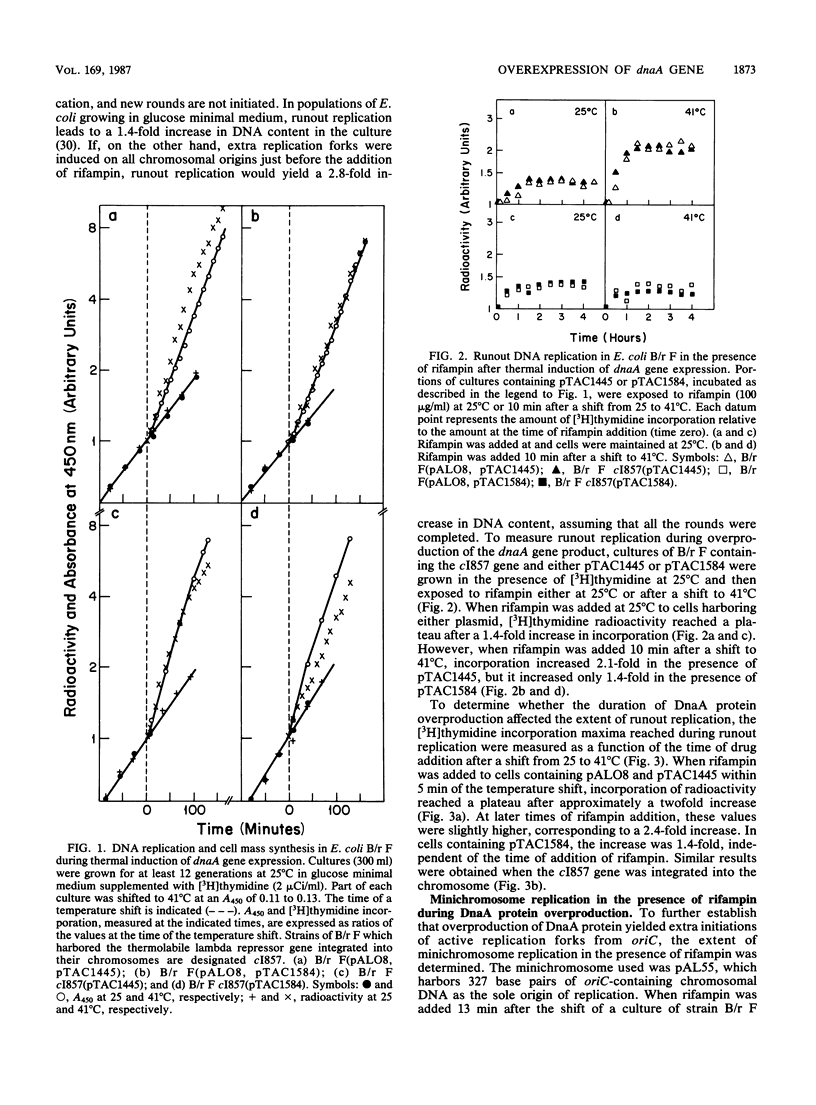
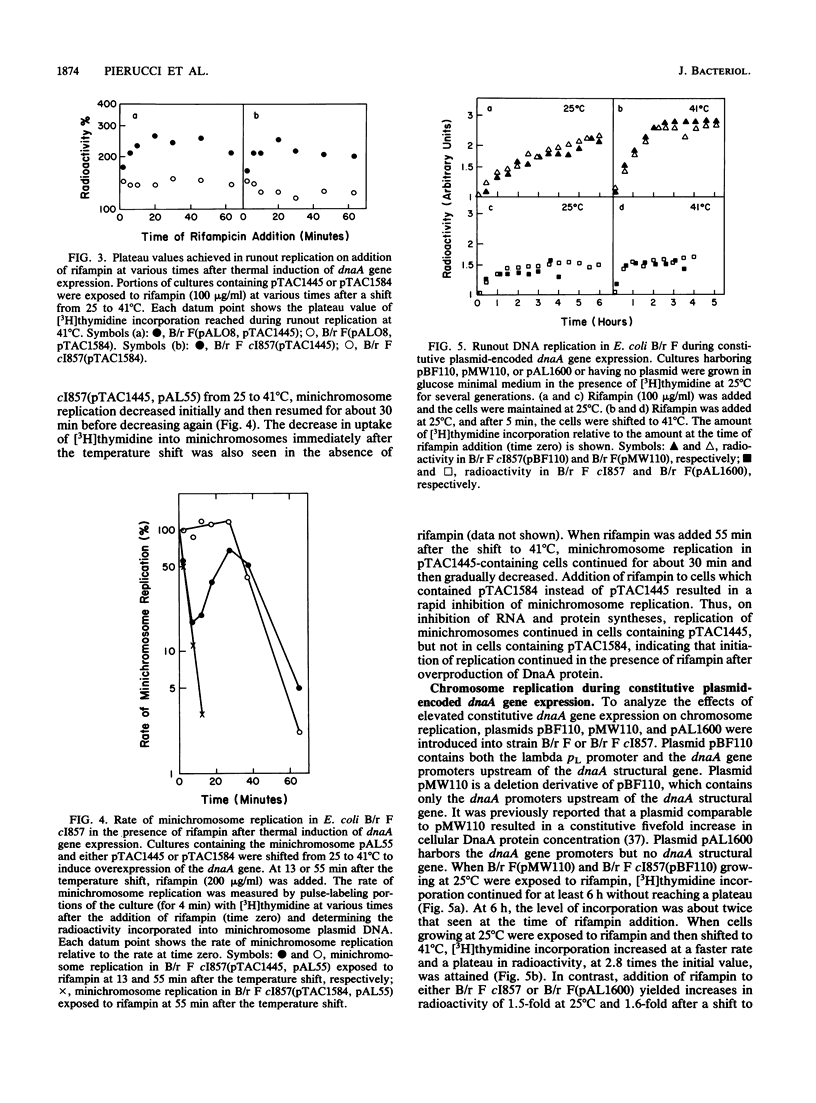
The replication of chromosomes and minichromosomes in Escherichia coli B/r was examined under conditions in which the dnaA gene product was overproduced. Increased levels of the DnaA protein were achieved by thermoinduction of the dnaA gene, under the control of the lambda pL promoter, or by cellular maintenance of multicopy plasmids carrying the dnaA gene under the control of its own promoters. Previous work has shown that overproduction of DnaA protein stimulates replication of the chromosomal origin, oriC, but that the newly initiated forks do not progress along the length of the chromosome (T. Atlung, K. V. Rasmussen, E. Clausen, and F. G. Hansen, p. 282-297, in M. Schaechter, F. C. Neidhardt, J. L. Ingraham, and N. O. Kjeldgaard, ed., The Molecular Biology of Bacterial Growth, 1985). In the present study, it was found that overproduction of DnaA protein caused both a two- to threefold increase in the amount of residual chromosome replication and an extended synthesis of minichromosome DNA in the presence of rifampin. The amount of residual chromosome replication was consistent with the appearance of functional replication forks on the majority of the chromosomes. Since the rate of DNA accumulation and the cellular DNA/mass ratios were not increased significantly by overexpression of the dnaA gene, we concluded that the addition of rifampin either enabled stalled replication forks to proceed beyond oriC or enabled new forks to initiate on both chromosomes and minichromosomes, or both.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atlung T., Clausen E. S., Hansen F. G. Autoregulation of the dnaA gene of Escherichia coli K12. Mol Gen Genet. 1985;200(3):442–450. doi: 10.1007/BF00425729. [DOI] [PubMed] [Google Scholar]
- Atlung T., Løbner-Olesen A., Hansen F. G. Overproduction of DnaA protein stimulates initiation of chromosome and minichromosome replication in Escherichia coli. Mol Gen Genet. 1987 Jan;206(1):51–59. doi: 10.1007/BF00326535. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal R. M., Dennis P. P. Gene expression in Escherichia coli B/r during partial rifampicin-mediated restrictions of transcription initiation. Mol Gen Genet. 1978 Sep 20;165(1):79–86. doi: 10.1007/BF00270379. [DOI] [PubMed] [Google Scholar]
- Braun R. E., O'Day K., Wright A. Autoregulation of the DNA replication gene dnaA in E. coli K-12. Cell. 1985 Jan;40(1):159–169. doi: 10.1016/0092-8674(85)90319-8. [DOI] [PubMed] [Google Scholar]
- Bremer H., Churchward G. Initiation of chromosome replication in Escherichia coli after induction of dnaA gene expression from a lac promoter. J Bacteriol. 1985 Nov;164(2):922–924. doi: 10.1128/jb.164.2.922-924.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carl P. L. Escherichia coli mutants with temperature-sensitive synthesis of DNA. Mol Gen Genet. 1970;109(2):107–122. doi: 10.1007/BF00269647. [DOI] [PubMed] [Google Scholar]
- Chakraborty T., Yoshinaga K., Lother H., Messer W. Purification of the E. coli dnaA gene product. EMBO J. 1982;1(12):1545–1549. doi: 10.1002/j.1460-2075.1982.tb01353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchward G., Holmans P., Bremer H. Increased expression of the dnaA gene has no effect on DNA replication in a dnaA+ strain of Escherichia coli. Mol Gen Genet. 1983;192(3):506–508. doi: 10.1007/BF00392197. [DOI] [PubMed] [Google Scholar]
- Frey J., Chandler M., Caro L. Overinitiation of chromosome and plasmid replication in a dna Acos mutant of Escherichia coli K12. Evidence for dnaA-dnaB interactions. J Mol Biol. 1984 Oct 25;179(2):171–183. doi: 10.1016/0022-2836(84)90464-9. [DOI] [PubMed] [Google Scholar]
- Frey J., Chandler M., Caro L. The initiation of chromosome replication in a dnaAts46 and a dnaA+ strain at various temperatures. Mol Gen Genet. 1981;182(2):364–366. doi: 10.1007/BF00269686. [DOI] [PubMed] [Google Scholar]
- Fuller R. S., Funnell B. E., Kornberg A. The dnaA protein complex with the E. coli chromosomal replication origin (oriC) and other DNA sites. Cell. 1984 Oct;38(3):889–900. doi: 10.1016/0092-8674(84)90284-8. [DOI] [PubMed] [Google Scholar]
- Fuller R. S., Kornberg A. Purified dnaA protein in initiation of replication at the Escherichia coli chromosomal origin of replication. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5817–5821. doi: 10.1073/pnas.80.19.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen F. G., Hansen E. B., Atlung T. The nucleotide sequence of the dnaA gene promoter and of the adjacent rpmH gene, coding for the ribosomal protein L34, of Escherichia coli. EMBO J. 1982;1(9):1043–1048. doi: 10.1002/j.1460-2075.1982.tb01294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen F. G., Rasmussen K. V. Regulation of the dnaA product in Escherichia coli. Mol Gen Genet. 1977 Oct 20;155(2):219–225. doi: 10.1007/BF00393163. [DOI] [PubMed] [Google Scholar]
- Hayward R. S., Fyfe S. K. Non-coordinate expression of the neighbouring genes rplL and rpoB,C of Escherichia coli. Mol Gen Genet. 1978 Mar 20;160(1):77–80. doi: 10.1007/BF00275121. [DOI] [PubMed] [Google Scholar]
- Helmstetter C. E., Pierucci O. DNA synthesis during the division cycle of three substrains of Escherichia coli B/r. J Mol Biol. 1976 Apr 15;102(3):477–486. doi: 10.1016/0022-2836(76)90329-6. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Mordoh J., Jacob F. On the process of cellular division in Escherichia coli. 3. Thermosensitive mutants of Escherichia coli altered in the process of DNA initiation. J Mol Biol. 1970 Nov 14;53(3):369–387. doi: 10.1016/0022-2836(70)90072-0. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Ryter A., Jacob F. Thermosensitive mutants of E. coli affected in the processes of DNA synthesis and cellular division. Cold Spring Harb Symp Quant Biol. 1968;33:677–693. doi: 10.1101/sqb.1968.033.01.077. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Yamada M., Nishimura A., Oka A., Sugimoto K., Asada K., Takanami M. The DNA replication origin (ori) of Escherichia coli: structure and function of the ori-containing DNA fragment. Prog Nucleic Acid Res Mol Biol. 1981;26:33–48. doi: 10.1016/s0079-6603(08)60393-1. [DOI] [PubMed] [Google Scholar]
- Kellenberger-Gujer G., Podhajska A. J., Caro L. A cold sensitive dnaA mutant of E. coli which overinitiates chromosome replication at low temperature. Mol Gen Genet. 1978 Jun 1;162(1):9–16. doi: 10.1007/BF00333845. [DOI] [PubMed] [Google Scholar]
- Leonard A. C., Helmstetter C. E. Cell cycle-specific replication of Escherichia coli minichromosomes. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5101–5105. doi: 10.1073/pnas.83.14.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lother H., Kölling R., Kücherer C., Schauzu M. dnaA protein-regulated transcription: effects on the in vitro replication of Escherichia coli minichromosomes. EMBO J. 1985 Feb;4(2):555–560. doi: 10.1002/j.1460-2075.1985.tb03664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui M., Oka A., Takanami M., Yasuda S., Hirota Y. Sites of dnaA protein-binding in the replication origin of the Escherichia coli K-12 chromosome. J Mol Biol. 1985 Aug 5;184(3):529–533. doi: 10.1016/0022-2836(85)90299-2. [DOI] [PubMed] [Google Scholar]
- McKenney K., Shimatake H., Court D., Schmeissner U., Brady C., Rosenberg M. A system to study promoter and terminator signals recognized by Escherichia coli RNA polymerase. Gene Amplif Anal. 1981;2:383–415. [PubMed] [Google Scholar]
- Nakamura Y., Yura T. Effects of rifampicin on synthesis and functional activity of DNA-dependent RNA polymerase in Escherichia coli. Mol Gen Genet. 1976 Jun 15;145(3):227–237. doi: 10.1007/BF00325817. [DOI] [PubMed] [Google Scholar]
- Oka A., Sugimoto K., Takanami M., Hirota Y. Replication origin of the Escherichia coli K-12 chromosome: the size and structure of the minimum DNA segment carrying the information for autonomous replication. Mol Gen Genet. 1980 Apr;178(1):9–20. doi: 10.1007/BF00267207. [DOI] [PubMed] [Google Scholar]
- Pierucci O. Chromosome replication and cell division in Escherichia coli at various temperatures of growth. J Bacteriol. 1972 Feb;109(2):848–854. doi: 10.1128/jb.109.2.848-854.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard R. H., Zaritsky A. Effect of thymine concentration on the replication velocity of DNA in a thymineless mutant of Escherichia coli. Nature. 1970 Apr 11;226(5241):126–131. doi: 10.1038/226126a0. [DOI] [PubMed] [Google Scholar]
- Projan S. J., Carleton S., Novick R. P. Determination of plasmid copy number by fluorescence densitometry. Plasmid. 1983 Mar;9(2):182–190. doi: 10.1016/0147-619x(83)90019-7. [DOI] [PubMed] [Google Scholar]
- Rasmussen K. V., Atlung T., Kerszman G., Hansen G. E., Hansen F. G. Conditional change of DNA replication control in an RNA polymerase mutant of Escherichia coli. J Bacteriol. 1983 Apr;154(1):443–451. doi: 10.1128/jb.154.1.443-451.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAECHTER M., MAALOE O., KJELDGAARD N. O. Dependency on medium and temperature of cell size and chemical composition during balanced grown of Salmonella typhimurium. J Gen Microbiol. 1958 Dec;19(3):592–606. doi: 10.1099/00221287-19-3-592. [DOI] [PubMed] [Google Scholar]
- Schaus N., O'Day K., Peters W., Wright A. Isolation and characterization of amber mutations in gene dnaA of escherichia coli K-12. J Bacteriol. 1981 Feb;145(2):904–913. doi: 10.1128/jb.145.2.904-913.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuitje A. R., de Wind N., van der Spek J. C., Pors T. H., Meijer M. Dissection of promoter sequences involved in transcriptional activation of the Escherichia coli replication origin. Nucleic Acids Res. 1986 Mar 11;14(5):2333–2344. doi: 10.1093/nar/14.5.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M., Ohmori H., Hiraga S. A novel type of E. coli mutants with increased chromosomal copy number. Mol Gen Genet. 1983;192(1-2):51–60. doi: 10.1007/BF00327646. [DOI] [PubMed] [Google Scholar]
- Zahn G., Messer W. Control of the initiation of DNA replication in Escherichia coli. II. Function of the dnaA product. Mol Gen Genet. 1979 Jan 10;168(2):197–209. doi: 10.1007/BF00431445. [DOI] [PubMed] [Google Scholar]
- von Meyenburg K., Hansen F. G., Riise E., Bergmans H. E., Meijer M., Messer W. Origin of replication, oriC, of the Escherichia coli K12 chromosome: genetic mapping and minichromosome replication. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):121–128. doi: 10.1101/sqb.1979.043.01.018. [DOI] [PubMed] [Google Scholar]


