Abstract
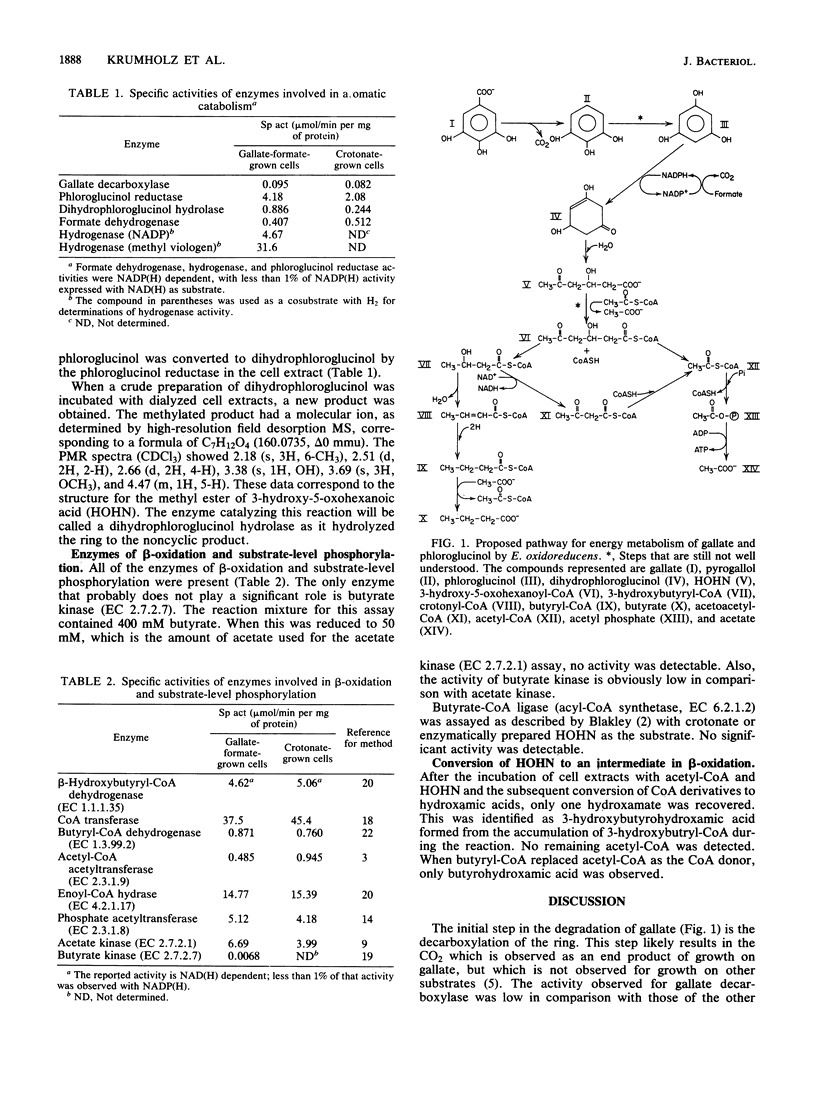
The pathway for the anaerobic catabolism of gallic acid by Eubacterium oxidoreducans was studied by using both in vivo and cell-free systems. Cells grown with gallate and crotonate, but with no formate or H2, excreted pyrogallol and phloroglucinol into the medium. Gallate was decarboxylated by crude cell extracts, with pyrogallol as the only detectable product. Whole cells converted pyrogallol to phloroglucinol. A phloroglucinol reductase catalyzed the conversion of phloroglucinol to dihydrophloroglucinol when NADPH was used as the source of electrons. Both formate dehydrogenase (EC 1.2.1.43) and hydrogenase (EC 1.18.99.1) were present in cell extracts of gallate-formate-grown cells. These two enzymes were both NADP linked. Since either H2 or formate is required for cell growth with gallate or phloroglucinol, these results suggest that the oxidation of the reduced substrate may be indirectly linked to the reduction of phloroglucinol. A dihydrophloroglucinol hydrolase was present, which hydrolyzed dihydrophloroglucinol to 3-hydroxy-5-oxohexanoate. This six-carbon ring cleavage product then presumably can be broken down by a series of reactions similar to beta-oxidation. These reactions cleaved the six-carbon acid to 3-hydroxybutyryl-coenzyme A yielding acetate and butyrate as end products. A number of key enzymes involved in beta-oxidation and substrate-level phosphorylation were demonstrated in cell extracts.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balba M. T., Evans W. C. The methanogenic biodegradation of catechol by a microbial consortium: evidence for the production of phenol through cis-benzenediol. Biochem Soc Trans. 1980 Aug;8(4):452–453. doi: 10.1042/bst0080452. [DOI] [PubMed] [Google Scholar]
- Blakley E. R. The microbial degradation of cyclohexanecarboxylic acid by a beta-oxidation pathway with simultaneous induction to the utilization of benzoate. Can J Microbiol. 1978 Jul;24(7):847–855. doi: 10.1139/m78-141. [DOI] [PubMed] [Google Scholar]
- Drake H. L. Demonstration of hydrogenase in extracts of the homoacetate-fermenting bacterium Clostridium thermoaceticum. J Bacteriol. 1982 May;150(2):702–709. doi: 10.1128/jb.150.2.702-709.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumholz L. R., Crawford R. L., Hemling M. E., Bryant M. P. A rumen bacterium degrading quercetin and trihydroxybenzenoids with concurrent use of formate or H2. Prog Clin Biol Res. 1986;213:211–214. [PubMed] [Google Scholar]
- Ljungdahl L. G., Andreesen J. R. Formate dehydrogenase, a selenium--tungsten enzyme from Clostridium thermoaceticum. Methods Enzymol. 1978;53:360–372. doi: 10.1016/s0076-6879(78)53042-5. [DOI] [PubMed] [Google Scholar]
- Patel T. R., Jure K. G., Jones G. A. Catabolism of phloroglucinol by the rumen anaerobe coprococcus. Appl Environ Microbiol. 1981 Dec;42(6):1010–1017. doi: 10.1128/aem.42.6.1010-1017.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TWAROG R., WOLFE R. S. ROLE OF BUTYRYL PHOSPHATE IN THE ENERGY METABOLISM OF CLOSTRIDIUM TETANOMORPHUM. J Bacteriol. 1963 Jul;86:112–117. doi: 10.1128/jb.86.1.112-117.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Henninger H., Wenning J., Decker K. The energy metabolism of Clostridium kluyveri. Eur J Biochem. 1968 Apr 3;4(2):173–180. doi: 10.1111/j.1432-1033.1968.tb00189.x. [DOI] [PubMed] [Google Scholar]
- Tsai C. G., Gates D. M., Ingledew W. M., Jones G. A. Products of anaerobic phloroglucinol degradation by Coprococcus sp. Pe15. Can J Microbiol. 1976 Feb;22(2):159–164. doi: 10.1139/m76-022. [DOI] [PubMed] [Google Scholar]
- Tung K. K., Wood W. A. Purification, new assay, and properties of coenzyme A transferase from Peptostreptococcus elsdenii. J Bacteriol. 1975 Dec;124(3):1462–1474. doi: 10.1128/jb.124.3.1462-1474.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks G., Shapiro M., Burns R. O., Wakil S. J. Control of fatty acid metabolism. I. Induction of the enzymes of fatty acid oxidation in Escherichia coli. J Bacteriol. 1969 Feb;97(2):827–836. doi: 10.1128/jb.97.2.827-836.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle P. J., Lunt D. O., Evans W. C. Anaerobic photometabolism of aromatic compounds by Rhodopseudomonas sp. Biochem Soc Trans. 1976;4(3):490–491. doi: 10.1042/bst0040490. [DOI] [PubMed] [Google Scholar]
- Williamson G., Engel P. C. Butyryl-CoA dehydrogenase from Megasphaera elsdenii. Specificity of the catalytic reaction. Biochem J. 1984 Mar 1;218(2):521–529. doi: 10.1042/bj2180521. [DOI] [PMC free article] [PubMed] [Google Scholar]



