Abstract
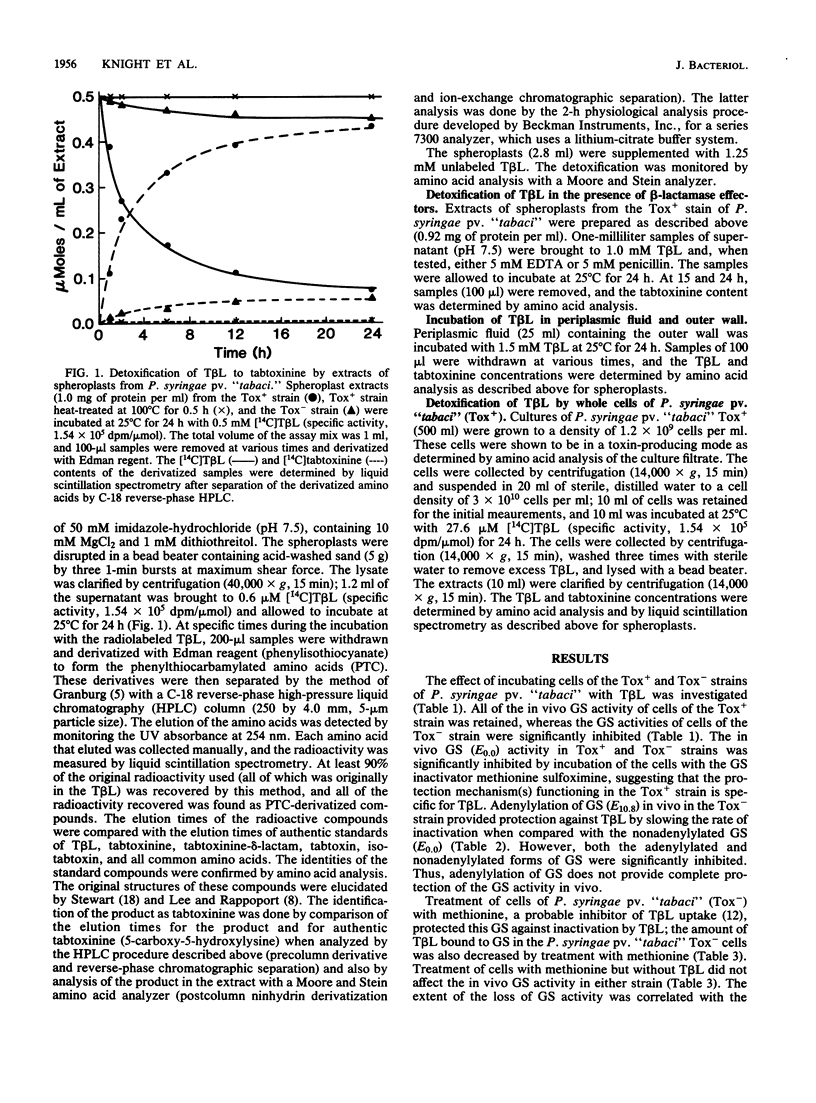
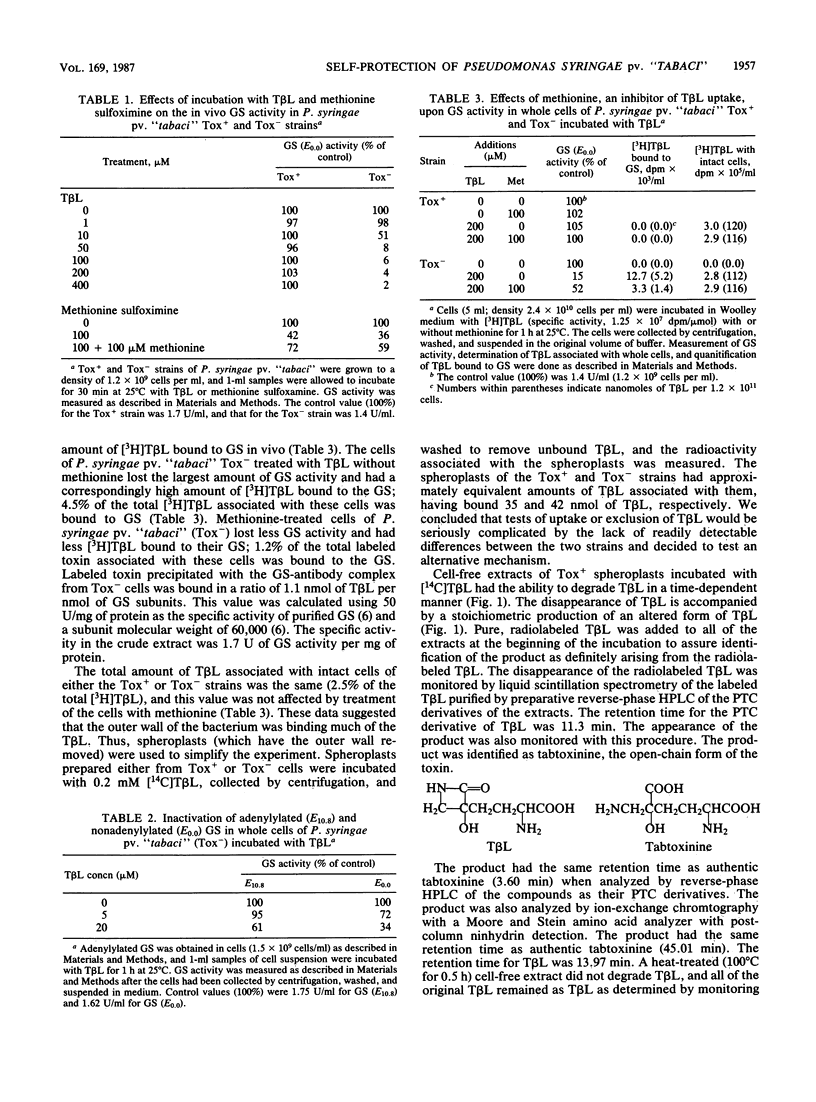
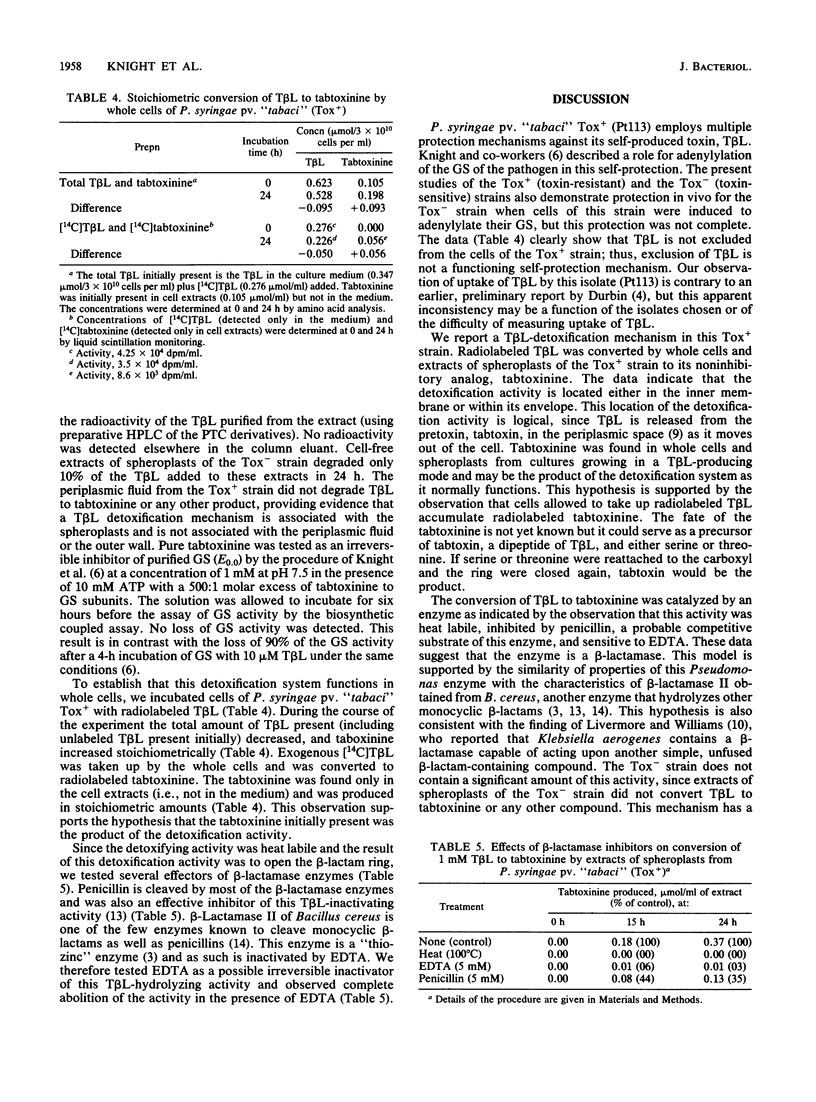
An extracellular toxin, tabtoxinine-beta-lactam (T beta L), is produced by Pseudomonas syringae pv. "tabaci." This toxin irreversibly inhibits its target, glutamine synthetase; yet P. syringae pv. "tabaci" retains significant amounts of glutamine synthetase activity during toxin production in culture. As part of our investigation of the self-protection of P. syringae pv. "tabaci," we compared the effects of T beta L on Tox+ (T beta L-producing, insensitive to T beta L) and Tox- (T beta L nonproducing, sensitive to T beta L) strains. The extent of protection afforded to the Tox- strain when induced to adenylylate glutamine synthetase was tested. We concluded that an additional protection mechanism was required. A detoxification activity was found in the Tox+ strain which opens the beta-lactam ring of T beta L to produce the inactive, open-chain form, tabtoxinine. Whole cells of the Tox+ strain incubated for 24 h with [14C]T beta L (0.276 mumol/3 X 10(10) cells) contained [14C]tabtoxinine (0.056 mumol), and the medium contained T beta L (0.226 mumol). Extracts of spheroplasts of the Tox+ stain also converted T beta L to tabtoxinine, whereas extracts of the Tox- strain did not alter T beta L. The conversion was time dependent and stoichiometric and was destroyed by boiling for 30 min or by the addition of 5 mM EDTA. Penicillin, a possible substrate and competitive inhibitor of this lactamase activity, inhibited the conversion of T beta L to tabtoxinine. Periplasmic fluid did not catalyze the conversion of T beta L.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender R. A., Janssen K. A., Resnick A. D., Blumenberg M., Foor F., Magasanik B. Biochemical parameters of glutamine synthetase from Klebsiella aerogenes. J Bacteriol. 1977 Feb;129(2):1001–1009. doi: 10.1128/jb.129.2.1001-1009.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Davies R. B., Abraham E. P. Metal cofactor requirements of beta-lactamase II. Biochem J. 1974 Oct;143(1):129–135. doi: 10.1042/bj1430129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight T. J., Durbin R. D., Langston-Unkefer P. J. Role of glutamine synthetase adenylylation in the self-protection of Pseudomonas syringae subsp. "tabaci" from its toxin, tabtoxinine-beta-lactam. J Bacteriol. 1986 Apr;166(1):224–229. doi: 10.1128/jb.166.1.224-229.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston-Unkefer P. J., Robinson A. C., Knight T. J., Durbin R. D. Inactivation of pea seed glutamine synthetase by the toxin, tabtoxinine-beta-lactam. J Biol Chem. 1987 Feb 5;262(4):1608–1613. [PubMed] [Google Scholar]
- Lee D. L., Rapoport H. Synthesis of tabtoxinine-delta-lactam. J Org Chem. 1975 Nov 28;40(24):3491–3495. doi: 10.1021/jo00912a005. [DOI] [PubMed] [Google Scholar]
- Livermore D. M., Williams J. D. In-vitro activity of the monobactam, SQ 26,776, against Gram-negative bacteria and its stability to their beta-lactamases. J Antimicrob Chemother. 1981 Dec;8 (Suppl E):29–37. doi: 10.1093/jac/8.suppl_e.29. [DOI] [PubMed] [Google Scholar]
- Meins F., Jr, Abrams M. L. How methionine and glutamine prevent inhibition of growth by methionine sulfoximine. Biochim Biophys Acta. 1972 Apr 14;266(1):307–311. doi: 10.1016/0005-2736(72)90146-0. [DOI] [PubMed] [Google Scholar]
- Pratt R. F., Anderson E. G., Odeh I. Certain monocyclic beta-lactams are beta-lactamase substrates: nocardicin A and desthiobenzylpenicillin. Biochem Biophys Res Commun. 1980 Apr 29;93(4):1266–1273. doi: 10.1016/0006-291x(80)90626-9. [DOI] [PubMed] [Google Scholar]
- Sinden S. L., Durbin R. D. Glutamine synthetase inhibition: possible mode of action of wildfire toxin from Pseudomonas tabaci. Nature. 1968 Jul 27;219(5152):379–380. doi: 10.1038/219379a0. [DOI] [PubMed] [Google Scholar]
- Stadtman E. R., Smyrniotis P. Z., Davis J. N., Wittenberger M. E. Enzymic procedures for determining the average state of adenylylation of Escherichia coli glutamine synthetase. Anal Biochem. 1979 May;95(1):275–285. doi: 10.1016/0003-2697(79)90217-3. [DOI] [PubMed] [Google Scholar]
- Stewart W. W. Isolation and proof of structure of wildfire toxin. Nature. 1971 Jan 15;229(5281):174–178. doi: 10.1038/229174a0. [DOI] [PubMed] [Google Scholar]
- Thomas M. D., Langston-Unkefer P. J., Uchytil T. F., Durbin R. D. Inhibition of Glutamine Synthetase from Pea by Tabtoxinine-beta-lactam. Plant Physiol. 1983 Apr;71(4):912–915. doi: 10.1104/pp.71.4.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOLLEY D. W., PRINGLE R. B., BRAUN A. C. Isolation of the phytopathogenic toxin of Pseudomonas tabaci, an antagonist of methionine. J Biol Chem. 1952 May;197(1):409–417. [PubMed] [Google Scholar]


