Abstract
We have shown that heat shock induces rapid dephosphorylation of τ in both female and male rats followed by hyperphosphorylation only in female rats. To investigate the role of gonadal hormones, rats were ovariectomized (OVX), orchiectomized (ORX), or sham-gonadectomized and received replacement therapy with estradiol benzoate (EB), testosterone propionate (TP), or sesame oil (SO) vehicle for 2–3 weeks, respectively. At 0, 3, 6, and 12 hr after heat shock, immunoblot analysis of SDS cerebral extracts was performed using phosphate-dependent and -independent anti-τ antibodies. Seven groups of rats were analyzed: (i) sham-OVX + SO; (ii) OVX + SO; (iii) OVX + EB; (iv) sham-ORX + SO; (v) ORX + SO; (vi) ORX + TP; and (vii) ORX. In all seven groups, there was dephosphorylation of τ at 0 hr after heat shock. In all three groups of female rats, there was hyperphosphorylation of τ at 3 hr after heat shock, and its degree and temporal pattern were identical between the OVX + SO and OVX + EB groups. In male rats, there was hyperphosphorylation of τ at 3 hr after heat shock in both ORX + SO and ORX groups, and its degree was reduced in the ORX + TP group. Thus, dephosphorylation of τ is gonadal hormone-independent, but while its hyperphosphorylation is estrogen-independent it is prevented by androgens. Because τ is abnormally hyperphosphorylated in Alzheimer disease, which is more frequent in women than men, these findings suggest that androgens may exert a neuroprotective effect.
The prevalence of Alzheimer disease (AD) after age 65 is roughly two to three times higher in women than men (1). Because estrogens prevent neuronal atrophy (2), exert a neuroprotective effect against various toxic insults (3), and may synergize with neurotrophic factors (4–6), a deficiency of estrogens has been suggested to play a role in the pathogenesis of AD. However, estrogen neurotoxicity also has been postulated to be involved in brain aging and degeneration (7). So far, in many studies of postmenopausal women, demonstration of a beneficial effect of estrogen replacement therapy in reducing the risk or delaying the onset of AD has been controversial (8, 9). On the other hand, a possible advantageous role of androgens in reducing AD occurrence or severity has not been investigated yet. This is in spite of the century-old suggestion that androgens may rejuvenate aged men (10), the demonstration that androgens inhibit the stress response (11–13), and the increasing evidence that stressful stimuli play a role in the etiopathogenesis of AD (14–16).
One histologic hallmark of AD is the neurofibrillary tangle. Neurofibrillary tangles are perikaryal aggregates of abnormal filaments, the so-called paired helical filaments, which consist mostly of abnormally hyperphosphorylated τ (17, 18), and appear to be conjugated with the stress-inducible protein ubiquitin (16). τ is a group of ≈50–66-kDa phosphopolypeptides, but although many τ protein kinases and phosphatases have been described, the lack of appropriate animal models hindered so far the elucidation of the cascade of events that lead to its hyperphosphorylation. We have recently shown that heat shock induces rapid dephosphorylation of τ in both female and male rats followed by hyperphosphorylation similar to that seen in AD only in female rats (19). In this communication, we have used this simple rat model to study the modification of dephosphorylation–hyperphosphorylation of τ by gonadal hormones. We have found that the dephosphorylation of τ is gonadal hormone-independent. Its hyperphosphorylation, however, is also estrogen-independent but is prevented by androgens.
MATERIALS AND METHODS
Gonadectomy.
Age-matched 2- to 3-month-old male and female Sprague–Dawley rats were anesthetized by i.p. injection of 0.2–0.3 ml/300 g of a rat cocktail containing ketamine, xylazine, and acepromazine. Under aseptic conditions, rats were orchiectomized (ORX), ovariectomized (OVX), or sham-gonadectomized through midscrotal or bilateral dorsal incisions, respectively.
Replacement Therapy with Gonadal Hormones.
Beginning on the day of gonadectomy, male and female rats received, under ether anesthesia, daily s.c. injections of 1 mg/100 g of testosterone propionate (TP) or 20 μg/100 g of estradiol benzoate (EB), respectively, in 0.1 ml of sesame oil (SO) vehicle for 2–3 weeks. TP and EB are slow-release, long-acting derivatives of testosterone and estradiol, respectively. Sham-gonadectomized rats received daily s.c. injections of equal volume of SO vehicle. The following six groups of animals were compared: (i) sham-ORX + SO (n = 26); (ii) ORX + SO (n = 24); (iii) ORX + TP (n = 30); (iv) sham-OVX + SO (n = 34); (v) OVX + SO (n = 24); and (vi) OVX + EB (n = 23). Also, 25 ORX rats received no daily s.c. injections of TP or SO and were compared with intact female and male rats. In addition, two groups of rats were ORX and received daily s.c. injections of 0.5 (n = 5) or 2.0 (n = 5) mg/100 g of TP for 3 weeks but were not heat-shocked. Rats were maintained in a temperature (21°-22°C)- and light (12-hr light/dark schedule with lights on at 0700 hr)-controlled environment and fed food and water ad libitum until heat shock. The efficacy of gonadectomies and replacement therapies was verified by weighing the ventral prostate and/or observing the size of thymus at the time the rats were killed.
Heat Shock.
Under ether anesthesia, rats were injected with a final dose of gonadal hormone or SO, sedated with pentobarbital [15–20 mg/kg (body weight)], and heat-shocked as described (19). When the rectal temperature reached 42°C, the door of the incubator was opened to a 37°C environment for another 15 min. At the end of this period the rectal temperature rose to 42.2°-42.9°C, and the rats were transferred to room temperature. The heat-shock process lasted ≈1 hr. OVX, ORX, and sham-gonadectomized control rats were sedated and given a final dose of gonadal hormone or SO but were not heat-shocked. They were killed 1, 4, 7, and 13 hr after sedation. As we have shown (19), sedation has no effect on the phosphorylation state of τ.
Preparation and Immunoblot Analysis of SDS Cerebral Extracts.
Preparation and immunoblot analysis of SDS cerebral extracts was done as described (14, 19). In brief, 0, 3, 6, and 12 hr after heat shock, the heat-shocked and control rats were killed under pentobarbital anesthesia (40 mg/kg) by intracardiac perfusion with 100 mM Tris⋅HCl, pH 7.6/6 mM EGTA at 4°C. The brain (excluding the brainstem and cerebellum) was homogenized by boiling in Laemmli sample buffer (20) and centrifuged, and the protein content was assayed in samples of the supernatant (SDS extracts) by using a modification (14) of the method of Lowry et al. (21). One hundred micrograms of protein of SDS extracts from control and heat-shocked rats were loaded in duplicate in each alternate lane of 5–12.5% linear gradient polyacrylamide gels and electrophoresed at a constant current of 30 mA. After electrophoresis, the proteins were transferred to nitrocellulose membranes and immunostained with anti-τ mAbs using the peroxidase-antiperoxidase technique (22) with and/or without previous dephosphorylation of electroblotted proteins with type VII-L alkaline phosphatase from bovine intestinal mucosa (Sigma) exactly as described (14, 19). The following four anti-τ antibodies were used: Tau-1, an IgG2a protein that recognizes a phosphate-dependent nonphosphorylated epitope and requires nonphosphorylated Ser199/202 within its epitope (Pro162-Gly210) (23), PHF-1, which requires phosphorylated Ser396 within its epitope (18, 24), and Tau-5 (25) and Tau-46, which recognize phosphate-independent epitopes in the middle (Ser210-Arg230) (23) and most carboxyl terminus (Leu428-Leu441) (23) of τ, respectively.
For quantitative immunoblot analysis of the Tau-1 epitope, the electroblotted proteins were incubated successively with Tau-1 and 125I-labeled protein A (type NEX-146L, DuPont/NEN), followed by autoradiography and counting of radioactivity in a γ counter, as described (14, 19). SDS extracts from 81 female and 114 male gonadectomized control and heat-shocked rats were analyzed at 0, 3, 6, and 12 hr after heat shock in 76 pairs of immunoblots, with the first immunoblot in each pair probed without dephosphorylation and the second after dephosphorylation. Approximately 760 values of “total” τ (recognized after dephosphorylation with alkaline phosphatase) and an equal number of values of nonphosphorylated τ (recognized without dephosphorylation) were obtained and used to calculate the ratio of total τ to nonphosphorylated τ as an indicator of the degree of phosphorylation of the Tau-1 epitope, i.e., the higher the ratio the higher the degree of phosphorylation. Although no stoichiometric phosphate analysis of τ was performed, we used the terms dephosphorylation and hyperphosphorylation of τ when the ratio of total τ to nonphosphorylated τ was lower or higher in heat-shocked than in control rats, respectively, and the qualitative immunoblot analysis indicated so. The effects of gonadectomy and time after heat shock on the levels of nonphosphorylated τ, total τ, and the ratio of total τ to nonphosphorylated τ were analyzed using the general linear model two-way ANOVA procedure. Differences between group means were tested using Tukey’s studentized range test.
Testosterone and Estradiol Concentrations in Serum.
The serum levels of testosterone and estradiol were determined in blood samples obtained by cardiac puncture just before terminal perfusion by using RIA kits.
RESULTS
The Heat Shock-Induced Hyperphosphorylation of τ Is Estrogen-Independent.
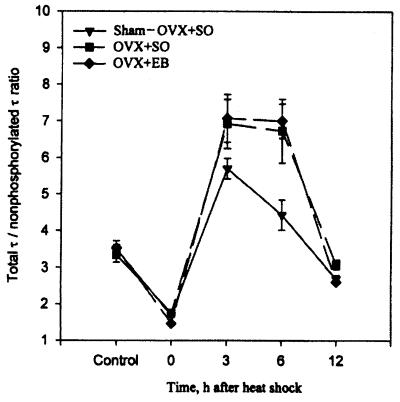
In all three groups of OVX rats (i.e., sham-OVX + SO, OVX + SO, and OVX + EB), there was hyperphosphorylation of τ at 3 and 6 hr after heat shock. This was qualitatively exemplified by upward gel mobility shift with resultant changes in banding and appearance of an additional 68-kDa τ polypeptide recognized by all four anti-τ antibodies (Fig. 1). Notably, however, these changes were revealed by Tau-1 only after dephosphorylation and were accompanied by a concomitant decrease in staining intensity of τ isoforms revealed without previous dephosphorylation, which indicated a precursor-product relationship between the two forms of τ (Fig. 1). In addition, quantitative analysis showed a highly significant increase in the ratio of total τ to nonphosphorylated τ between control and heat-shocked rats (Table 1). Importantly, however, the degree of hyperphosphorylation of τ was identical between the groups of OVX + SO and OVX + EB rats (Fig. 2 and Table 1). In addition, the degree of hyperphosphorylation of τ was higher and lasted longer in both OVX + SO and OVX + EB rats in comparison with sham-OVX rats (Fig. 2 and Table 1). Also, as was expected (26), OVX + EB rats weighed less than OVX + SO rats at the time of heat shock [205 ± 10 (n = 21) vs. 246 ± 7 (n = 24) g, P < 0.001].
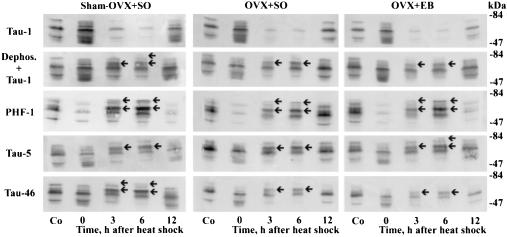
Figure 1.
The heat shock-induced hyperphosphorylation of τ is estrogen-independent. Immunoblots of SDS cerebral extracts from female control (Co) and heat-shocked rats. Tau-1 was used without and with previous dephosphorylation (dephos.) of electroblotted proteins. All three groups of sham-OVX + SO, OVX + SO, and OVX + EB exhibited the following: (i) dephosphorylation of τ at 0 hr after heat shock exemplified by intense staining with Tau-1 without previous dephosphorylation, the simplified banding pattern of three discrete 52-, 60-, and 66-kDa τ isoforms seen by PHF-1, and the appearance of new weakly stained τ polypeptides recognized by Tau-1, Tau-5, and Tau-46; (ii) upward mobility shift at 3 and 6 hr after heat shock evidenced by accentuation of 64- and 68-kDa τ polypeptides (arrows), recognized by PHF-1, Tau-5, Tau-46, and Tau-1 after previous dephosphorylation; and (iii) reduced staining intensity of τ isoforms detected by Tau-1 without previous dephosphorylation at 3 and 6 hr after heat shock.
Table 1.
Quantitative analysis of the Tau-1 epitope on immunoblots of OVX female rat cerebrum 0–12 hr after heat shock with and without daily replacement therapy with EB [20 μg/100 g (body weight)] for 2–3 weeks
|
125I-labeled protein A, cpm × 10−3/100 μg of extract protein
|
|||||
|---|---|---|---|---|---|
| Control | hr after heat shock
|
||||
| 0 | 3 | 6 | 12 | ||
| Sham-OVX + SO | n = 10 | n = 5 | n = 6 | n = 8 | n = 5 |
| Nonphosphorylated | 14.0 ± 1.7 | 29.2 ± 2.5 | 7.4 ± 1.1 | 11.3 ± 1.7 | 17.1 ± 1.2 |
| (0.0001) | (0.0020) | ||||
| Total | 43.1 ± 1.8 | 48.9 ± 5.1 | 39.6 ± 2.0 | 42.0 ± 2.7 | 44.6 ± 1.5 |
| Total/nonphosphorylated | 3.5 ± 0.4 | 1.7 ± 0.1 | 5.7 ± 0.5 | 4.4 ± 0.8 | 2.7 ± 0.3 |
| (0.0319) | |||||
| OVX + SO | n = 5 | n = 4 | n = 5 | n = 5 | n = 5 |
| Nonphosphorylated | 10.1 ± 1.3 | 16.5 ± 4.5 | 4.7 ± 1.6 | 5.0 ± 1.0 | 12.0 ± 0.8 |
| (0.0188) | (0.0370) | (0.0469) | |||
| Total | 31.8 ± 3.6 | 29.6 ± 9.5 | 25.8 ± 6.0 | 26.6 ± 5.8 | 36.6 ± 2.8 |
| Total/nonphosphorylated | 3.3 ± 0.4 | 1.7 ± 0.2 | 6.9 ± 1.5 | 6.7 ± 1.9 | 3.1 ± 0.3 |
| (0.0055) | (0.0084) | ||||
| OVX + EB | n = 5 | n = 4 | n = 6 | n = 5 | n = 3 |
| Nonphosphorylated | 7.7 ± 0.8 | 23.2 ± 0.9 | 5.0 ± 1.0 | 4.9 ± 0.8 | 13.7 ± 0.5 |
| (0.0001) | (0.0426) | ||||
| Total | 26.4 ± 1.4 | 33.8 ± 1.8 | 29.6 ± 1.7 | 32.5 ± 5.3 | 32.0 ± 2.0 |
| Total/nonphosphorylated | 3.5 ± 0.2 | 1.5 ± 0.1 | 7.1 ± 1.3 | 7.0 ± 1.0 | 2.3 ± 0.1 |
| (0.0042) | (0.0069) | ||||
Nonphosphorylated and total are defined as the epitope recognized by Tau-1 without and with alkaline phosphatase treatment of electroblotted proteins before immunoblotting, respectively. Data are mean ± SE values. Shown in parentheses are the values of P < 0.05 in comparison with the respective control. n, number of rats.
Figure 2.
The heat shock-induced hyperphosphorylation of τ is estrogen-independent. Note that in OVX rats the degree of hyperphosphorylation of τ at 3 and 6 hr after heat shock is identical with and without replacement therapy with EB. The data values are also shown in Table 1.
Heat Shock-Induced Hyperphosphorylation of τ Also Occurs in ORX + SO and ORX Rats and Can Be Reduced by Testosterone.
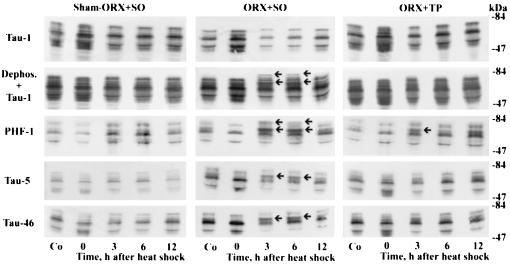
As is shown in Figs. 3 and 4 and Table 2, hyperphosphorylation of τ also occurred at 3 hr after heat shock in ORX + SO rats as was indicated by the criteria of upward gel mobility shift, changes in banding patterns, and significant increase in the ratio of total τ to nonphosphorylated τ (P = 0.0027 and P = 0.0191 in comparison with the control and sham-ORX + SO, respectively). Importantly, however, administration of TP markedly reduced, both qualitatively and quantitatively, the degree of hyperphosphorylation of τ. In addition, the “protective” effect of androgens against hyperphosphorylation of τ is further indicated in Fig. 5 where the possibly confounding stress of daily s.c. injections under ether anesthesia was avoided, but at 3 hr after heat shock the ratio of total τ to nonphosphorylated τ was significantly higher in ORX than in intact male rats (P = 0.0001).
Figure 3.
Androgens prevent the heat shock-induced hyperphosphorylation of τ. Immunoblots of SDS cerebral extracts from control (Co) and heat-shocked male rats. Note the dephosphorylation of τ at 0 hr after heat shock in all three groups (sham-ORX + SO, ORX + SO, and ORX + TP) as explained in Fig. 1; the hyperphosphorylation of τ at 3 and 6 hr after heat shock in the ORX + SO group as explained in Fig. 1 (arrows); and the marked reduction in hyperphosphorylation of τ at 3 and 6 hr after heat shock in the ORX + TP group.
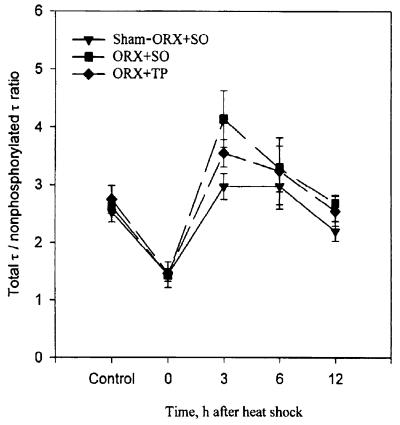
Figure 4.
Androgens prevent the heat shock-induced hyperphosphorylation of τ. Note the hyperphosphorylation of τ in ORX + SO rats 3 hr after heat shock (P = 0.0027, in comparison with its control) and its marked reduction in ORX + TP rats. Data values are also shown in Table 2.
Table 2.
Quantitative analysis of the Tau-1 epitope on immunoblots of ORX male rat cerebrum 0–12 hr after heat shock with and without daily replacement therapy with TP [1 mg/100 g (body weight)] for 2–3 weeks
|
125I-labeled protein A, cpm × 10−3/100 μg of extract protein
|
|||||
|---|---|---|---|---|---|
| Control | hr after heat shock
|
||||
| 0 | 3 | 6 | 12 | ||
| Sham-ORX + SO | n = 6 | n = 4 | n = 5 | n = 7 | n = 4 |
| Nonphosphorylated | 22.2 ± 4.0 | 40.3 ± 13.2 | 21.3 ± 4.0 | 23.4 ± 3.8 | 24.1 ± 5.6 |
| (0.0040) | |||||
| Total | 54.6 ± 8.1 | 49.6 ± 10.4 | 59.9 ± 7.4 | 62.0 ± 6.3 | 51.1 ± 11.0 |
| Total/nonphosphorylated | 2.5 ± 0.2 | 1.4 ± 0.2 | 3.0 ± 0.2 | 3.0 ± 0.4 | 2.2 ± 0.2 |
| (0.0313) | |||||
| ORX + SO | n = 5 | n = 5 | n = 5 | n = 5 | n = 4 |
| Nonphosphorylated | 16.3 ± 1.3 | 34.2 ± 4.0 | 10.5 ± 1.5 | 15.0 ± 2.3 | 12.7 ± 0.4 |
| (0.0118) | |||||
| Total | 42.6 ± 3.1 | 45.2 ± 5.0 | 40.9 ± 3.0 | 45.6 ± 2.5 | 34.2 ± 1.3 |
| Total/nonphosphorylated | 2.6 ± 0.1 | 1.4 ± 0.1 | 4.1 ± 0.5 | 3.3 ± 0.4 | 2.7 ± 0.2 |
| (0.0169) | (0.0027) | ||||
| ORX + TP | n = 9 | n = 4 | n = 6 | n = 7 | n = 4 |
| Nonphosphorylated | 19.5 ± 2.9 | 33.9 ± 3.0 | 11.0 ± 1.1 | 18.6 ± 4.0 | 18.5 ± 2.0 |
| (0.0133) | |||||
| Total | 51.1 ± 7.6 | 49.4 ± 4.2 | 38.8 ± 4.0 | 48.9 ± 5.6 | 47.3 ± 7.0 |
| Total/nonphosphorylated | 2.8 ± 0.2 | 1.5 ± 0.1 | 3.5 ± 0.2 | 3.2 ± 0.6 | 2.6 ± 0.3 |
| (0.0068) | |||||
Definitions are as in Table 1. Data are mean ± SE values. Shown in parentheses are the values of P < 0.05 in comparison with the respective control. n, number of rats.
Figure 5.
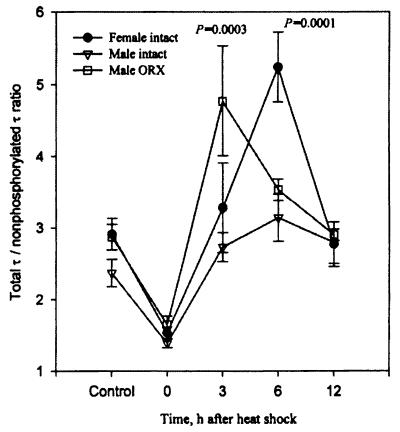
Androgens prevent the heat shock-induced hyperphosphorylation of τ. In comparison with intact males, there is significantly higher hyperphosphorylation of τ in ORX rats at 3 hr after heat shock. The values of P shown are in comparisons with the respective non-heat-shocked controls. The data for intact male and female rats were published previously (19) and are shown here for the sake of comparison.
Testosterone Decreases the Degree of Phosphorylation of the Tau-1 Epitope.
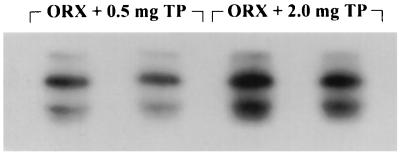
To investigate further whether the concentration of circulating testosterone (Table 3) has any effect on the degree of phosphorylation of τ, 0.5 mg or 2.0 mg/100 g TP was given daily s.c. for 3 weeks to ORX rats that were not heat-shocked. As is shown in Fig. 6, the Tau-1 site was less phosphorylated, i.e., more intensely stained, after administration of 2.0 rather than 0.5 mg of TP, and the total τ to nonphosphorylated τ ratios were 2.4 ± 0.1 (n = 4) vs. 2.9 ± 0.3 (n = 5), respectively.
Table 3.
Gonadal hormone concentrations in sera and weights of ventral prostate of rats
| Treatment | Testosterone,* ng/ml | Estradiol,† pg/ml | Prostate, mg |
|---|---|---|---|
| Sham-ORX + SO | 2.3 ± 0.6 (n = 15) | 41.4 ± 6.1 (n = 7)‡ | 450 ± 15 (n = 18) |
| ORX + SO | <MCD (n = 5) | 46.6 ± 7.8 (n = 7)‡ | 48 ± 2.5 (n = 20) |
| ORX + TP (1 mg/100 g) | 33.5 ± 0.7 (n = 21) | — | 731 ± 22 (n = 25) |
| ORX + TP (0.5 mg/100 g) | 9.6 ± 1.4 (n = 5) | — | 735 ± 40 (n = 5) |
| ORX + TP (2 mg/100 g) | 49.6 ± 2.6 (n = 4) | — | 862 ± 41 (n = 5) |
| Sham-OVX + SO | — | 36.4 ± 6.4 (n = 10) | — |
| OVX + EB (20 μg/100 g) | — | 750.0 ± 37.4 (n = 7) | — |
n, number of rats; MCD, minimal concentration detectable; ∗ and †, the minimum detectable concentrations were 0.08 ng/ml and 4.7 pg/ml, respectively; ‡, not significantly different (P > 0.05) from each other. Data are mean + SE values.
Figure 6.
The higher the concentration of circulating androgens the less the phosphorylation of the Tau-1 epitope. Duplicate immunoblot of SDS cerebral extracts from non-heat-shocked ORX rats were probed with Tau-1 and 125I-labeled protein A 3 weeks after orchiectomy and daily replacement therapy with either 0.5 or 2.0 mg/100 g of body weight of TP. Note the more intense staining, i.e., less phosphorylation, after injections of 2.0 rather than 0.5 mg of TP.
The Heat Shock-Induced Rapid Dephosphorylation of τ Is Gonadal Hormone-Independent.
In all seven groups of rats, i.e., sham-OVX + SO, OVX + SO, OVX + EB, sham-ORX + SO, ORX + SO, ORX + TP, and ORX, there was dephosphorylation of τ at 0 hr after heat shock as was evidenced by (i) accentuation and attenuation of τ isoforms recognized by Tau-1 and PHF-1, respectively, and appearance of new τ polypeptides recognized by Tau-1, Tau-5, and Tau-46, but not PHF-1 (Figs. 1 and 3); and (ii) highly statistically significant increase in the amount of nonphosphorylated τ, whereas the amount of total τ remained unchanged, and resultant decrease in the ratio of total τ to nonphosphorylated τ (Figs. 2, 4, and 5; Tables 1 and 2).
Other Findings.
Table 3 shows that there was no difference in serum concentrations of estradiol between sham-ORX + SO and ORX + SO rats, indicating, in addition to the above findings, that withdrawal of circulating estrogens played no role in the heat shock-induced hyperphosphorylation of τ in ORX + SO rats. Also, female rats had a more robust response than male rats to even mildly stressful stimuli such as daily s.c. injections of SO vehicle under ether anesthesia, as indicated by the significantly higher (P = 0.0207) total τ to nonphosphorylated τ ratio in control sham-OVX + SO than in control sham-ORX + SO rats (Tables 1 and 2). There was no significant difference between female and male intact control rats (P = 0.1230) (Fig. 5).
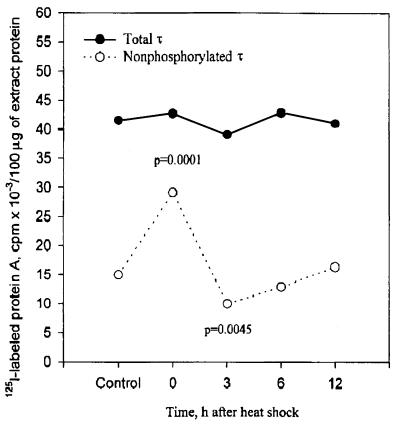
To test for significant effects of gonadectomy with and without replacement therapy and time after heat shock on the levels of nonphosphorylated τ, total τ, and the ratio of total τ to nonphosphorylated τ, two-way ANOVA was used on female and male rats. Only the results of the overall model are shown. Nonphosphorylated τ was significant for both females (F = 16.00, df = 14, 80, P < 0.0001) and males (F = 3.73, df = 14, 79, P < 0.0001), and total τ was significant for females (F = 3.89, df = 14, 80, P < 0.0001) but not significant for males (F = 1.28, df = 14, 80, P < 0.2468). The ratio of total τ to nonphosphorylated τ was significant for both females (F = 5.44, df = 14, 80, P < 0.0001) and males (F = 4.94, df = 14, 79, P < 0.0001). In addition, when all the values of total τ from control and each time point after heat shock from all six gonadectomized groups shown in Tables 1 and 2 were combined, the total τ was remarkably unchanged. In contrast, the nonphosphorylated τ was significantly increased (P = 0.0001) and decreased (P = 0.0045) at 0 and 3 hr after heat shock, respectively. These results support a precursor-product relationship between the two forms of τ (Fig. 7).
Figure 7.
Hyperphosphorylation of τ at 3 hr after heat shock is caused by increased phosphorylation of pre-existing τ. Data values shown in Tables 1 and 2 from all six gonadectomized groups at each time point after heat shock and non-heat-shocked controls were combined. Note the constant amounts of total τ, and the statistically significant increase and decrease in nonphosphorylated τ at 0 and 3 hr after heat shock, respectively. The values of P shown are based on comparison with the control values.
Immunoblot analysis using the SMI 31 and SMI 34 anti-phosphoneurofilament and the SMI 33 anti-nonphosphoneurofilament antibodies showed no detectable differences between control and heat-shocked rats (data not shown).
In addition to the prostatic atrophy and hypertrophy shown in Table 3, thymic atrophy was observed in both OVX and ORX rats with gonadal hormone replacement therapy, and thymic hyperplasia was seen in both OVX and ORX rats that received no replacement therapy (data not shown).
DISCUSSION
Our data showed that the heat shock-induced hyperphosphorylation of τ is estrogen-independent. In male rats, the hyperphosphorylation response occurred only after removal of circulating androgens by orchiectomy and its degree was reduced by replacement therapy with testosterone. On the other hand, the rapid dephosphorylation of τ was independent of gonadal hormones, both estrogens, and androgens. Because it has been shown that androgens inhibit the stress response (11–13), these findings suggest that hyperphosphorylation of τ is a component of the adaptive response to heat shock. It is also known that an extensive interrelationship exists between the hypothalamo-pituitary-adrenal and -gonadal axes, as exemplified by the severe reduction in circulating androgens in chronically stressed military trainees (27). In addition, in the hippocampus, androgens down-regulate the glucocorticoid receptor mRNA (13), and protect against the chronic stress-induced death of pyramidal cells (28). Therefore, in view of these facts, a role for androgens in the attenuation of the heat shock-induced hyperphosphorylation of τ is very likely.
In all three groups of OVX rats there was hyperphosphorylation of τ after heat shock, but its degree was higher in both OVX + SO and OVX + EB than in sham-OVX + SO. This result is most likely due to the removal of the inhibiting effect of circulating androgens in OVX rats, because the ovaries are the major source of androgens in female rats (29). On the other hand, the hyperphosphorylation of τ in ORX rats is certainly caused by the exaggerated stress response after removal of circulating androgens (11–13). This hyperphosphorylation response is independent of any estrogen effect because our data (Table 3) and previous studies (30) show that the levels of circulating estrogens remain unchanged after orchiectomy. Furthermore, while replacement therapy of OVX rats with EB had no effect, TP reduced or prevented the hyperphosphorylation of τ in most of the ORX rats. In all gonadectomized rats the hyperphosphorylation of τ peaked at 3 hr rather than at 6 hr after heat shock as was previously seen in intact female rats (ref. 14 and Fig. 5). This finding suggests that removal of the inhibitory effect of androgens, in both male and female rats, increases the rate of phosphorylation of τ.
The replacement doses of TP and EB used in this study achieved supraphysiological serum levels of testosterone and estradiol. Although similar or higher doses have been used in previous studies (28, 31–33), it has been shown that higher testosterone levels are required to suppress the stress response in ORX than intact rats (12). Perhaps the physiologic circadian variation of testosterone levels (34) are more effective than constant replacement levels. However, prolonged administration of supraphysiologic doses of testosterone to normal men increased muscle size and strength and had no side effects (35).
What is the mechanism, then, of the initial dephosphorylation and subsequent hyperphosphorylation of τ? Based on in vitro studies, many non-proline-directed and proline-directed protein kinases (36), including the stress-activated (c-Jun NH2-terminal) protein kinase (37) have been found to act on τ. Among the protein phosphatases, the Ser/Thr protein phosphatase 2A appears to be the enzyme with the highest specificity for τ phosphorylated in vitro (38) and, in cultured cells, associates with microtubules and dephosphorylates τ (39). τ has multiple phosphorylation sites. As usually happens with multiphosphorylation processes, its first modification should be regulated by second messenger-activated protein kinases such as protein kinase A, Ca2+/calmodulin kinase II, and others, followed by phosphorylation with multi-purpose kinases (40). Activation of protein phosphatase 2A by ceramide (41), which is formed during heat shock (42), could explain the initial dephosphorylation of τ. Ceramide, however, activates also the stress-activated protein kinase (43). Perhaps τ becomes hyperphosphorylated through prolonged activation of the stress-activated protein kinase, which also can lead to growth arrest and apoptosis (44), in synergy with other protein kinases and/or inactivation of phosphatases.
How could androgens prevent the hyperphosphorylation of τ? Only speculation can be offered at this time. Gonadal hormones have both organizational and activational effects and thus play a role in the development, maintenance, function, and regeneration of the nervous system (45). In addition to the long-term genomic effects mediated by ligand-activated intracellular receptors (46), neuroactive gonadal steroids rapidly alter neuronal excitability through neurotransmitter-gated ion channels (47). Three enzymes play a major role in the metabolism of testosterone in the brain of the rat. (i) The aromatase cytochrome P450 aromatizes testosterone to estrogens, but has a limited distribution to the medial basal hypothalamus, preoptic area, and limbic regions, i.e., areas related to reproductive neuroendocrine function; its activity is high during the perinatal period, but very low in the adult rat (48). (ii) The 5α-reductase reduces testosterone to the more potent dihydrotestosterone and is also present at high levels in the cerebral cortex (49). (iii) The oxidoreductase 3α-hydroxysteroid dehydrogenase works in concert with 5α-reductase to convert testosterone to dihydrotestosterone and the weak androgen 3α-androstanediol (50). Based on these facts and our findings, it is highly unlikely, if not impossible, that aromatization of testosterone to estrogens prevents the hyperphosphorylation of τ in male rats. In this heat-shock model, the 72-kDa stress-inducible heat-shock protein becomes detectable at 3 hr and remains so by 24 hr after heat shock (19). It has been shown that the heat-shock proteins form aporeceptor complexes with the androgen, estrogen, and glucocorticoid receptors, keeping them in a conformation highly responsive only to ligand (51). Furthermore, the androgen, glucocorticoid, mineralocorticoid, and progesterone receptors bind to the same hormone response element, which differs from that recognized by the estrogen receptor (46). Thus, it is possible that the androgen receptor inhibits the stress response by competing with the glucocorticoid receptor for binding to the same response element. Heat-shock proteins also may play a modifying role in this process. These hypotheses, genomic and/or nongenomic, should be investigated further.
Ever since Aristotle described the developmental and behavioral effects of orchiectomy more than 2,000 years ago in Historia Animalium (52), androgens have been found to play a role in the regulation of sexuality, aggression, emotion, cognition, and personality (53). Although andropause in a strict sense does not occur, there is a 50% decrease of free testosterone blood levels from 20 to 80 years of age (54). If abnormal hyperphosphorylation of τ is detrimental to mental function, as indicated by available evidence (55, 56), then our findings suggest that androgens may exert a primary neuroprotective effect against AD. This contrasts with any likely beneficial actions of estrogens which may be secondary to their systemic effects such as cardiovascular protection. The possibility, then, of using androgen replacement therapy in elderly men and, perhaps, in postmenopausal women as a preventive measure against AD should be investigated.
Acknowledgments
I thank Dr. B. M. Sanborn for her advice on the protocols for the gonadal hormone replacement therapy, Drs. L. I. Binder, S. G. Greenberg, and J. Q. Trojanowski for supplying the anti-τ antibodies, Diagnostic Systems Laboratories for the gift of RIA kits, Myrna Khan for the statistical evaluation of the data, P. Cho and J. Check for the computer-generated composite figures, Dr. S. J. Norris for critical review, and Chris Haverkorn for typing the manuscript.
ABBREVIATIONS
- AD
Alzheimer disease
- EB
estradiol benzoate
- ORX
orchiectomized
- OVX
ovariectomized
- SO
sesame oil
- TP
testosterone propionate
References
- 1.Jorm A F, Korten A E, Henderson A S. Acta Psychiatr Scand. 1987;76:465–479. doi: 10.1111/j.1600-0447.1987.tb02906.x. [DOI] [PubMed] [Google Scholar]
- 2.Gould E, Woolley C, Frankfurt M, McEwen B. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodman Y D, Bruce A J, Cheng B, Mattson M P. J Neurochem. 1996;66:1836–1844. doi: 10.1046/j.1471-4159.1996.66051836.x. [DOI] [PubMed] [Google Scholar]
- 4.Toran-Allerand C D, Miranda R C, Bentham W D L, Sohrabji F, Brown T J, Hochberg R B, MacLusky N J. Proc Natl Acad Sci USA. 1992;89:4668–4672. doi: 10.1073/pnas.89.10.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McMillan P J, Singer C A, Dorsa D M. J Neurosci. 1996;16:1860–1865. doi: 10.1523/JNEUROSCI.16-05-01860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibbs R B, Wu D, Hersh L B, Pfaff D W. Exp Neurol. 1994;129:70–80. doi: 10.1006/exnr.1994.1148. [DOI] [PubMed] [Google Scholar]
- 7.Schipper H M. Neurobiol Aging. 1996;17:467–480. doi: 10.1016/0197-4580(96)00014-0. [DOI] [PubMed] [Google Scholar]
- 8.Brenner D E, Kukull W A, Stergachis A, Van Belle G, Bowen J D, McCormick W C, Teri L, Larson E B. Am J Epidemiol. 1994;140:262–267. doi: 10.1093/oxfordjournals.aje.a117245. [DOI] [PubMed] [Google Scholar]
- 9.Tang M-X, Jacobs D, Stern Y, Marden K, Schofield P, Gurland B, Andrews H, Mayeux R. Lancet. 1996;348:429–432. doi: 10.1016/S0140-6736(96)03356-9. [DOI] [PubMed] [Google Scholar]
- 10.Brown-Sequard C E. Lancet. 1889;2:105–107. [Google Scholar]
- 11.Bingaman E W, Magnuson D J, Gray T S, Handa R J. Neuroendocrinology. 1994;59:228–234. doi: 10.1159/000126663. [DOI] [PubMed] [Google Scholar]
- 12.Viau V, Meaney M J. J Neurosci. 1996;16:1866–1876. doi: 10.1523/JNEUROSCI.16-05-01866.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerr J E, Beck S G, Handa R J. J Neuroendocrinol. 1996;8:439–447. doi: 10.1046/j.1365-2826.1996.04735.x. [DOI] [PubMed] [Google Scholar]
- 14.Papasozomenos S Ch, Su Y. Proc Natl Acad Sci USA. 1991;88:4543–4547. doi: 10.1073/pnas.88.10.4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hatzinger M, Z’Brun A, Hemmeter U, Seifritz E, Baumann F, Holsboer-Trachsler E, Heuser I J. Neurobiol Aging. 1995;16:205–209. doi: 10.1016/0197-4580(94)00159-6. [DOI] [PubMed] [Google Scholar]
- 16.Mori H J, Kondo I, Ihara Y. Science. 1987;235:1641–1644. doi: 10.1126/science.3029875. [DOI] [PubMed] [Google Scholar]
- 17.Grundke-Iqbal I, Iqbal K, Tung Y-C, Quinlay M, Wisniewski H M, Binder L I. Proc Natl Acad Sci USA. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenberg S G, Davies P. Proc Natl Acad Sci USA. 1990;87:5827–5831. doi: 10.1073/pnas.87.15.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papasozomenos S Ch. J Neurochem. 1996;66:1140–1149. doi: 10.1046/j.1471-4159.1996.66031140.x. [DOI] [PubMed] [Google Scholar]
- 20.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Lowry O H, Rosebrough N J, Farr A L, Randall R J. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 22.Sternberger L A, Hardy P H, Cuculis J J, Meyer H G. J Histochem Cytochem. 1970;18:315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
- 23.Carmel G, Mager E M, Binder L I, Kuret J. J Biol Chem. 1996;51:32789–32795. doi: 10.1074/jbc.271.51.32789. [DOI] [PubMed] [Google Scholar]
- 24.Lang E, Szendrei G I, Lee V M-Y, Otvos L. Biochem Biophys Res Comm. 1992;87:783–790. doi: 10.1016/0006-291x(92)91264-q. [DOI] [PubMed] [Google Scholar]
- 25.LoPresti P, Szuchet S, Papasozomenos S Ch, Zinkowski R P, Binder L I. Proc Natl Acad Sci USA. 1995;92:10369–10373. doi: 10.1073/pnas.92.22.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smimomura Y, Shimizu H, Takahashi M, Vehara Y, Kobayashi I, Kobayashi S. Exp Clin Endocrinol. 1990;95:281–285. doi: 10.1055/s-0029-1210966. [DOI] [PubMed] [Google Scholar]
- 27.Bernton E, Hoover D, Galloway R, Popp K. Ann NY Acad Sci. 1995;774:217–231. [PubMed] [Google Scholar]
- 28.Mizoguchi K, Tatshuhide T, De-Hua C, Tabira T. Neurosci Lett. 1992;138:157–160. doi: 10.1016/0304-3940(92)90495-s. [DOI] [PubMed] [Google Scholar]
- 29.Dupon C, Kim M H. J Endocrinol. 1973;59:653–654. doi: 10.1677/joe.0.0590653. [DOI] [PubMed] [Google Scholar]
- 30.Abdelgadir S E, Resko J A, Ojeda S R, Lephart E D, McPhaul M J, Roselli C E. Endocrinology. 1994;135:395–401. doi: 10.1210/endo.135.1.8013375. [DOI] [PubMed] [Google Scholar]
- 31.Lacasa D, Agli B, Mur B, Dieudonne M N, Giudelli Y. J Endocrinol. 1993;138:493–501. doi: 10.1677/joe.0.1380493. [DOI] [PubMed] [Google Scholar]
- 32.Steinberger E, Chowdhury M, Tcholakian R. Andrologia. 1977;9:307–312. doi: 10.1111/j.1439-0272.1977.tb01675.x. [DOI] [PubMed] [Google Scholar]
- 33.Zhou Y, Watters J J, Dorsa D M. Endocrinology. 1996;137:2163–2166. doi: 10.1210/endo.137.5.8612562. [DOI] [PubMed] [Google Scholar]
- 34.Keating R J, Tcholakian R K. Endocrinology. 1979;104:184–188. doi: 10.1210/endo-104-1-184. [DOI] [PubMed] [Google Scholar]
- 35.Bhasin S, Stover T W, Berman N, Callegari C, Clevenger B, Phillips J, Bunnell T J, Tricker R, Shirazi A, Casaburi R. N Engl J Med. 1996;335:1–7. doi: 10.1056/NEJM199607043350101. [DOI] [PubMed] [Google Scholar]
- 36.Morishima-Kawashima M, Hasegawa M, Takio K, Suzuki M, Yoshida H, Titani K, Ihara Y. J Biol Chem. 1995;270:823–829. doi: 10.1074/jbc.270.2.823. [DOI] [PubMed] [Google Scholar]
- 37.Mohit A A, Martin J H, Miller C A. Neuron. 1995;14:67–78. doi: 10.1016/0896-6273(95)90241-4. [DOI] [PubMed] [Google Scholar]
- 38.Goedert M, Jakes R, Qi Z, Wang J H, Cohen P. J Neurochem. 1995;65:2804–2807. doi: 10.1046/j.1471-4159.1995.65062804.x. [DOI] [PubMed] [Google Scholar]
- 39.Sontag E, Nunbhakdi-Craig V, Lee G, Bloom G S, Mumby M C. Neuron. 1996;17:1201–1207. doi: 10.1016/s0896-6273(00)80250-0. [DOI] [PubMed] [Google Scholar]
- 40.Singh T J, Haque N, Grundke-Iqbal I, Iqbal K. FEBS Lett. 1995;358:267–272. doi: 10.1016/0014-5793(94)01445-7. [DOI] [PubMed] [Google Scholar]
- 41.Law B, Rossie S. J Biol Chem. 1995;270:12808–12813. doi: 10.1074/jbc.270.21.12808. [DOI] [PubMed] [Google Scholar]
- 42.Chang Y, Abe A, Shayman J A. Proc Natl Acad Sci USA. 1995;92:12275–12279. doi: 10.1073/pnas.92.26.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verheij M, Bose R, Lin X H, Yao B, Jarvis W D, Grant S, Birrer M J, Szabo E, Zon L I, Kyriakis J M, Haimovitz-Friedman A, Fuks Z, Kolesnick R N. Nature (London) 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y-R, Wang X, Templeton D, Davis R J, Tan T-H. J Biol Chem. 1996;271:31929–31936. doi: 10.1074/jbc.271.50.31929. [DOI] [PubMed] [Google Scholar]
- 45.Garcia-Sequra L M, Chowen J A, Rarducz A, Naftolin F. Prog Neurobiol. 1994;44:279–307. doi: 10.1016/0301-0082(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 46.Mangelsdorf D J, Thummel C, Beato M, Herrlich P, Schülz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans R M. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paul S M, Purdy R H. FASEB J. 1992;6:2311–2322. [PubMed] [Google Scholar]
- 48.Lephart E D. Brain Res Rev. 1996;22:1–26. [PubMed] [Google Scholar]
- 49.Resko J A, Roselli C E. Neuroprotocols. 1992;1:27–34. [Google Scholar]
- 50.Martini L, Melcangi R C, Maggi R. J Steroid Biochem Mol Biol. 1993;47:195–205. doi: 10.1016/0960-0760(93)90075-8. [DOI] [PubMed] [Google Scholar]
- 51.Bohen S P, Kralli A, Yamamoto K R. Science. 1995;268:1303–1304. doi: 10.1126/science.7761850. [DOI] [PubMed] [Google Scholar]
- 52.Dorfman R I, Shipley R A. Androgens: Biochemistry, Physiology, and Clinical Significance. New York: Wiley; 1956. pp. 5–9. [Google Scholar]
- 53.Rubinow D R, Schmidt P J. Am J Psych. 1996;153:974–984. doi: 10.1176/ajp.153.8.974. [DOI] [PubMed] [Google Scholar]
- 54.Vermeulen A, Kaufman J M. Horm Res. 1995;43:25–28. doi: 10.1159/000184233. [DOI] [PubMed] [Google Scholar]
- 55.Papasozomenos S Ch. Lab Invest. 1989;60:375–388. [PubMed] [Google Scholar]
- 56.Arriagada P V, Growdon J H, Hedley-Whyte E T, Human B T. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]