Abstract
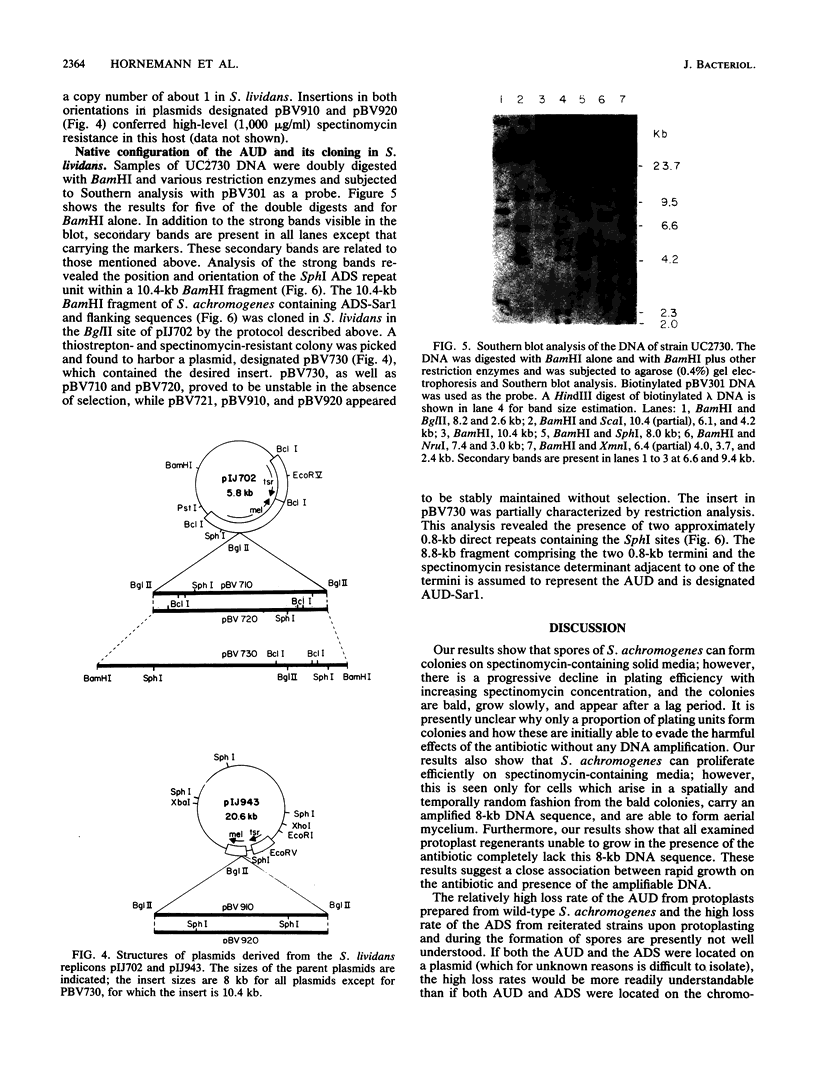
Streptomyces achromogenes subsp. rubradiris plated at low density on 1,000 micrograms of spectinomycin per ml initially produces slow-growing, bald colonies from which arise, in a spatially and temporally random fashion, foci of rapidly growing aerial mycelium-forming cells whose DNA contains an approximately 200- to 300-fold amplification of an 8-kilobase (kb) sequence. This sequence was cloned in Escherichia coli on pBR322 and physically characterized. It was separately cloned also in Streptomyces lividans as a BglII fragment and shown to impart high-level resistance to spectinomycin in an orientation-independent manner when present in either the high-copy-number vector pIJ702 or the unit-copy-number vector pIJ943. A spectinomycin resistance determinant was shown to reside on a 1.7-kb SphI-BglII subfragment. Analysis of Southern blots of restriction enzyme digests of wild-type S. achromogenes DNA probed with the labeled 8-kb DNA sequence resulted in the identification and subsequent cloning in S. lividans of a 10.4-kb BamHI fragment which probably includes the complete 8.8-kb amplifiable unit of DNA. This unit is present in wild-type S. achromogenes and in the initially slow-growing, bald colonies arising on 1,000 micrograms of spectinomycin per ml as a single copy. It carries two 0.8-kb direct repeats at its termini as well as the spectinomycin resistance determinant close to one of these termini. About 5% of protoplast regenerants from wild-type S. achromogenes and 77% of protoplast regenerants from the rapidly growing strains lost both the ability to grow on spectinomycin at 10 micrograms/ml and the sequences that hybridize with the 8-kb probe DNA. The 1.7-kb Bg/II-SphI resistance fragment, when introduced via the vector pIJ702 into an S. achromogenes strain sensitive to 10 microgram of spectinomycin per ml, permitted its vigorous growth on 1,000 micrograms of the antibiotic per ml.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Boyer H. W., Helling R. B. Construction of biologically functional bacterial plasmids in vitro. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3240–3244. doi: 10.1073/pnas.70.11.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman S. E., Hershberger C. L. Amplified DNA in Streptomyces fradiae. J Bacteriol. 1983 Aug;155(2):459–466. doi: 10.1128/jb.155.2.459-466.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman S. E., Rosteck P. R., Jr, Hershberger C. L. A 2.2-kilobase repeated DNA segment is associated with DNA amplification in Streptomyces fradiae. J Bacteriol. 1985 Jan;161(1):199–206. doi: 10.1128/jb.161.1.199-206.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HICKEY R. J., TRESNER H. D. A cobalt-containing medium for sporulation of Streptomyces species. J Bacteriol. 1952 Dec;64(6):891–892. doi: 10.1128/jb.64.6.891-892.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa M., Hintermann G., Simonet J. M., Crameri R., Piret J., Hütter R. Certain chromosomal regions in Streptomyces glaucescens tend to carry amplifications and deletions. Mol Gen Genet. 1985;200(3):375–384. doi: 10.1007/BF00425720. [DOI] [PubMed] [Google Scholar]
- Hepburn A. G., Hindley J. Small-scale techniques for the analysis of recombinant plasmids. J Biochem Biophys Methods. 1979 Oct;1(5):299–308. doi: 10.1016/0165-022x(79)90004-6. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A., Kieser T., Wright H. M., Bibb M. J. Plasmids, recombination and chromosome mapping in Streptomyces lividans 66. J Gen Microbiol. 1983 Jul;129(7):2257–2269. doi: 10.1099/00221287-129-7-2257. [DOI] [PubMed] [Google Scholar]
- Katz E., Thompson C. J., Hopwood D. A. Cloning and expression of the tyrosinase gene from Streptomyces antibioticus in Streptomyces lividans. J Gen Microbiol. 1983 Sep;129(9):2703–2714. doi: 10.1099/00221287-129-9-2703. [DOI] [PubMed] [Google Scholar]
- Langer P. R., Waldrop A. A., Ward D. C. Enzymatic synthesis of biotin-labeled polynucleotides: novel nucleic acid affinity probes. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6633–6637. doi: 10.1073/pnas.78.11.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary J. J., Brigati D. J., Ward D. C. Rapid and sensitive colorimetric method for visualizing biotin-labeled DNA probes hybridized to DNA or RNA immobilized on nitrocellulose: Bio-blots. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4045–4049. doi: 10.1073/pnas.80.13.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydiate D. J., Malpartida F., Hopwood D. A. The Streptomyces plasmid SCP2*: its functional analysis and development into useful cloning vectors. Gene. 1985;35(3):223–235. doi: 10.1016/0378-1119(85)90001-0. [DOI] [PubMed] [Google Scholar]
- Marinus M. G., Morris N. R. Pleiotropic effects of a DNA adenine methylation mutation (dam-3) in Escherichia coli K12. Mutat Res. 1975 Apr;28(1):15–26. doi: 10.1016/0027-5107(75)90309-7. [DOI] [PubMed] [Google Scholar]
- Orlova V. A., Danilenko V. N. Mul'tiplikatsiia fragmenta DNK u Streptomyces antibioticus--produtsent oleandomitsina. Antibiotiki. 1983 Mar;28(3):163–167. [PubMed] [Google Scholar]
- Peterson B. C., Rownd R. H. Drug resistance gene amplification of plasmid NR1 derivatives with various amounts of resistance determinant DNA. J Bacteriol. 1985 Mar;161(3):1042–1048. doi: 10.1128/jb.161.3.1042-1048.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potekhin Ia A., Danilenko V. N. Determinant ustoichivosti k kanamitsinu Streptomyces rimosus: amplifikatsiia v sostave khromosomy i obratimaia geneticheskaia nestabil'nost'. Mol Biol (Mosk) 1985 May-Jun;19(3):805–817. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stonesifer J., Matsushima P., Baltz R. H. High frequency conjugal transfer of tylosin genes and amplifiable DNA in Streptomyces fradiae. Mol Gen Genet. 1986 Mar;202(3):348–355. doi: 10.1007/BF00333261. [DOI] [PubMed] [Google Scholar]
- Tlsty T. D., Albertini A. M., Miller J. H. Gene amplification in the lac region of E. coli. Cell. 1984 May;37(1):217–224. doi: 10.1016/0092-8674(84)90317-9. [DOI] [PubMed] [Google Scholar]