Abstract
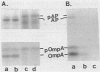
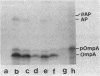
The effects of several membrane antibiotics and other agents on ATP-dependent protein translocation were examined in membrane vesicles under conditions where no significant proton motive force was present. The membrane perturbants ethanol and procaine abolished ATP-dependent protein translocation. Phenethyl alcohol at low concentrations abolished translocation, whereas at high concentrations it allowed precursors to be translocated but inhibited their processing. Translocation of precursors promoted by phenethyl alcohol was temperature dependent and occurred without an added energy source but was enhanced by ATP. However, such precursors could not be further processed to mature forms upon removal of the alcohol. The membrane-active antibiotics polymyxin B and gramicidin S were strong inhibitors of translocation, whereas gramicidin D, cerulenin, and mycobacillin had no effect even at higher concentrations, indicating some specificity in interference with protein translocation. Duramycin, an antibiotic previously shown to affect protein-lipid interaction, severely impaired protein translocation. These results showed that membrane structures play important roles, either directly or indirectly, in protein translocation. Chelating agents 1,10-phenanthroline and EDTA, but not EGTA [ethylene glycol-bis(beta-aminoethyl ether)-N,N,N',N'-tetraacetic acid], also abolished protein translocation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briggs M. S., Gierasch L. M. Exploring the conformational roles of signal sequences: synthesis and conformational analysis of lambda receptor protein wild-type and mutant signal peptides. Biochemistry. 1984 Jul 3;23(14):3111–3114. doi: 10.1021/bi00309a001. [DOI] [PubMed] [Google Scholar]
- Briggs M. S., Gierasch L. M., Zlotnick A., Lear J. D., DeGrado W. F. In vivo function and membrane binding properties are correlated for Escherichia coli lamB signal peptides. Science. 1985 May 31;228(4703):1096–1099. doi: 10.1126/science.3158076. [DOI] [PubMed] [Google Scholar]
- Chen L. L., Tai P. C. Roles of H+-ATPase and proton motive force in ATP-dependent protein translocation in vitro. J Bacteriol. 1986 Jul;167(1):389–392. doi: 10.1128/jb.167.1.389-392.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Rhoads D., Tai P. C. Alkaline phosphatase and OmpA protein can be translocated posttranslationally into membrane vesicles of Escherichia coli. J Bacteriol. 1985 Mar;161(3):973–980. doi: 10.1128/jb.161.3.973-980.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Tai P. C. ATP is essential for protein translocation into Escherichia coli membrane vesicles. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4384–4388. doi: 10.1073/pnas.82.13.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Tai P. C., Briggs M. S., Gierasch L. M. Protein translocation into Escherichia coli membrane vesicles is inhibited by functional synthetic signal peptides. J Biol Chem. 1987 Feb 5;262(4):1427–1429. [PubMed] [Google Scholar]
- Chen L., Tai P. C. Effects of nucleotides on ATP-dependent protein translocation into Escherichia coli membrane vesicles. J Bacteriol. 1986 Nov;168(2):828–832. doi: 10.1128/jb.168.2.828-832.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiRienzo J. M., Inouye M. Lipid fluidity-dependent biosynthesis and assembly of the outer membrane proteins of E. coli. Cell. 1979 May;17(1):155–161. doi: 10.1016/0092-8674(79)90303-9. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Steitz T. A. The spontaneous insertion of proteins into and across membranes: the helical hairpin hypothesis. Cell. 1981 Feb;23(2):411–422. doi: 10.1016/0092-8674(81)90136-7. [DOI] [PubMed] [Google Scholar]
- Fishman Y., Rottem S., Citri N. Evidence linking penicillinase formation and secretion to lipid metabolism in Bacillus licheniformis. J Bacteriol. 1978 May;134(2):434–439. doi: 10.1128/jb.134.2.434-439.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman Y., Rottem S., Citri N. Preferential suppression of normal exoenzyme formation by membrane-modifying agents. J Bacteriol. 1980 Mar;141(3):1435–1438. doi: 10.1128/jb.141.3.1435-1438.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halegoua S., Inouye M. Translocation and assembly of outer membrance proteins of Escherichia coli. Selective accumulation of precursors and novel assembly intermediates caused by phenethyl alcohol. J Mol Biol. 1979 May 5;130(1):39–61. doi: 10.1016/0022-2836(79)90551-5. [DOI] [PubMed] [Google Scholar]
- Kimura K., Izui K. Importance of membrane fluidity in the induction of alkaline phosphatase, a periplasmic enzyme, in Escherichia coli. Biochem Biophys Res Commun. 1976 Jun 7;70(3):900–906. doi: 10.1016/0006-291x(76)90676-8. [DOI] [PubMed] [Google Scholar]
- Lazdunski C., Baty D., Pagès J. M. Procaine, a local anesthetic interacting with the cell membrane, inhibits the processing of precursor forms of periplasmic proteins in Escherichia coli. Eur J Biochem. 1979 May 2;96(1):49–57. doi: 10.1111/j.1432-1033.1979.tb13012.x. [DOI] [PubMed] [Google Scholar]
- Lory S., Tai P. C., Davis B. D. Mechanism of protein excretion by gram-negative bacteria: Pseudomonas aeruginosa exotoxin A. J Bacteriol. 1983 Nov;156(2):695–702. doi: 10.1128/jb.156.2.695-702.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandl J., Paulus H. Effect of linear gramicidin on sporulation and intracellular ATP pools of Bacillus brevis. Arch Microbiol. 1985 Dec;143(3):248–252. doi: 10.1007/BF00411244. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Racker E. Inhibitory effect of duramycin on partial reactions catalyzed by (Na+,K+)-adenosinetriphosphatase from dog kidney. Biochemistry. 1984 Jan 17;23(2):385–389. doi: 10.1021/bi00297a031. [DOI] [PubMed] [Google Scholar]
- Nesmeyanova M. A. On the possible participation of acid phospholipids in the translocation of secreted proteins through the bacterial cytoplasmic membrane. FEBS Lett. 1982 Jun 7;142(2):189–193. doi: 10.1016/0014-5793(82)80131-2. [DOI] [PubMed] [Google Scholar]
- PAULUS H., GRAY E. THE BIOSYNTHESIS OF POLYMYXIN B BY GROWING CULTURES OF BACILLUS POLYMYXA. J Biol Chem. 1964 Mar;239:865–871. [PubMed] [Google Scholar]
- Pages J. M., Lazdunski C. Maturation of exported proteins in Escherichia coli. Requirement for energy, site and kinetics of processing. Eur J Biochem. 1982 Jun;124(3):561–566. [PubMed] [Google Scholar]
- Pagès J. M., Piovant M., Varenne S., Lazdunski C. Mechanistic aspects of the transfer of nascent periplasmic proteins across the cytoplasmic membrane in Escherichia coli. Eur J Biochem. 1978 May 16;86(2):589–602. doi: 10.1111/j.1432-1033.1978.tb12343.x. [DOI] [PubMed] [Google Scholar]
- Palva E. T., Hirst T. R., Hardy S. J., Holmgren J., Randall L. Synthesis of a precursor to the B subunit of heat-labile enterotoxin in Escherichia coli. J Bacteriol. 1981 Apr;146(1):325–330. doi: 10.1128/jb.146.1.325-330.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit-Glatron M. F., Chambert R. Levansucrase of Bacillus subtilis: Conclusive evidence that its production and export are unrelated to fatty-acid synthesis but modulated by membrane-modifying agents. Eur J Biochem. 1981 Oct;119(3):603–611. doi: 10.1111/j.1432-1033.1981.tb05650.x. [DOI] [PubMed] [Google Scholar]
- Racker E., Riegler C., Abdel-Ghany M. Stimulation of glycolysis by placental polypeptides and inhibition by duramycin. Cancer Res. 1984 Apr;44(4):1364–1367. [PubMed] [Google Scholar]
- Rhoads D. B., Tai P. C., Davis B. D. Energy-requiring translocation of the OmpA protein and alkaline phosphatase of Escherichia coli into inner membrane vesicles. J Bacteriol. 1984 Jul;159(1):63–70. doi: 10.1128/jb.159.1.63-70.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. P. Cotranslational secretion of diphtheria toxin and alkaline phosphatase in vitro: involvement of membrane protein(s). J Bacteriol. 1980 Mar;141(3):1142–1147. doi: 10.1128/jb.141.3.1142-1147.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone D. K., Xie X. S., Racker E. Inhibition of clathrin-coated vesicle acidification by duramycin. J Biol Chem. 1984 Mar 10;259(5):2701–2703. [PubMed] [Google Scholar]
- Tribhuvan R. C., Pilgaokar A. K., Pradhan D. S., Sreenivasan A. Effect of phenethyl alcohol on induction of alkaline phosphatase in Escherichia coli. Biochem Biophys Res Commun. 1970 Oct 9;41(1):244–250. doi: 10.1016/0006-291x(70)90495-x. [DOI] [PubMed] [Google Scholar]
- Tribhuwan R. C., Pradhan D. S. Induction of alkaline phosphatase in Escherichia coli: effect of procaine hydrochloride. J Bacteriol. 1977 Aug;131(2):431–437. doi: 10.1128/jb.131.2.431-437.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M., Vaara T. Polycations sensitize enteric bacteria to antibiotics. Antimicrob Agents Chemother. 1983 Jul;24(1):107–113. doi: 10.1128/aac.24.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatvin M. B., Smith K. M., Siegel F. L. Translocation of nascent non-signal sequence protein in heated Escherichia coli. J Biol Chem. 1986 Jun 15;261(17):8070–8075. [PubMed] [Google Scholar]