Abstract
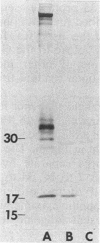
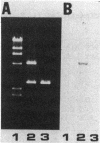
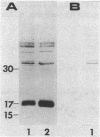
DNA obtained from the Sheila Smith strain of Rickettsia rickettsii was digested to completion with the restriction endonucleases BamHI and SalI and ligated with the plasmid vector pUC19. The ligation mixture was used to transform Escherichia coli. A total of 465 bacterial clones were screened for antigen production with hyperimmune rabbit serum. One of the reactive clones, containing a recombinant plasmid designated pSS124, was solubilized and subjected to immunoblot analysis and revealed expression of a 17-kilodalton protein reactive with anti-R. rickettsii serum that comigrated with an antigen from R. rickettsii. A 1.6-kilobase PstI-BamHI fragment from pSS124 was subcloned and continued to direct synthesis of the 17-kilodalton antigen. The nucleotide sequence was determined for this 1.6-kilobase subclone, which encompassed the gene encoding the polypeptide as well as flanking regions containing potential regulatory sequences. The open reading frame consisted of 477 nucleotides that specified a 159-amino-acid protein with a calculated molecular weight of 16,840. The deduced amino acid sequence contained a hydrophobic sequence near the amino terminus that resembled signal peptides described for E. coli. The carboxy terminus was hydrophilic in nature and probably contained the exposed epitopes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan I., Cunningham T. M., Lovett M. A. Molecular cloning of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1984 Sep;45(3):637–641. doi: 10.1128/iai.45.3.637-641.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker R. L., List R. H., Mann R. E., Hayes S. F., Thomas L. A. Characterization of monoclonal antibodies protecting mice against Rickettsia rickettsii. J Infect Dis. 1985 Jun;151(6):1052–1060. doi: 10.1093/infdis/151.6.1052. [DOI] [PubMed] [Google Scholar]
- Anacker R. L., Philip R. N., Williams J. C., List R. H., Mann R. E. Biochemical and immunochemical analysis of Rickettsia rickettsii strains of various degrees of virulence. Infect Immun. 1984 Jun;44(3):559–564. doi: 10.1128/iai.44.3.559-564.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Conformational parameters for amino acids in helical, beta-sheet, and random coil regions calculated from proteins. Biochemistry. 1974 Jan 15;13(2):211–222. doi: 10.1021/bi00699a001. [DOI] [PubMed] [Google Scholar]
- Eisemann C. S., Osterman J. V. Proteins of typhus and spotted fever group rickettsiae. Infect Immun. 1976 Jul;14(1):155–162. doi: 10.1128/iai.14.1.155-162.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Gold L., Pribnow D., Schneider T., Shinedling S., Singer B. S., Stormo G. Translational initiation in prokaryotes. Annu Rev Microbiol. 1981;35:365–403. doi: 10.1146/annurev.mi.35.100181.002053. [DOI] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Hsu C. P., Itakura K., Inouye M. Requirement for signal peptide cleavage of Escherichia coli prolipoprotein. Science. 1983 Jul 1;221(4605):59–61. doi: 10.1126/science.6344218. [DOI] [PubMed] [Google Scholar]
- Krause D. C., Winkler H. H., Wood D. O. Cosmid cloning of Rickettsia prowazekii antigens in Escherichia coli K-12. Infect Immun. 1985 Jan;47(1):157–165. doi: 10.1128/iai.47.1.157-165.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nano F. E., Caldwell H. D. Expression of the chlamydial genus-specific lipopolysaccharide epitope in Escherichia coli. Science. 1985 May 10;228(4700):742–744. doi: 10.1126/science.2581315. [DOI] [PubMed] [Google Scholar]
- Pedersen C. E., Jr, Walters V. D. Comparative electrophoresis of spotted fever group rickettsial proteins. Life Sci. 1978 Feb;22(7):583–587. doi: 10.1016/0024-3205(78)90337-5. [DOI] [PubMed] [Google Scholar]
- Silhavy T. J., Benson S. A., Emr S. D. Mechanisms of protein localization. Microbiol Rev. 1983 Sep;47(3):313–344. doi: 10.1128/mr.47.3.313-344.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. S., Kuo C. C., Newport G., Agabian N. Molecular cloning and expression of Chlamydia trachomatis major outer membrane protein antigens in Escherichia coli. Infect Immun. 1985 Mar;47(3):713–718. doi: 10.1128/iai.47.3.713-718.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Coolbaugh J. C., Williams J. C. Separation of viable Rickettsia typhi from yolk sac and L cell host components by renografin density gradient centrifugation. Appl Microbiol. 1975 Sep;30(3):456–463. doi: 10.1128/am.30.3.456-463.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Walker D. H., Peacock M. G., Stewart S. T. Humoral immune response to Rocky Mountain spotted fever in experimentally infected guinea pigs: immunoprecipitation of lactoperoxidase 125I-labeled proteins and detection of soluble antigens of Rickettsia rickettsii. Infect Immun. 1986 Apr;52(1):120–127. doi: 10.1128/iai.52.1.120-127.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood D. O., Atkinson W. H., Sikorski R. S., Winkler H. H. Expression of the Rickettsia prowazekii citrate synthase gene in Escherichia coli. J Bacteriol. 1983 Jul;155(1):412–416. doi: 10.1128/jb.155.1.412-416.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]