Abstract
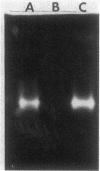
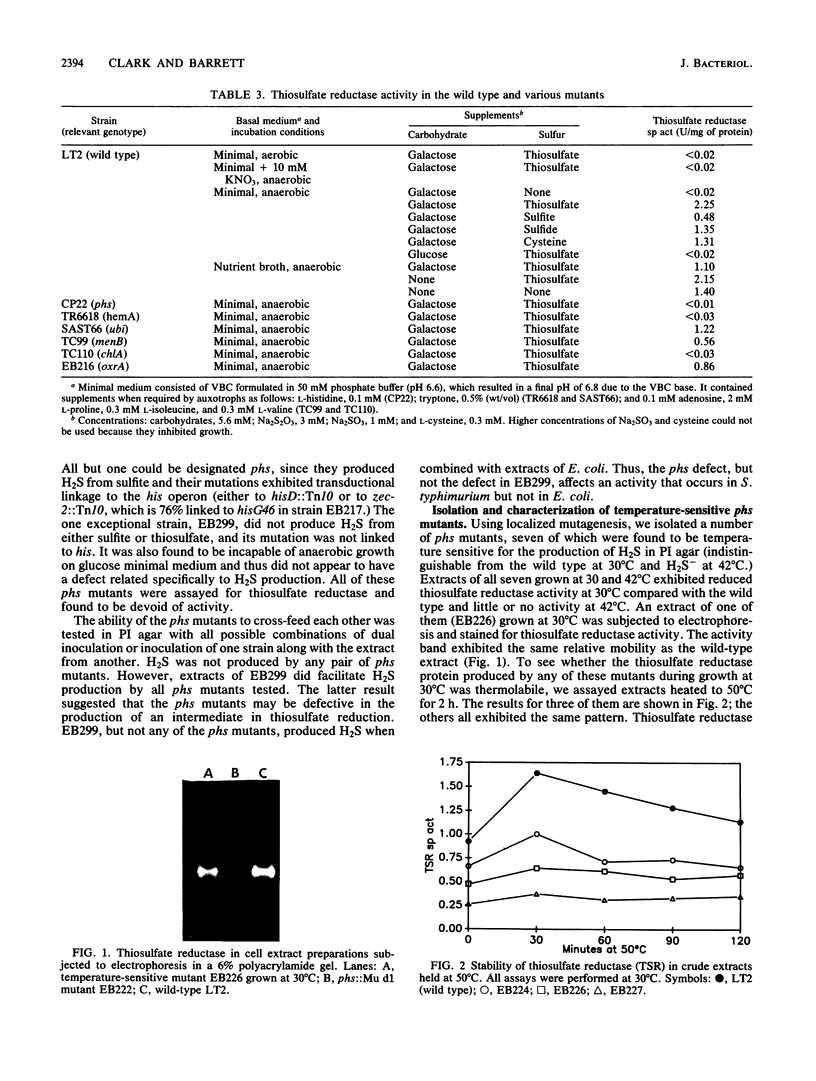
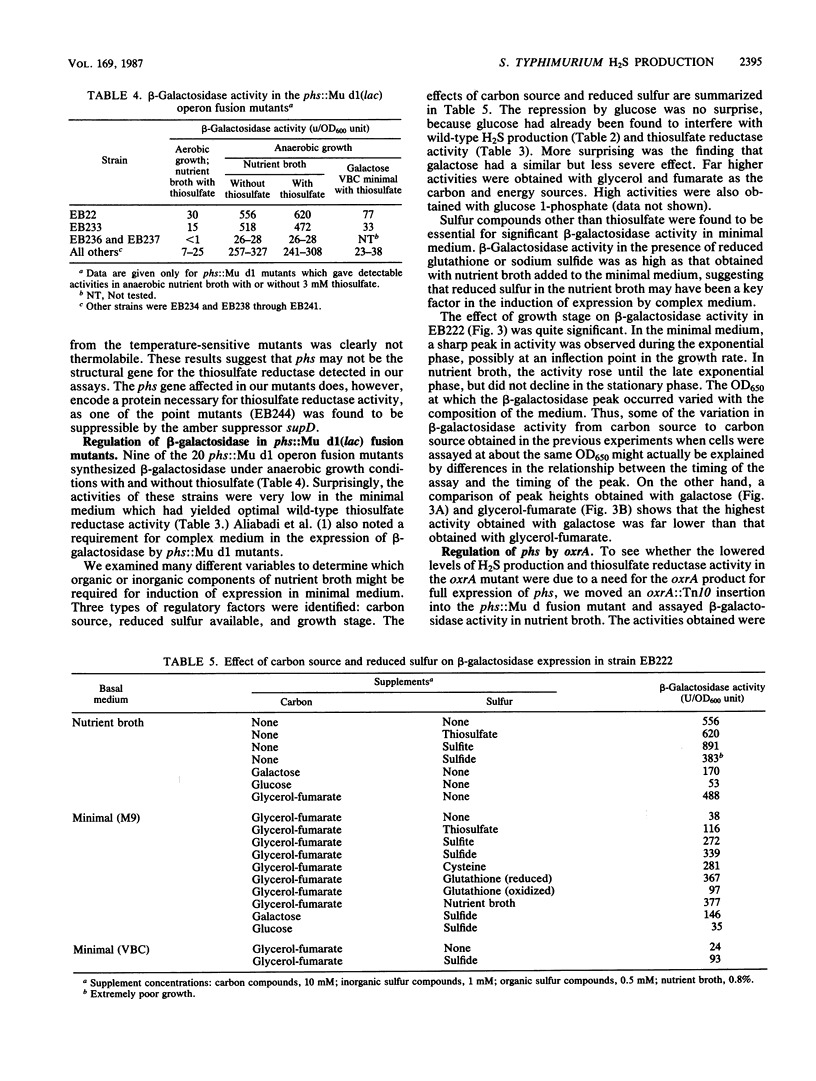
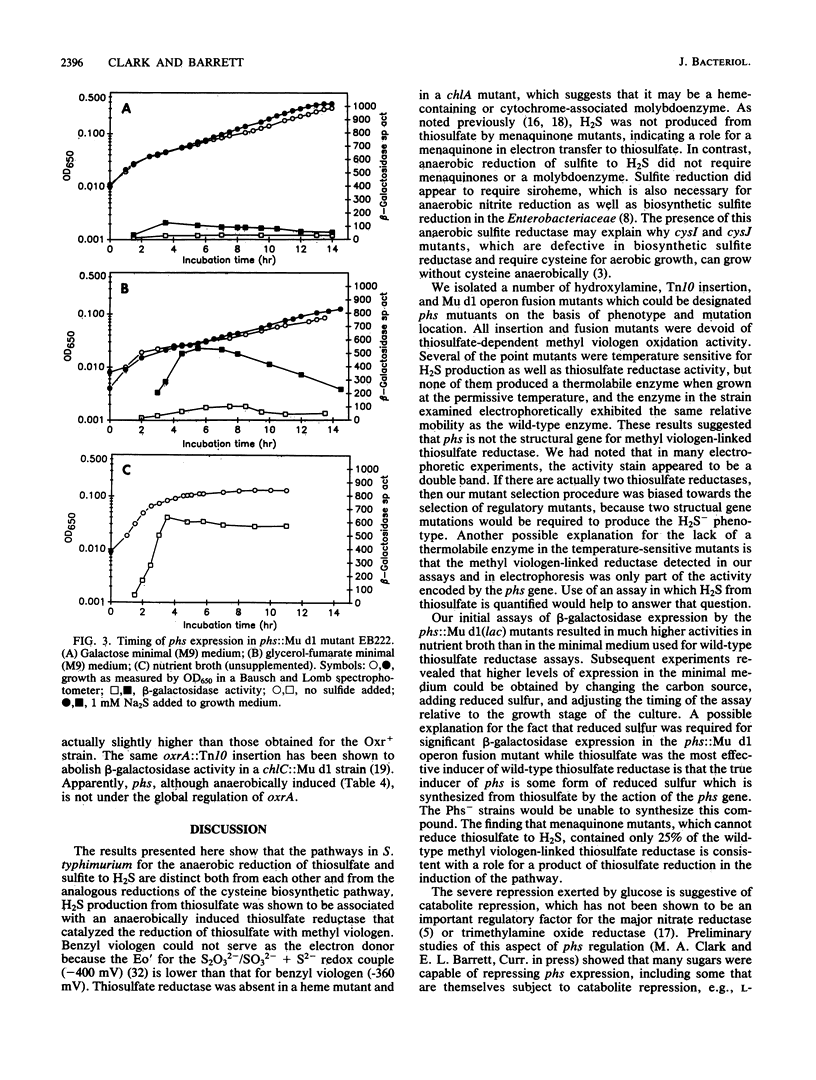
Salmonella typhimurium produces H2S from thiosulfate or sulfite. The respective pathways for the two reductions must be distinct as mutants carrying motations in phs, chlA, and menB reduced sulfite, but not thiosulfate, to H2S, and glucose repressed the production of H2S from thiosulfate while it stimulated its production from sulfite. The phs and chlA mutants also lacked a methyl viologen-linked thiosulfate reductase activity present in anaerobically grown wild-type cultures. A number of hydroxylamine, transposon Tn10 insertion, and Mu d1(Apr lac) operon fusion mutants defective in phs were characterized. One of the hydroxylamine mutants was an amber mutant, as indicated by suppression of its mutation in a supD background. The temperature-sensitive phs mutants produced H2S and methyl viologen-linked thiosulfate reductase at 30 degrees C but not at 42 degrees C. The reductases in all such mutants grown at 30 degrees C were as thermostable as the wild-type enzyme and did not differ in electrophoretic relative mobility, suggesting that phs is not the structural gene for thiosulfate reductase. Expression of beta-galactosidase in phs::Mu d1(Apr lac) mutants was dependent on anaerobiosis and the presence of reduced sulfur. It was also strongly influenced by carbon source and growth stage. The results are consistent with a model in which the phs gene encodes a regulatory protein essential for the reduction of thiosulfate to hydrogen sulfide.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aliabadi Z., Warren F., Mya S., Foster J. W. Oxygen-regulated stimulons of Salmonella typhimurium identified by Mu d(Ap lac) operon fusions. J Bacteriol. 1986 Mar;165(3):780–786. doi: 10.1128/jb.165.3.780-786.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. L., Chang G. W. Cysteine auxotrophs of Salmonella typhimurium which grow without cysteine in a hydrogen/carbon dioxide atmosphere. J Gen Microbiol. 1979 Dec;115(2):513–516. doi: 10.1099/00221287-115-2-513. [DOI] [PubMed] [Google Scholar]
- Barrett E. L., Kwan H. S., Macy J. Anaerobiosis, formate, nitrate, and pyrA are involved in the regulation of formate hydrogenlyase in Salmonella typhimurium. J Bacteriol. 1984 Jun;158(3):972–977. doi: 10.1128/jb.158.3.972-977.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. L., Riggs D. L. Evidence of a second nitrate reductase activity that is distinct from the respiratory enzyme in Salmonella typhimurium. J Bacteriol. 1982 May;150(2):563–571. doi: 10.1128/jb.150.2.563-571.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Casse F., Pascal M. C., Chippaux M. A mutant of Salmonella typhimurium deficient in tetrathionate reductase activity. Mol Gen Genet. 1972;119(1):71–74. doi: 10.1007/BF00270446. [DOI] [PubMed] [Google Scholar]
- Cole J. A., Newman B. M., White P. Biochemical and genetic characterization of nirB mutants of Escherichia coli K 12 pleiotropically defective in nitrite and sulphite reduction. J Gen Microbiol. 1980 Oct;120(2):475–483. doi: 10.1099/00221287-120-2-475. [DOI] [PubMed] [Google Scholar]
- Hartman P. E. Some improved methods in P22 transduction. Genetics. 1974 Apr;76(4):625–631. doi: 10.1093/genetics/76.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinojosa-Leon M., Dubourdieu M., Sanchez-Crispin J. A., Chippaux M. Tetrathionate reductase of Salmonella thyphimurium: a molybdenum containing enzyme. Biochem Biophys Res Commun. 1986 Apr 29;136(2):577–581. doi: 10.1016/0006-291x(86)90479-1. [DOI] [PubMed] [Google Scholar]
- Hong J. S., Ames B. N. Localized mutagenesis of any specific small region of the bacterial chromosome. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3158–3162. doi: 10.1073/pnas.68.12.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson D. J., Higgins C. F. Anaerobic and leucine-dependent expression of a peptide transport gene in Salmonella typhimurium. J Bacteriol. 1984 Oct;160(1):131–136. doi: 10.1128/jb.160.1.131-136.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaprálek F. The physiological role of tetrathionate respiration in growing citrobacter. J Gen Microbiol. 1972 Jun;71(1):133–139. doi: 10.1099/00221287-71-1-133. [DOI] [PubMed] [Google Scholar]
- Kwan H. S., Barrett E. L. Map locations and functions of Salmonella typhimurium men genes. J Bacteriol. 1984 Sep;159(3):1090–1092. doi: 10.1128/jb.159.3.1090-1092.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan H. S., Barrett E. L. Purification and properties of trimethylamine oxide reductase from Salmonella typhimurium. J Bacteriol. 1983 Sep;155(3):1455–1458. doi: 10.1128/jb.155.3.1455-1458.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan H. S., Barrett E. L. Roles for menaquinone and the two trimethylamine oxide (TMAO) reductases in TMAO respiration in Salmonella typhimurium: Mu d(Apr lac) insertion mutations in men and tor. J Bacteriol. 1983 Sep;155(3):1147–1155. doi: 10.1128/jb.155.3.1147-1155.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan H. S., Wong K. K. A general method for isolation of Mu d1-8(Aprlac) operon fusions in Salmonella typhimurium LT2 from Tn10 insertion strains: chlC::Mu d1-8. Mol Gen Genet. 1986 Nov;205(2):221–224. doi: 10.1007/BF00430431. [DOI] [PubMed] [Google Scholar]
- LEINWEBER F. J., MONTY K. J. THE METABOLISM OF THIOSULFATE IN SALMONELLA TYPHIMURIUM. J Biol Chem. 1963 Nov;238:3775–3780. [PubMed] [Google Scholar]
- Lund K., DeMoss J. A. Association-dissociation behavior and subunit structure of heat-released nitrate reductase from Escherichia coli. J Biol Chem. 1976 Apr 25;251(8):2207–2216. [PubMed] [Google Scholar]
- Maloy S. R., Nunn W. D. Selection for loss of tetracycline resistance by Escherichia coli. J Bacteriol. 1981 Feb;145(2):1110–1111. doi: 10.1128/jb.145.2.1110-1111.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCready R. G., Grinenko V. A., Krouse H. R. Sulfur isotope fractionation by Proteus vulgaris and Salmonella heidelberg during the reduction of thiosulfate. Can J Microbiol. 1980 Oct;26(10):1173–1177. doi: 10.1139/m80-196. [DOI] [PubMed] [Google Scholar]
- Oltmann L. F., Schoenmaker G. S., Stouthamer A. H. Solubilization and purification of a cytoplasmic membrane bound enzyme catalyzing tetrathionate and thiosulphate reduction in Proteus mirabilis. Arch Mikrobiol. 1974 Jun 7;98(1):19–30. doi: 10.1007/BF00425264. [DOI] [PubMed] [Google Scholar]
- Padron A. P., Dockstader W. B. Selective medium for hydrogen sulfide production by salmonellae. Appl Microbiol. 1972 Jun;23(6):1107–1112. doi: 10.1128/am.23.6.1107-1112.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel L. M., Davis P. S. Reduced nicotinamide adenine dinucleotide phosphate-sulfite reductase of enterobacteria. IV. The Escherichia coli hemoflavoprotein: subunit structure and dissociation into hemoprotein and flavoprotein components. J Biol Chem. 1974 Mar 10;249(5):1587–1598. [PubMed] [Google Scholar]
- Smith H. O., Levine M. A phage P22 gene controlling integration of prophage. Virology. 1967 Feb;31(2):207–216. doi: 10.1016/0042-6822(67)90164-x. [DOI] [PubMed] [Google Scholar]
- Stouthamer A. H. A genetical and biochemical study of chlorate-resistant mutants of Salmonella typhimurium. Antonie Van Leeuwenhoek. 1969;35(4):505–521. doi: 10.1007/BF02219168. [DOI] [PubMed] [Google Scholar]
- Strauch K. L., Lenk J. B., Gamble B. L., Miller C. G. Oxygen regulation in Salmonella typhimurium. J Bacteriol. 1985 Feb;161(2):673–680. doi: 10.1128/jb.161.2.673-680.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley F. W. The Relation Between Chemical Composition of Peptones and Hydrogen Sulphide Production by Bacteria. J Bacteriol. 1923 May;8(3):287–295. doi: 10.1128/jb.8.3.287-295.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Voll M. J., Cohen L. A., Germida J. J. his-Linked hydrogen sulfide locus of Salmonella typhimurium and its expression in Escherichia coli. J Bacteriol. 1979 Sep;139(3):1082–1084. doi: 10.1128/jb.139.3.1082-1084.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voll M. J., Shiller L. M., Castrilli J. His-linked hydrogen sulfide locus in Salmonella typhimurium. J Bacteriol. 1974 Nov;120(2):902–905. doi: 10.1128/jb.120.2.902-905.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]