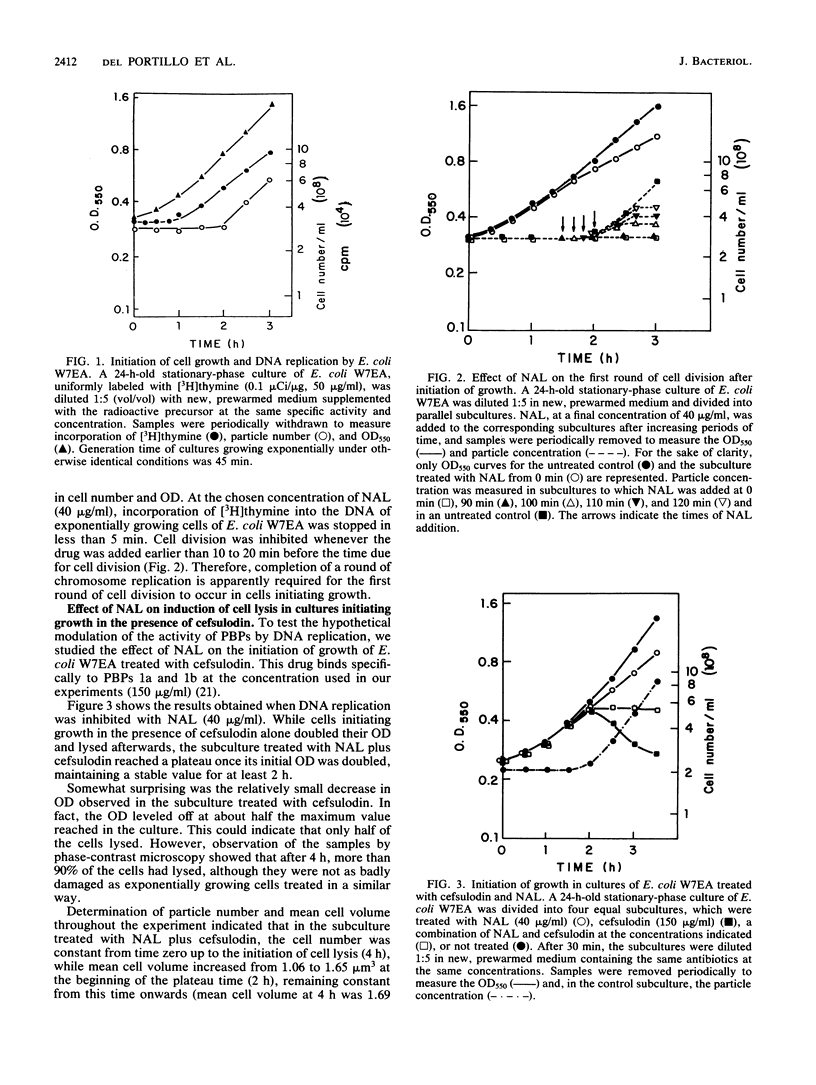
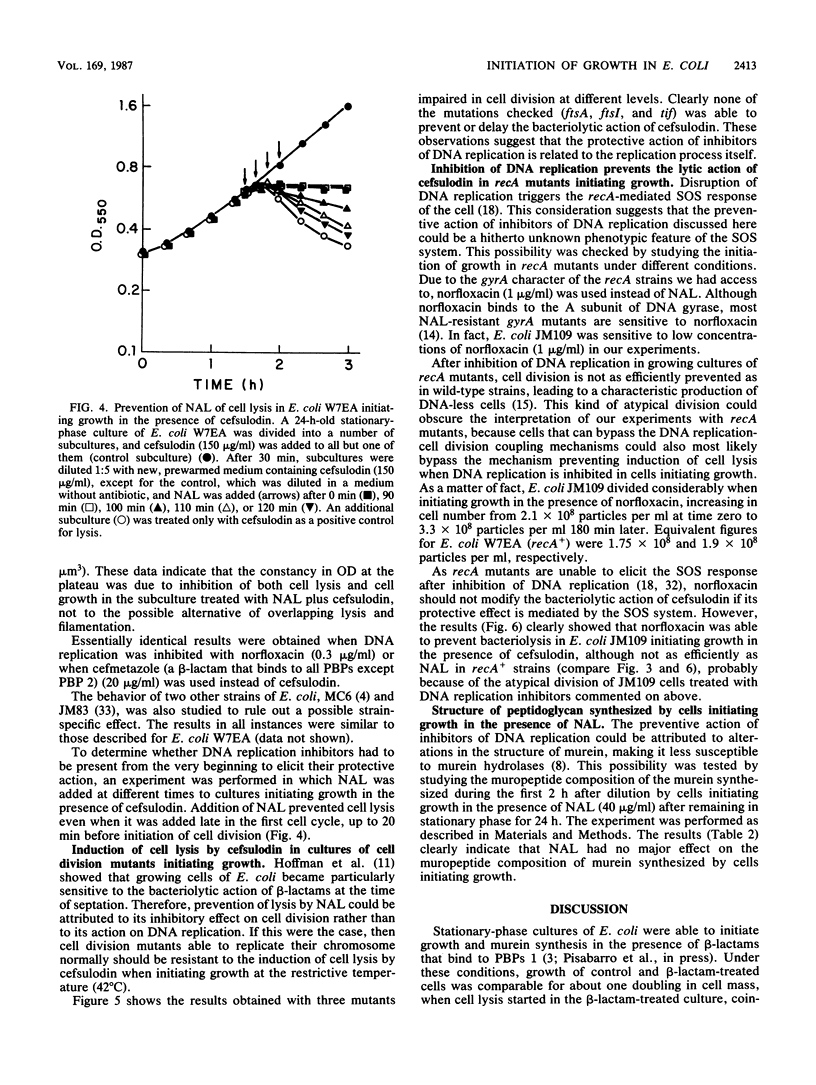
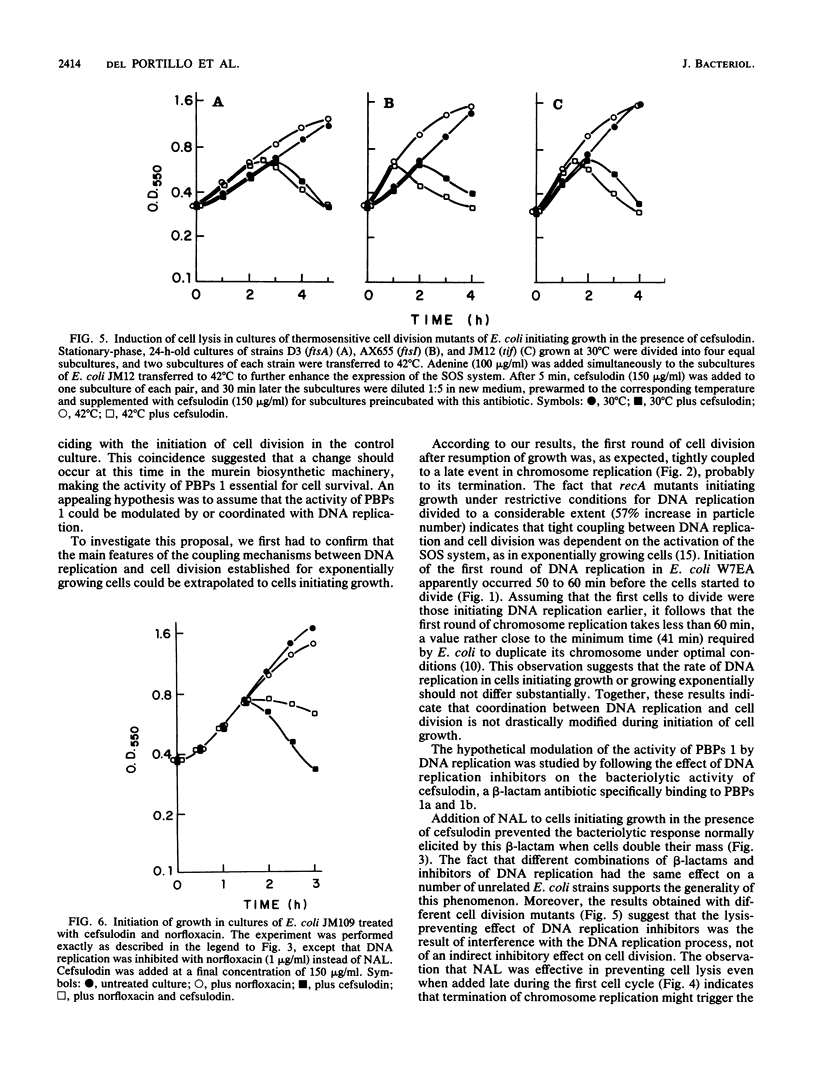
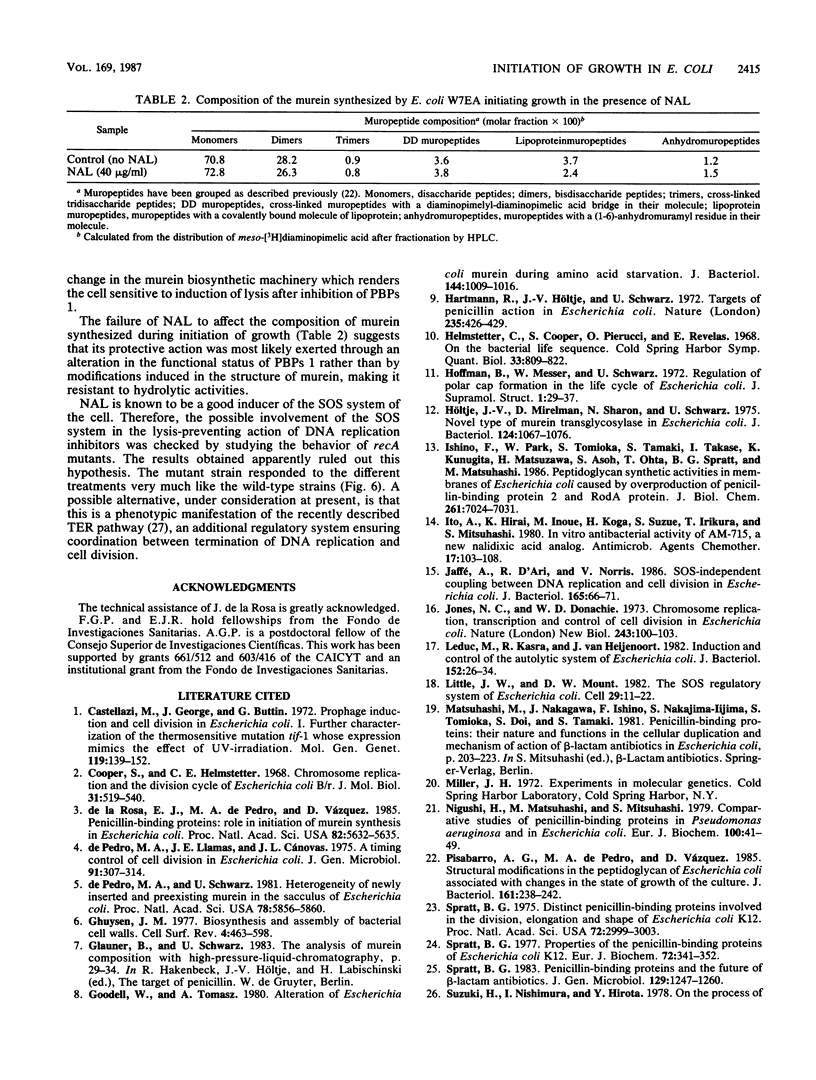
Abstract
Resting cells of Escherichia coli are able to initiate growth and murein biosynthesis in the presence of beta-lactam antibiotics binding to penicillin-binding proteins (PBPs) 1a and 1b (E. J. de la Rosa, M. A. de Pedro, and D. Vázquez, Proc. Natl. Acad. Sci. USA 82:5632-5635, 1985). Under these conditions, cells elongate normally until they approach the first doubling in mass, the time at which cell lysis starts. Assuming that coupling between DNA replication and cell division both in cells starting growth and in growing cells is essentially similar, triggering of the lytic response in the beta-lactam-treated cells coincides with the termination of the first round of DNA replication. This coincidence suggests that both events are interrelated. We investigated this possibility by studying the initiation of growth in cultures of wild-type strains and in cell division mutants treated with beta-lactams inhibiting PBPs 1a and 1b and with the DNA replication inhibitor nalidixic acid. Addition of nalidixic acid, even late in the first cell cycle, prevented the lytic response of the cells to the blockade of PBPs 1a and 1b. The effect of nalidixic acid is more likely due to its action on DNA replication itself than to its indirect inhibitory effect on cell division or to its ability to induce the SOS system of the cell. These observations favor the idea that the cell wall biosynthetic machinery might be modulated by DNA replication at precise periods during cell growth.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Castellazzi M., George J., Buttin G. Prophage induction and cell division in E. coli. I. Further characterization of the thermosensitive mutation tif-1 whose expression mimics the effect of UV irradiation. Mol Gen Genet. 1972;119(2):139–152. doi: 10.1007/BF00269133. [DOI] [PubMed] [Google Scholar]
- Cooper S., Helmstetter C. E. Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):519–540. doi: 10.1016/0022-2836(68)90425-7. [DOI] [PubMed] [Google Scholar]
- Goodell W., Tomasz A. Alteration of Escherichia coli murein during amino acid starvation. J Bacteriol. 1980 Dec;144(3):1009–1016. doi: 10.1128/jb.144.3.1009-1016.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann R., Höltje J. V., Schwarz U. Targets of penicillin action in Escherichia coli. Nature. 1972 Feb 25;235(5339):426–429. doi: 10.1038/235426a0. [DOI] [PubMed] [Google Scholar]
- Helmstetter C., Cooper S., Pierucci O., Revelas E. On the bacterial life sequence. Cold Spring Harb Symp Quant Biol. 1968;33:809–822. doi: 10.1101/sqb.1968.033.01.093. [DOI] [PubMed] [Google Scholar]
- Hoffmann B., Messer W., Schwarz U. Regulation of polar cap formation in the life cycle of Escherichia coli. J Supramol Struct. 1972;1(1):29–37. doi: 10.1002/jss.400010105. [DOI] [PubMed] [Google Scholar]
- Höltje J. V., Mirelman D., Sharon N., Schwarz U. Novel type of murein transglycosylase in Escherichia coli. J Bacteriol. 1975 Dec;124(3):1067–1076. doi: 10.1128/jb.124.3.1067-1076.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishino F., Park W., Tomioka S., Tamaki S., Takase I., Kunugita K., Matsuzawa H., Asoh S., Ohta T., Spratt B. G. Peptidoglycan synthetic activities in membranes of Escherichia coli caused by overproduction of penicillin-binding protein 2 and rodA protein. J Biol Chem. 1986 May 25;261(15):7024–7031. [PubMed] [Google Scholar]
- Ito A., Hirai K., Inoue M., Koga H., Suzue S., Irikura T., Mitsuhashi S. In vitro antibacterial activity of AM-715, a new nalidixic acid analog. Antimicrob Agents Chemother. 1980 Feb;17(2):103–108. doi: 10.1128/aac.17.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffé A., D'Ari R., Norris V. SOS-independent coupling between DNA replication and cell division in Escherichia coli. J Bacteriol. 1986 Jan;165(1):66–71. doi: 10.1128/jb.165.1.66-71.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N. C., Donachie W. D. Chromosome replication, transcription and control of cell division in Escherichia coli. Nat New Biol. 1973 May 23;243(125):100–103. [PubMed] [Google Scholar]
- Leduc M., Kasra R., van Heijenoort J. Induction and control of the autolytic system of Escherichia coli. J Bacteriol. 1982 Oct;152(1):26–34. doi: 10.1128/jb.152.1.26-34.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Noguchi H., Matsuhashi M., Mitsuhashi S. Comparative studies of penicillin-binding proteins in Pseudomonas aeruginosa and Escherichia coli. Eur J Biochem. 1979 Oct;100(1):41–49. doi: 10.1111/j.1432-1033.1979.tb02031.x. [DOI] [PubMed] [Google Scholar]
- Pisabarro A. G., de Pedro M. A., Vázquez D. Structural modifications in the peptidoglycan of Escherichia coli associated with changes in the state of growth of the culture. J Bacteriol. 1985 Jan;161(1):238–242. doi: 10.1128/jb.161.1.238-242.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Penicillin-binding proteins and the future of beta-lactam antibiotics. The Seventh Fleming Lecture. J Gen Microbiol. 1983 May;129(5):1247–1260. doi: 10.1099/00221287-129-5-1247. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Tormo A., Dopazo A., de la Campa A. G., Aldea M., Vicente M. Coupling between DNA replication and cell division mediated by the FtsA protein in Escherichia coli: a pathway independent of the SOS response, the "TER" pathway. J Bacteriol. 1985 Nov;164(2):950–953. doi: 10.1128/jb.164.2.950-953.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tormo A., Fernández-Cabrera C., Vicente M. The ftsA gene product: a possible connection between DNA replication and septation in Escherichia coli. J Gen Microbiol. 1985 Feb;131(2):239–244. doi: 10.1099/00221287-131-2-239. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Walker J. R., Kovarik A., Allen J. S., Gustafson R. A. Regulation of bacterial cell division: temperature-sensitive mutants of Escherichia coli that are defective in septum formation. J Bacteriol. 1975 Aug;123(2):693–703. doi: 10.1128/jb.123.2.693-703.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman D. J., Strominger J. L. Penicillin-binding proteins and the mechanism of action of beta-lactam antibiotics. Annu Rev Biochem. 1983;52:825–869. doi: 10.1146/annurev.bi.52.070183.004141. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- de Pedro M. A., Llamas J. E., Cánovas J. L. A timing control of cell division in Escherichia coli. J Gen Microbiol. 1975 Dec;91(2):307–314. doi: 10.1099/00221287-91-2-307. [DOI] [PubMed] [Google Scholar]
- de Pedro M. A., Schwarz U. Heterogeneity of newly inserted and preexisting murein in the sacculus of Escherichia coli. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5856–5860. doi: 10.1073/pnas.78.9.5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Rosa E. J., de Pedro M. A., Vázquez D. Penicillin binding proteins: role in initiation of murein synthesis in Escherichia coli. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5632–5635. doi: 10.1073/pnas.82.17.5632. [DOI] [PMC free article] [PubMed] [Google Scholar]



