Abstract
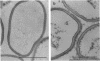
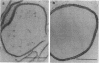
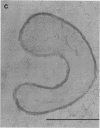
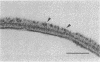
The fine structure of the Staphylococcus aureus cell wall was determined by electron microscopy with the new technique of rapid freezing and substitution fixation. The surface of the cell wall was covered with a fuzzy coat which consisted of fine fibers or an electron-dense mass. Morphological examination of the cell wall, which was treated sequentially with sodium dodecyl sulfate, trypsin, and trichloroacetic acid, revealed that this coat was partially removed by trypsin digestion and was completely removed by trichloroacetic acid extraction but was not affected by sodium dodecyl sulfate treatment, suggesting that the fuzzy coat consists mostly of a complex of teichoic acids and proteins. This was confirmed by the application of the concanavalin A-ferritin technique for teichoic acid and antiferritin immunoglobulin G technique for protein A.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. J. The estimation of phosphorus. Biochem J. 1940 Jun;34(6):858–865. doi: 10.1042/bj0340858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amako K., Murata K., Umeda A. Structure of the envelope of Escherichia coli observed by the rapid-freezing and substitution fixation method. Microbiol Immunol. 1983;27(1):95–99. doi: 10.1111/j.1348-0421.1983.tb03571.x. [DOI] [PubMed] [Google Scholar]
- Amako K., Okada K., Miake S. Evidence for the presence of a capsule in Vibrio vulnificus. J Gen Microbiol. 1984 Oct;130(10):2741–2743. doi: 10.1099/00221287-130-10-2741. [DOI] [PubMed] [Google Scholar]
- Amako K., Takade A. The fine structure of Bacillus subtilis revealed by the rapid-freezing and substitution-fixation method. J Electron Microsc (Tokyo) 1985;34(1):13–17. [PubMed] [Google Scholar]
- Amako K., Umeda A. Cross wall synthesis and the arrangement of the wall polymers in the cell wall of Staphylococcus spp. Microbiol Immunol. 1984;28(12):1293–1301. doi: 10.1111/j.1348-0421.1984.tb00787.x. [DOI] [PubMed] [Google Scholar]
- Amako K., Umeda A. Regular arrangement of wall polymers in staphylococci. J Gen Microbiol. 1979 Aug;113(2):421–424. doi: 10.1099/00221287-113-2-421. [DOI] [PubMed] [Google Scholar]
- Birdsell D. C., Doyle R. J., Morgenstern M. Organization of teichoic acid in the cell wall of Bacillus subtilis. J Bacteriol. 1975 Feb;121(2):726–734. doi: 10.1128/jb.121.2.726-734.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coley J., Tarelli E., Archibald A. R., Baddiley J. The linkage between teichoic acid and peptidoglycan in bacterial cell walls. FEBS Lett. 1978 Apr 1;88(1):1–9. doi: 10.1016/0014-5793(78)80594-8. [DOI] [PubMed] [Google Scholar]
- Dubochet J., McDowall A. W., Menge B., Schmid E. N., Lickfeld K. G. Electron microscopy of frozen-hydrated bacteria. J Bacteriol. 1983 Jul;155(1):381–390. doi: 10.1128/jb.155.1.381-390.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesbrecht P., Wecke J., Reinicke B. On the morphogenesis of the cell wall of staphylococci. Int Rev Cytol. 1976;44:225–318. doi: 10.1016/s0074-7696(08)61651-4. [DOI] [PubMed] [Google Scholar]
- Hobot J. A., Carlemalm E., Villiger W., Kellenberger E. Periplasmic gel: new concept resulting from the reinvestigation of bacterial cell envelope ultrastructure by new methods. J Bacteriol. 1984 Oct;160(1):143–152. doi: 10.1128/jb.160.1.143-152.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobot J. A., Villiger W., Escaig J., Maeder M., Ryter A., Kellenberger E. Shape and fine structure of nucleoids observed on sections of ultrarapidly frozen and cryosubstituted bacteria. J Bacteriol. 1985 Jun;162(3):960–971. doi: 10.1128/jb.162.3.960-971.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Phillips G. N., Jr, Flicker P. F., Cohen C., Manjula B. N., Fischetti V. A. Streptococcal M protein: alpha-helical coiled-coil structure and arrangement on the cell surface. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4689–4693. doi: 10.1073/pnas.78.8.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REISSIG J. L., STORMINGER J. L., LELOIR L. F. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955 Dec;217(2):959–966. [PubMed] [Google Scholar]
- Reeder W. J., Ekstedt R. D. Study of the interaction of concanavalin A with staphylocccal teichoic acids. J Immunol. 1971 Feb;106(2):334–340. [PubMed] [Google Scholar]
- Umeda A., Ikebuchi T., Amako K. Localization of bacteriophage receptor, clumping factor, and protein A on the cell surface of Staphylococcus aureus. J Bacteriol. 1980 Feb;141(2):838–844. doi: 10.1128/jb.141.2.838-844.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]