Abstract
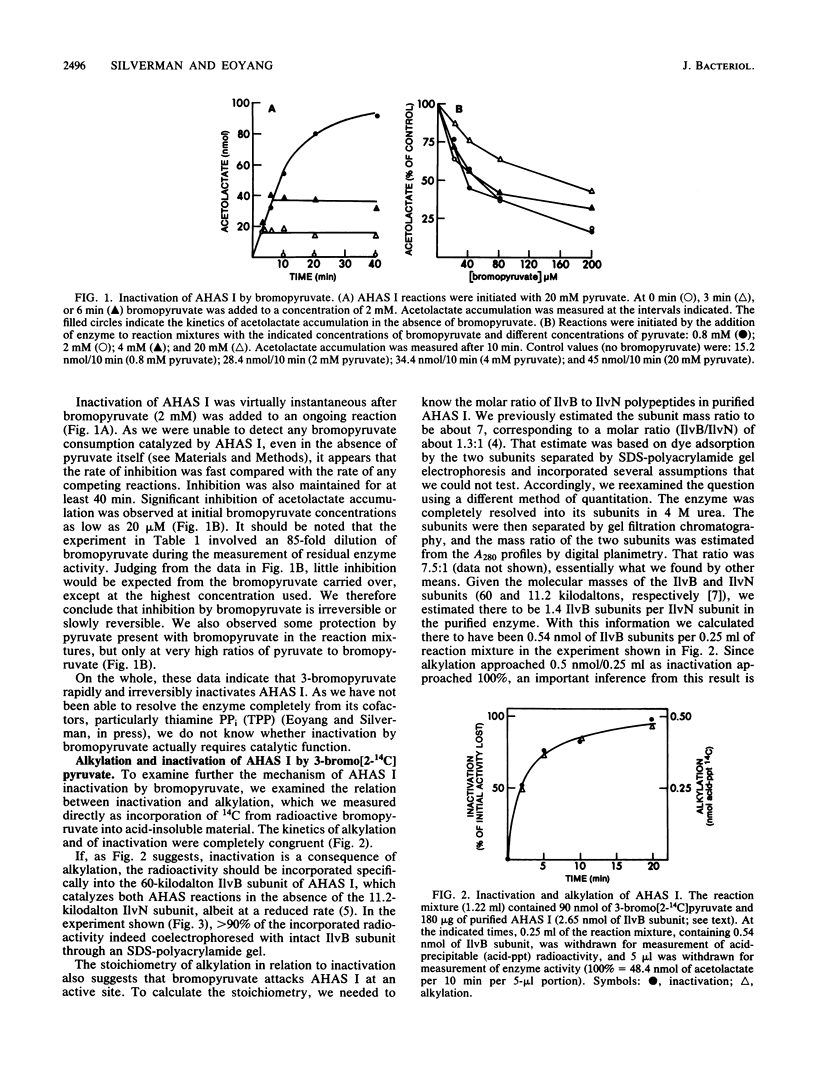
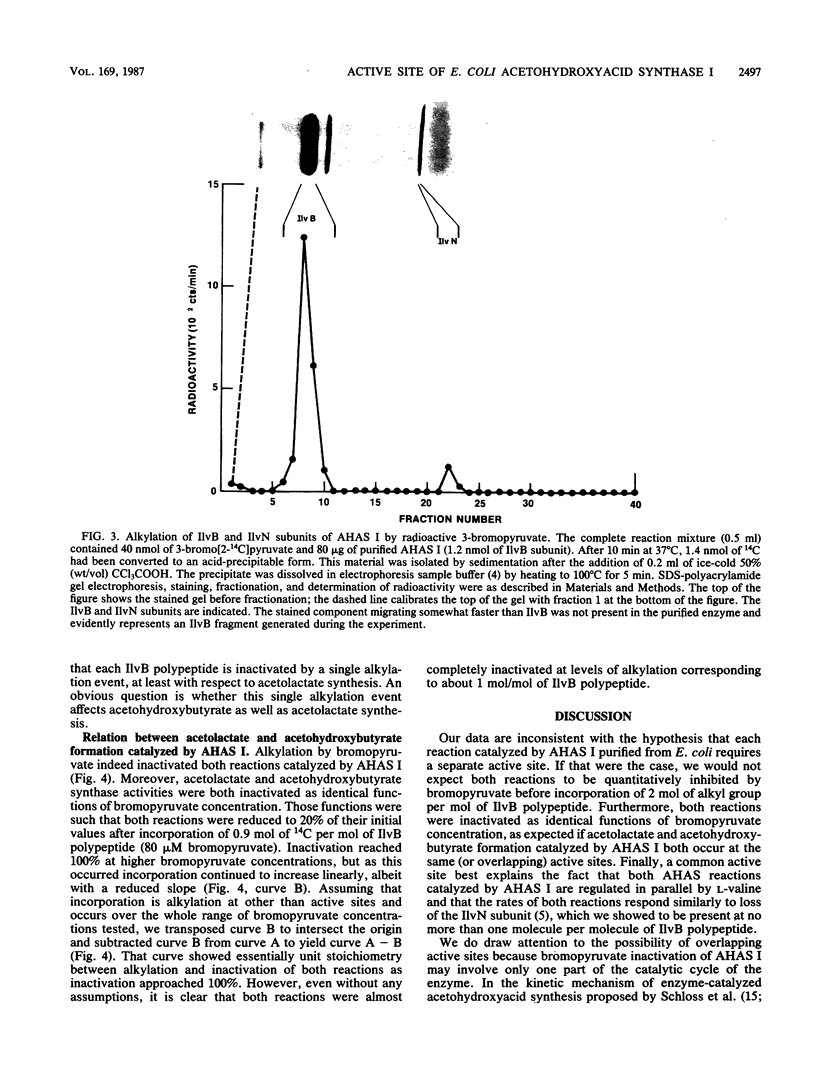
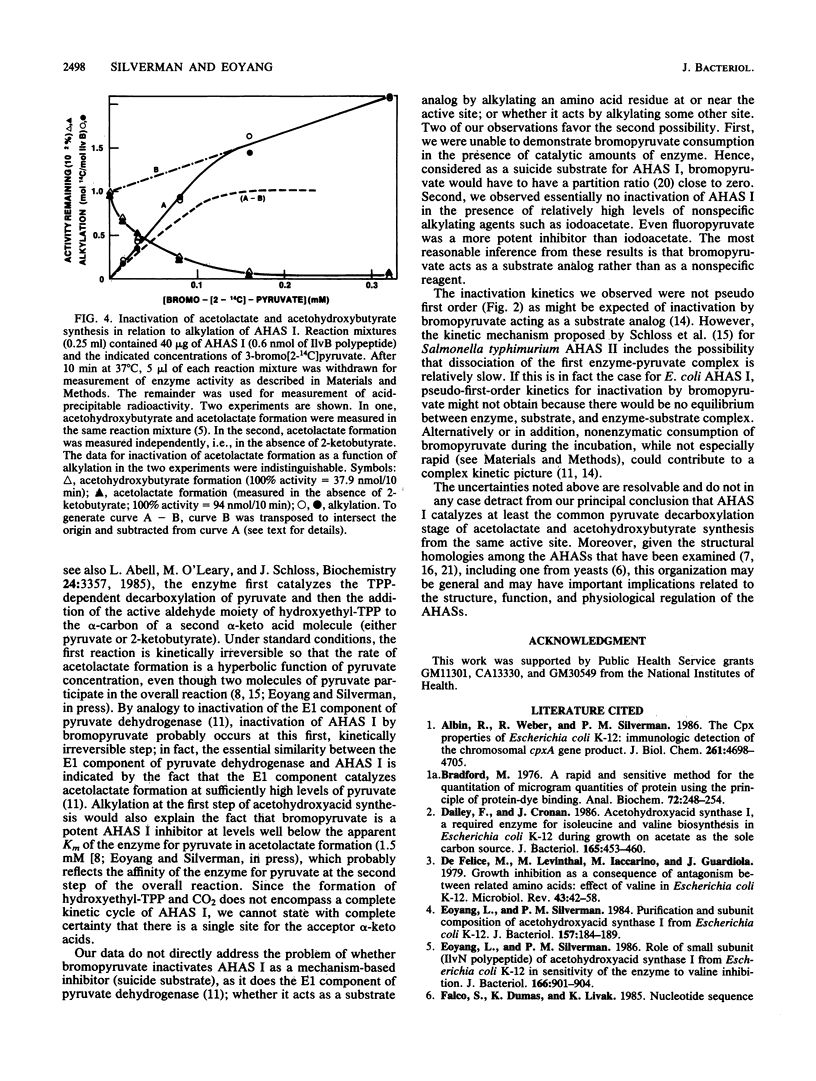
Acetohydroxyacid synthase I (AHAS I) purified from Escherichia coli K-12 was irreversibly inactivated by incubation with 3-bromopyruvate. Inactivation was specific, insofar as bromoacetate and iodoacetate were much less effective than bromopyruvate. Inactivation was accompanied by incorporation of radioactivity from 3-bromo[2-14C]pyruvate into acid-insoluble material. More than 95% of the incorporated radioactivity coelectrophoresed with the 60-kilodalton IlvB subunit of the enzyme through a sodium dodecyl sulfate-polyacrylamide gel; less than 5% coelectrophoresed with the 11.2-kilodalton IlvN subunit. The stoichiometry of incorporation at nearly complete inactivation was 1 mol of 14C per mol of IlvB polypeptide. These data indicate that bromopyruvate inactivates AHAS I by alkylating an amino acid at or near a single active site located in the IlvB subunit of the enzyme. We confirmed that this alkylation inactivated both AHAS reactions normally catalyzed by AHAS I. These results provide the first direct evidence that AHAS I catalyzes both acetohydroxybutyrate and acetolactate synthesis from the same active site.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albin R., Weber R., Silverman P. M. The Cpx proteins of Escherichia coli K12. Immunologic detection of the chromosomal cpxA gene product. J Biol Chem. 1986 Apr 5;261(10):4698–4705. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Dailey F. E., Cronan J. E., Jr Acetohydroxy acid synthase I, a required enzyme for isoleucine and valine biosynthesis in Escherichia coli K-12 during growth on acetate as the sole carbon source. J Bacteriol. 1986 Feb;165(2):453–460. doi: 10.1128/jb.165.2.453-460.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice M., Levinthal M., Iaccarino M., Guardiola J. Growth inhibition as a consequence of antagonism between related amino acids: effect of valine in Escherichia coli K-12. Microbiol Rev. 1979 Mar;43(1):42–58. doi: 10.1128/mr.43.1.42-58.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eoyang L., Silverman P. M. Purification and subunit composition of acetohydroxyacid synthase I from Escherichia coli K-12. J Bacteriol. 1984 Jan;157(1):184–189. doi: 10.1128/jb.157.1.184-189.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eoyang L., Silverman P. M. Role of small subunit (IlvN polypeptide) of acetohydroxyacid synthase I from Escherichia coli K-12 in sensitivity of the enzyme to valine inhibition. J Bacteriol. 1986 Jun;166(3):901–904. doi: 10.1128/jb.166.3.901-904.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falco S. C., Dumas K. S., Livak K. J. Nucleotide sequence of the yeast ILV2 gene which encodes acetolactate synthase. Nucleic Acids Res. 1985 Jun 11;13(11):4011–4027. doi: 10.1093/nar/13.11.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friden P., Donegan J., Mullen J., Tsui P., Freundlich M., Eoyang L., Weber R., Silverman P. M. The ilvB locus of Escherichia coli K-12 is an operon encoding both subunits of acetohydroxyacid synthase I. Nucleic Acids Res. 1985 Jun 11;13(11):3979–3993. doi: 10.1093/nar/13.11.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimminger H., Umbarger H. E. Acetohydroxy acid synthase I of Escherichia coli: purification and properties. J Bacteriol. 1979 Feb;137(2):846–853. doi: 10.1128/jb.137.2.846-853.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEAVITT R. I., UMBARGER H. E. Isoleucine and valine metabolism in Escherichia coli. X. The enzymatic formation of acetohydroxybutyrate. J Biol Chem. 1961 Sep;236:2486–2491. [PubMed] [Google Scholar]
- Lowe P. N., Perham R. N. Bromopyruvate as an active-site-directed inhibitor of the pyruvate dehydrogenase multienzyme complex from Escherichia coli. Biochemistry. 1984 Jan 3;23(1):91–97. doi: 10.1021/bi00296a015. [DOI] [PubMed] [Google Scholar]
- Lowry O. H., Carter J., Ward J. B., Glaser L. The effect of carbon and nitrogen sources on the level of metabolic intermediates in Escherichia coli. J Biol Chem. 1971 Nov;246(21):6511–6521. [PubMed] [Google Scholar]
- McEwen J., Silverman P. Mutations in genes cpxA and cpxB of Escherichia coli K-12 cause a defect in isoleucine and valine syntheses. J Bacteriol. 1980 Oct;144(1):68–73. doi: 10.1128/jb.144.1.68-73.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloche H. P. Bromopyruvate inactivation of 2-keto-3-deoxy-6-phosphogluconic aldolase. I. Kinetic evidence for active site specificity. Biochemistry. 1967 Aug;6(8):2273–2280. doi: 10.1021/bi00860a002. [DOI] [PubMed] [Google Scholar]
- Schloss J. V., Van Dyk D. E., Vasta J. F., Kutny R. M. Purification and properties of Salmonella typhimurium acetolactate synthase isozyme II from Escherichia coli HB101/pDU9. Biochemistry. 1985 Aug 27;24(18):4952–4959. doi: 10.1021/bi00339a034. [DOI] [PubMed] [Google Scholar]
- Squires C. H., De Felice M., Devereux J., Calvo J. M. Molecular structure of ilvIH and its evolutionary relationship to ilvG in Escherichia coli K12. Nucleic Acids Res. 1983 Aug 11;11(15):5299–5313. doi: 10.1093/nar/11.15.5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires C. H., De Felice M., Lago C. T., Calvo J. M. IlvHI locus of Salmonella typhimurium. J Bacteriol. 1983 Jun;154(3):1054–1063. doi: 10.1128/jb.154.3.1054-1063.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton A., Newman T., McEwen J., Silverman P. M., Freundlich M. Mutations in genes cpxA and cpxB of Escherichia coli K-12 cause a defect in acetohydroxyacid synthase I function in vivo. J Bacteriol. 1982 Aug;151(2):976–982. doi: 10.1128/jb.151.2.976-982.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbarger H. E. Amino acid biosynthesis and its regulation. Annu Rev Biochem. 1978;47:532–606. doi: 10.1146/annurev.bi.47.070178.002533. [DOI] [PubMed] [Google Scholar]
- Waley S. G. Kinetics of suicide substrates. Biochem J. 1980 Mar 1;185(3):771–773. doi: 10.1042/bj1850771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wek R. C., Hauser C. A., Hatfield G. W. The nucleotide sequence of the ilvBN operon of Escherichia coli: sequence homologies of the acetohydroxy acid synthase isozymes. Nucleic Acids Res. 1985 Jun 11;13(11):3995–4010. doi: 10.1093/nar/13.11.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]