Abstract
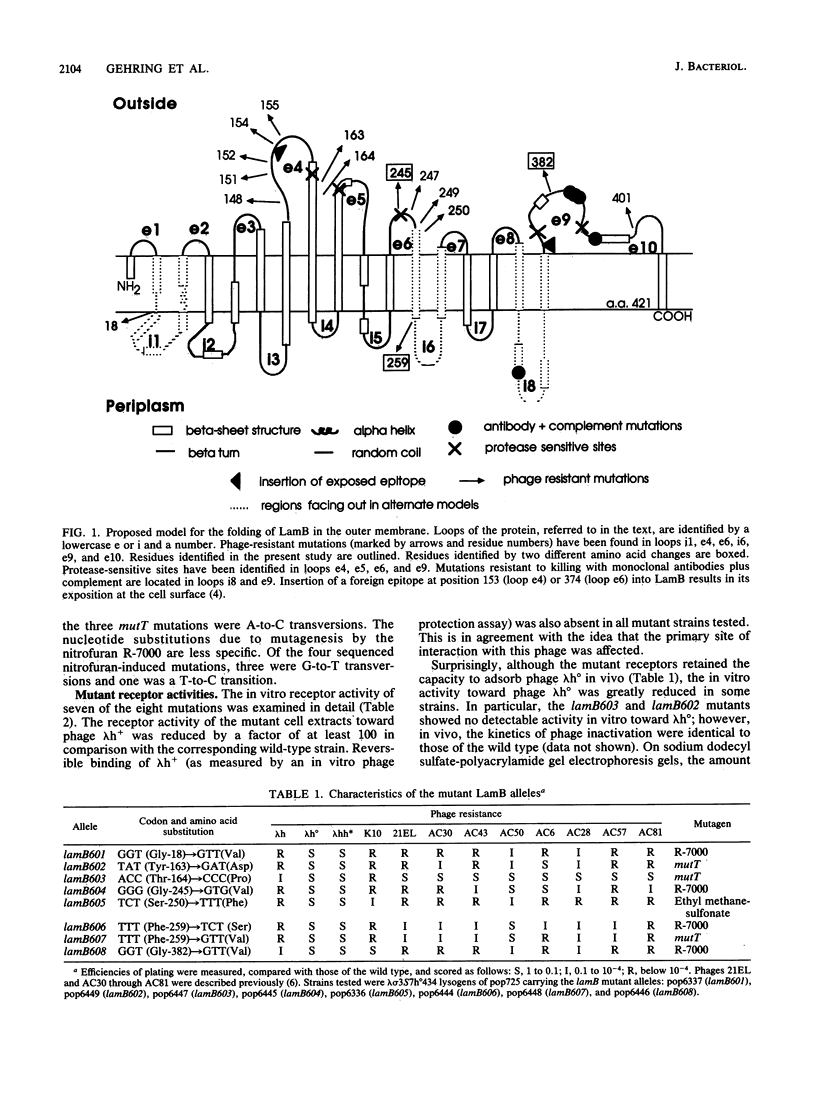
We have analyzed eight new phage-resistant missense mutations in lamB. These mutations identify five new amino acid residues essential for phage lambda adsorption. Two mutations at positions 245 and 382 affect residues which were previously identified, but lead to different amino acid changes. Three mutations at residues 163, 164, and 250 enlarge and confirm previously proposed phage receptor sites. Two different mutations at residue 259 and one at 18 alter residues previously suggested as facing the periplasmic face. The mutation at residue 18 implicates for the first time the amino-terminal region of the LamB protein in phage adsorption. The results are discussed in terms of the topology of the LamB protein.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boulain J. C., Charbit A., Hofnung M. Mutagenesis by random linker insertion into the lamB gene of Escherichia coli K12. Mol Gen Genet. 1986 Nov;205(2):339–348. doi: 10.1007/BF00430448. [DOI] [PubMed] [Google Scholar]
- Braun-Breton C., Hofnung M. In vivo and in vitro functional alterations of the bacteriophage lambda receptor in lamB missense mutants of Escherichia coli K-12. J Bacteriol. 1981 Dec;148(3):845–852. doi: 10.1128/jb.148.3.845-852.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapon C. Role of the catabolite activator protein in the maltose regulon of Escherichia coli. J Bacteriol. 1982 May;150(2):722–729. doi: 10.1128/jb.150.2.722-729.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbit A., Boulain J. C., Ryter A., Hofnung M. Probing the topology of a bacterial membrane protein by genetic insertion of a foreign epitope; expression at the cell surface. EMBO J. 1986 Nov;5(11):3029–3037. doi: 10.1002/j.1460-2075.1986.tb04602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbit A., Clement J. M., Hofnung M. Further sequence analysis of the phage lambda receptor site. Possible implications for the organization of the lamB protein in Escherichia coli K12. J Mol Biol. 1984 May 25;175(3):395–401. doi: 10.1016/0022-2836(84)90355-3. [DOI] [PubMed] [Google Scholar]
- Charbit A., Hofnung M. Isolation of different bacteriophages using the LamB protein for adsorption on Escherichia coli K-12. J Virol. 1985 Feb;53(2):667–671. doi: 10.1128/jvi.53.2.667-671.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clément J. M., Lepouce E., Marchal C., Hofnung M. Genetic study of a membrane protein: DNA sequence alterations due to 17 lamB point mutations affecting adsorption of phage lambda. EMBO J. 1983;2(1):77–80. doi: 10.1002/j.1460-2075.1983.tb01384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desaymard C., Débarbouillé M., Jolit M., Schwartz M. Mutations affecting antigenic determinants of an outer membrane protein of Escherichia coli. EMBO J. 1986 Jun;5(6):1383–1388. doi: 10.1002/j.1460-2075.1986.tb04371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudl R., MacIntyre S., Degen M., Henning U. Cell surface exposure of the outer membrane protein OmpA of Escherichia coli K-12. J Mol Biol. 1986 Apr 5;188(3):491–494. doi: 10.1016/0022-2836(86)90171-3. [DOI] [PubMed] [Google Scholar]
- Gabay J., Benson S., Schwartz M. Genetic mapping of antigenic determinants on a membrane protein. J Biol Chem. 1983 Feb 25;258(4):2410–2414. [PubMed] [Google Scholar]
- Hofnung M., Jezierska A., Braun-Breton C. lamB mutations in E. coli K12: growth of lambda host range mutants and effect of nonsense suppressors. Mol Gen Genet. 1976 May 7;145(2):207–213. doi: 10.1007/BF00269595. [DOI] [PubMed] [Google Scholar]
- Hofnung M., Lepouce E., Braun-Breton C. General method for fine mapping of the Escherichia coli K-12 lamB gene: localization of missense mutations affecting bacteriophage lambda adsorption. J Bacteriol. 1981 Dec;148(3):853–860. doi: 10.1128/jb.148.3.853-860.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleffel B., Garavito R. M., Baumeister W., Rosenbusch J. P. Secondary structure of a channel-forming protein: porin from E. coli outer membranes. EMBO J. 1985 Jun;4(6):1589–1592. doi: 10.1002/j.1460-2075.1985.tb03821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B. Rapid mapping of conditional and auxotrophic mutations in Escherichia coli K-12. J Bacteriol. 1973 Feb;113(2):798–812. doi: 10.1128/jb.113.2.798-812.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus J. M. The receptor protein of phage lambda: purification, characterization and preliminary electrical studies in planar lipid bilayers. Ann Microbiol (Paris) 1982 Jan;133A(1):27–32. [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul C., Rosenbusch J. P. Folding patterns of porin and bacteriorhodopsin. EMBO J. 1985 Jun;4(6):1593–1597. doi: 10.1002/j.1460-2075.1985.tb03822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall-Hazelbauer L., Schwartz M. Isolation of the bacteriophage lambda receptor from Escherichia coli. J Bacteriol. 1973 Dec;116(3):1436–1446. doi: 10.1128/jb.116.3.1436-1446.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roa M., Clément J. M. Location of a phage binding region on an outer membrane protein. FEBS Lett. 1980 Nov 17;121(1):127–129. doi: 10.1016/0014-5793(80)81280-4. [DOI] [PubMed] [Google Scholar]
- Roa M. Interaction of bacteriophage K10 with its receptor, the lamB protein of Escherichia coli. J Bacteriol. 1979 Nov;140(2):680–686. doi: 10.1128/jb.140.2.680-686.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkman S., Tsugita A., Schwartz M., Rosenbusch J. P. Topology of phage lambda receptor protein. Mapping targets of proteolytic cleavage in relation to binding sites for phage or monoclonal antibodies. J Biol Chem. 1984 Jun 25;259(12):7570–7576. [PubMed] [Google Scholar]
- Thirion J. P., Hofnung M. On some genetic aspects of phage lambda resistance in E. coli K12. Genetics. 1972 Jun;71(2):207–216. doi: 10.1093/genetics/71.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommassen J., van der Ley P., van Zeijl M., Agterberg M. Localization of functional domains in E. coli K-12 outer membrane porins. EMBO J. 1985 Jun;4(6):1583–1587. doi: 10.1002/j.1460-2075.1985.tb03820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel H., Jähnig F. Models for the structure of outer-membrane proteins of Escherichia coli derived from raman spectroscopy and prediction methods. J Mol Biol. 1986 Jul 20;190(2):191–199. doi: 10.1016/0022-2836(86)90292-5. [DOI] [PubMed] [Google Scholar]
- Weill-Thevenet N., Buisson J. P., Royer R., Hofnung M. Mutagenic activity of benzofurans and naphthofurans in the Salmonella/microsome assay: 2-nitro-7-methoxy-naphtho[2,1-b]furan (R7000), a new highly potent mutagenic agent. Mutat Res. 1981 Apr;88(4):355–362. doi: 10.1016/0165-1218(81)90027-6. [DOI] [PubMed] [Google Scholar]
- van der Ley P., Struyvé M., Tommassen J. Topology of outer membrane pore protein PhoE of Escherichia coli. Identification of cell surface-exposed amino acids with the aid of monoclonal antibodies. J Biol Chem. 1986 Sep 15;261(26):12222–12225. [PubMed] [Google Scholar]



