Abstract
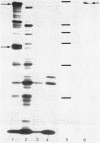
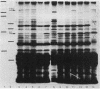
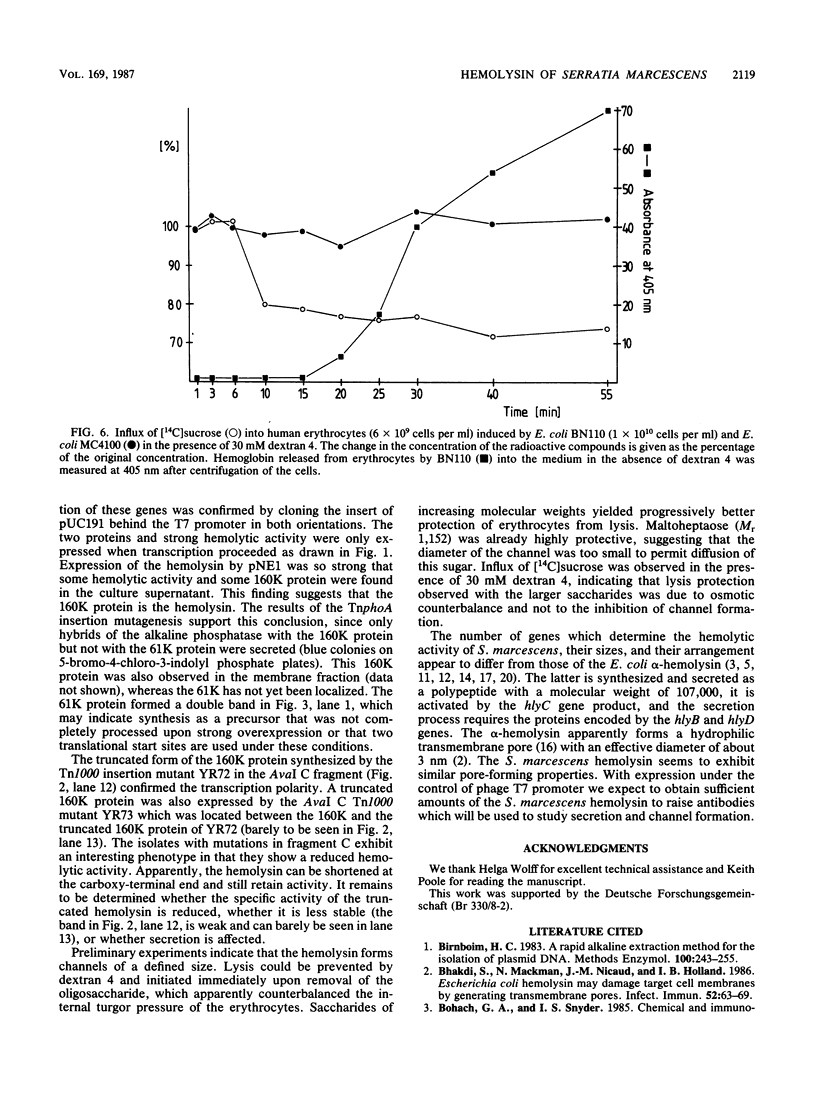
A cosmid bank of Serratia marcescens was established from which DNA fragments were cloned into the plasmid pBR322, which conferred the chromosomally encoded hemolytic activity to Escherichia coli K-12. By transposon mutagenesis with Tn1000 and Tn5 IS50L::phoA (TnphoA), the coding region was assigned to a DNA fragment, designated hly, comprising approximately 7 kilobases. Two proteins with molecular weights of 61,000 (61K protein) and 160,000 (160K protein) were expressed by the pBR322 derivatives and by a plasmid which contained the hly genes under the control of a phage T7 promoter and the T7 RNA polymerase. When strongly overexpressed the 160K protein was released by E. coli cells into the extracellular medium concomitant with hemolytic activity. The genes encoding the 61K and the 160K proteins were transcribed in the same direction. Mutants expressing a 160K protein truncated at the carboxy-terminal end were partially hemolytic. Hemolysis was progressively inhibited by saccharides with increasing molecular weights from maltotriose (Mr 504) to maltoheptaose (Mr 1,152) and was totally abolished by dextran 4 (Mr 4,000). This result and the observed influx of [14C]sucrose into erythrocytes in the presence of hemolytic E. coli transformants under osmotically protective conditions suggest the formation of defined transmembrane channels by the hemolysin.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhakdi S., Mackman N., Nicaud J. M., Holland I. B. Escherichia coli hemolysin may damage target cell membranes by generating transmembrane pores. Infect Immun. 1986 Apr;52(1):63–69. doi: 10.1128/iai.52.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol. 1983;100:243–255. doi: 10.1016/0076-6879(83)00059-2. [DOI] [PubMed] [Google Scholar]
- Braun V., Günther H., Neuss B., Tautz C. Hemolytic activity of Serratia marcescens. Arch Microbiol. 1985 May;141(4):371–376. doi: 10.1007/BF00428852. [DOI] [PubMed] [Google Scholar]
- Cavalieri S. J., Bohach G. A., Snyder I. S. Escherichia coli alpha-hemolysin: characteristics and probable role in pathogenicity. Microbiol Rev. 1984 Dec;48(4):326–343. doi: 10.1128/mr.48.4.326-343.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty T., Huhle B., Bergbauer H., Goebel W. Cloning, expression, and mapping of the Aeromonas hydrophila aerolysin gene determinant in Escherichia coli K-12. J Bacteriol. 1986 Jul;167(1):368–374. doi: 10.1128/jb.167.1.368-374.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Collins J. Escherichia coli plasmids packageable in vitro in lambda bacteriophage particles. Methods Enzymol. 1979;68:309–326. doi: 10.1016/0076-6879(79)68022-9. [DOI] [PubMed] [Google Scholar]
- Dauenhauer S. A., Hull R. A., Williams R. P. Cloning and expression in Escherichia coli of Serratia marcescens genes encoding prodigiosin biosynthesis. J Bacteriol. 1984 Jun;158(3):1128–1132. doi: 10.1128/jb.158.3.1128-1132.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmlee T., Pellett S., Lee E. Y., Welch R. A. Escherichia coli hemolysin is released extracellularly without cleavage of a signal peptide. J Bacteriol. 1985 Jul;163(1):88–93. doi: 10.1128/jb.163.1.88-93.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmlee T., Pellett S., Welch R. A. Nucleotide sequence of an Escherichia coli chromosomal hemolysin. J Bacteriol. 1985 Jul;163(1):94–105. doi: 10.1128/jb.163.1.94-105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer M. S. Uses of the transposon gamma delta in the analysis of cloned genes. Methods Enzymol. 1983;101:362–369. doi: 10.1016/0076-6879(83)01027-7. [DOI] [PubMed] [Google Scholar]
- Hohn B. In vitro packaging of lambda and cosmid DNA. Methods Enzymol. 1979;68:299–309. doi: 10.1016/0076-6879(79)68021-7. [DOI] [PubMed] [Google Scholar]
- Jorgensen S. E., Mulcahy P. F., Wu G. K., Louis C. F. Calcium accumulation in human and sheep erythrocytes that is induced by Escherichia coli hemolysin. Toxicon. 1983;21(5):717–727. doi: 10.1016/0041-0101(83)90277-5. [DOI] [PubMed] [Google Scholar]
- Juarez A., Hughes C., Vogel M., Goebel W. Expression and regulation of the plasmid-encoded hemolysin determinant of Escherichia coli. Mol Gen Genet. 1984;197(2):196–203. doi: 10.1007/BF00330963. [DOI] [PubMed] [Google Scholar]
- Kamata R., Yamamoto T., Matsumoto K., Maeda H. A serratial protease causes vascular permeability reaction by activation of the Hageman factor-dependent pathway in guinea pigs. Infect Immun. 1985 Jun;48(3):747–753. doi: 10.1128/iai.48.3.747-753.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Mackman N., Nicaud J. M., Gray L., Holland I. B. Genetical and functional organisation of the Escherichia coli haemolysin determinant 2001. Mol Gen Genet. 1985;201(2):282–288. doi: 10.1007/BF00425672. [DOI] [PubMed] [Google Scholar]
- Manoil C., Beckwith J. TnphoA: a transposon probe for protein export signals. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8129–8133. doi: 10.1073/pnas.82.23.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer J., König W., Hacker J., Goebel W. Bacterial adherence and hemolysin production from Escherichia coli induces histamine and leukotriene release from various cells. Infect Immun. 1985 Oct;50(1):271–278. doi: 10.1128/iai.50.1.271-278.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz G., Braun V. Cell-bound and secreted proteases of Serratia marcescens. J Bacteriol. 1985 Mar;161(3):1002–1009. doi: 10.1128/jb.161.3.1002-1009.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone K. J., Strominger J. L. Mechanism of action of bacitracin: complexation with metal ion and C 55 -isoprenyl pyrophosphate. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3223–3227. doi: 10.1073/pnas.68.12.3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomás J. M., Kay W. W. Effect of bacteriophage P1 lysogeny on lipopolysaccharide composition and the lambda receptor of Escherichia coli. J Bacteriol. 1984 Sep;159(3):1047–1052. doi: 10.1128/jb.159.3.1047-1052.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweig M., Cummings D. J. Structural proteins of bacteriophage T5. Virology. 1973 Feb;51(2):443–453. doi: 10.1016/0042-6822(73)90443-1. [DOI] [PubMed] [Google Scholar]