Abstract
Premature infants are routinely exposed to invasive medical procedures during neonatal intensive care treatment that are largely performed in the absence of anesthetics or analgesics. Data collected to date suggest that exposure to early insult during this time of increased plasticity alters the development of the CNS and influences future pain responses. As previous studies examining the impact of neonatal injury on nociception have been conducted primarily in males, the potential adverse effects on females is not known. Therefore, the present studies were conducted to determine whether neonatal injury differentially impacts male and female sensory thresholds in adulthood. A short lasting inflammatory response was evoked in male and female rats on the day of birth with an injection of carrageenan (CGN; 1% or 2%) into the right hind paw. Nociceptive thresholds were assessed using a noxious thermal stimulus at both adolescence (P40) and adulthood (P60). A more persistent inflammation was subsequently evoked in adult rats with an intraplantar injection of Complete Freund's adjuvant (CFA). Neonatally injured females exhibited significantly greater hypoalgesia at P60, and displayed enhanced inflammatory hyperalgesia following re-injury in adulthood compared to neonatally injured males and controls. These results demonstrate that the long-term adverse effects of neonatal injury are exacerbated in females, and may contribute to the higher prevalence, severity and duration of pain syndromes noted in women compared to men.
Keywords: sex differences, inflammation, neonate, pain
The neonatal period is a time of increased neurodevelopmental plasticity. Unlike other sensory systems, which require proper stimulation for appropriate development, maturation of nociceptive circuitry typically occurs in the absence of adequate stimuli (Lidow 2002; Fitzgerald 2004). Premature infants, however, are routinely exposed to invasive medical procedures during neonatal intensive care treatment (Anand 1998; Grunau et al. 2005; Peters et al. 2005). Hundreds of thousands of infants are born premature each year, and as a result of major medical and technological advances in neonatal care, infants born after 23 weeks' gestation are routinely kept alive (Qiu 2006). In order to increase their chances of survival these newborns experience an average of 14 noxious procedures per day in the Neonatal Intensive Care Unit (NICU), including heel lances, endotracheal intubation, respiratory and gastric suctioning, and catheter insertion (Simons et al. 2003).
Recent evidence indicates that nociceptive circuitry is both intact and functional during late gestation, and that premature infants are indeed responsive to noxious stimulation (Bartocci et al. 2006; Slater et al. 2006). Furthermore, growing clinical data suggests that exposure to noxious stimulation in premature neonates can lead to long-term alterations in subsequent physiological and behavioral responses to innocuous and noxious somatosensory stimulation (Anand 2000; Whitfield and Grunau 2000; Grunau et al. 2005). Several studies report that former preterm infants exposed to multiple invasive procedures during NICU care display dampened behavioral responses and enhanced physiological responses to subsequent pain (Anand 2000; Oberlander et al. 2000; Whitfield and Grunau 2000; Grunau et al. 2005).
Parallel evidence in non-human animal models also suggests that neonatal noxious stimulation is associated with long-term changes in somatosensory structure and function (Bhutta et al. 2001; Lidow 2002; Walker et al. 2003; Ren et al. 2004; Wang et al. 2004). Previous studies examining the impact of neonatal inflammation on adult somatosensory processing and dorsal horn physiology were conducted exclusively in males (Ruda et al. 2000; Lidow 2002; Ren et al. 2004). The neuroendocrine profile of the newborn laboratory rat is sexually dimorphic such that in males, there is a significant surge of testicular testosterone that is centrally aromatized to estrogen and ultimately results in the masculinization of the male brain (Balthazart and Ball 2006; Cornil et al. 2006). In females, the ovaries are quiescent and intracerebral estradiol remains low (Weisz and Ward 1980; Amateau et al. 2004; Balthazart and Ball 2006; Cornil et al. 2006). Estrogens have been shown to exert neuromodulatory and neuroprotective effects following acute and chronic central injuries (Garcia-Segura et al. 2001; Maggi et al. 2004; Amantea et al. 2005). Therefore, increased central levels of estrogen in males may attenuate the long-term adverse effects of neonatal injury, whereas in females the consequences of neonatal injury may be exacerbated. The aim of the current study was (1) determine whether neonatal inflammation differentially affects male and female sensory thresholds in adulthood and (2) to test whether the impact of neonatal injury on inflammation-induced hyperalgesia in adulthood is sexually dimorphic.
METHODS
Animals
Time-pregnant Sprague-Dawley rats were obtained on the 14th day of gestation (E14) (Zivic Miller) and housed individually. Animals were maintained on a 12:12h light:dark cycle, with food and water available ad libitum. On the day of birth (P0), sexing of the pups was determined by examination of the anogenital distance. All litters were reared identically, weaned at P21, and housed with same-sex littermates in groups of 2-3. All experiments adhered to the guidelines of the Committee for Research and Ethical Issues of IASP, and were approved by the Georgia State University Animal Care and Use Committee.
Early Life Manipulations
Acute neonatal injury was induced by unilateral hindpaw injection of carrageenan (CGN; 1% or 2% soln dissolved in sterile in saline; 5ul volume; Sigma, St. Louis MO) into the plantar surface of the right hindpaw within 12 hours of birth on P0, except for critical period experiments where neonatal injury was induced on P8 or P14. This inflammatory agent provides a well-established model of acute local inflammation that lasts for 12-72 hours (Lidow 2002; Ren et al. 2004). Control animals received either an equivolume of sterile saline into the right hindpaw or were “handled” in a similar manner and returned to their home cage. All pups within a litter received the same neonatal treatment.
Maternal Behavior
Mother-pup interactions were observed for 1 hour following the neonatal injury, and daily at 18:00 hours (1 hour prior to lights off) for 60 minutes from P0-P21. Maternal observations were conducted by both direct observations (in a manner so as not to disrupt the dam) and by videotape for later offline analysis. Specific maternal behaviors were recorded including pup licking/grooming, nursing posture (crouching), hovering over pups, pup retrieval, nest construction, eating/drinking, exploring, inactive/napping and self-grooming. Observations were conducted by an individual blind to the neonatal group assignment.
Baseline Nociceptive Behavior
On P40 and P60, baseline paw withdrawal latencies (PWL) in response to a noxious thermal stimulus were determined. Thermal testing was conducted using the Paw Thermal Stimulator (UCSD, San Diego, California). In this test, animals were placed in a clear plastic testing chamber on a glass surface and allowed to acclimate for a minimum of 30 minutes prior to testing. A radiant beam of light beneath the glass base was directed at the plantar surface of the each hindpaw and the withdrawal reflex latency was electronically measured (in seconds). Intact male and cycling female rats were tested separately. The average withdrawal latency of 3 trials was taken; all trials were separated by a 5-minute inter-trial interval. Application of the thermal stimulus to either paw was randomly determined. To avoid potential tissue damage, a 20-second automatic termination of the heat stimulus was imposed if a paw withdrawal did not occur. The testing apparatus was thoroughly cleaned between sessions. Body weight and paw diameter for right and left hindpaws were measured prior to baseline testing on P40 and P60. To characterize the estrous status of female rats, vaginal smears (using the saline lavage technique) were taken daily beginning two weeks prior to testing and continuing to the end of the experimental session. Proestrus was defined by the presence of nucleated epithelial cells in >90% of the total cell population; estrus was defined by the presence of cornified epithelial cells; diestrus-1 was defined as the presence of both leukocytes and cornified epithelial cells; diestrus-2 was defined as the relative absence of all cell types (Wang et al. 2006). All animals were smeared in the morning, approximately 3-4 hours after lights on. Vaginal smears were conducted a minimum of three hours prior to testing to minimize the potential effects of vaginal stimulation-produced analgesia (Komisaruk 1977).
Nociceptive Behavior Following Re-Inflammation
Following baseline PWL determination at P60, animals received an injection of Complete Freund's adjuvant (CFA; 1:1 CFA:saline soln; 200ul; Sigma) into the plantar surface of the right (neonatally injured) hindpaw. A separate group of animals received intraplantar CFA into the left hindpaw. CFA was used for re-injury as neonatally-injured animals may potentially develop antibodies against carrageenan (CGN), thereby limiting its potency for use as an inflammatory agent. Twenty-four hours following CFA-induced inflammation, paw diameter and PWLs were tested using the Paw Thermal Stimulator as described above.
Drugs
The opioid antagonist naloxone hydrochloride (1 mg/kg; Sigma; St. Louis, MO) was injected subcutaneously fifteen minutes prior to P60 testing. This dose of naloxone was chosen based on our previous observations demonstrating that 1 mg/kg of naloxone was effective in reversing the effects of systemic morphine but had no effect on nociceptive thresholds alone (Ji et al. 2006). Control animals received an equivolume of saline.
Statistical Analysis
Data were expressed as raw withdrawal latencies or difference scores. A maximal PWL of 20 seconds was used to prevent excessive tissue damage due to repeated application of a noxious thermal stimulus. All values are reported as Mean ± S.E.M. Data were analyzed for significant main effects of neonatal treatment and sex using ANOVA; p< 0.05 was considered statistically significant. Post-hoc tests using the method of Sheffe were conducted as warranted to determine significant mean differences. Where multiple comparisons were made, p values were adjusted accordingly using the Bonferroni adjustment.
RESULTS
Neonatal Injury Differentially Affects Male and Female Sensory Thresholds in Adulthood
Previous studies have reported that neonatally injured animals have significantly higher sensory thresholds in adulthood (Lidow 2002; Ren et al. 2004). These studies, however, were conducted primarily in males. As central estradiol levels are significantly elevated in males at this time point (Amateau et al. 2004), and estrogens have been shown to confer neuroprotection (Garcia-Segura et al. 2001; Maggi et al. 2004; Amantea et al. 2005), the present studies were conducted to determine if the long-term consequences of neonatal injury are exacerbated in females compared to males. Intraplantar administration of carrageenan (CGN; 1% or 2%) on the day of birth resulted in significantly longer paw withdrawal latencies (PWL) in comparison to control animals at postnatal day 60 (P60). Thermal hypoalgesia was present in both the previously injured (Figure 1A) and uninjured (Figure 1B) hindpaws. As shown in Figure 1, paw withdrawal latency for the injured paw increased over 50% in injured animals (1% and 2% CGN) compared with saline and handled controls (p<.0001). Furthermore, neonatally injured females displayed up to a 3 second longer latency in the injured paw compared to neonatally injured males (p=.0078) (Figure 1A). This increased hypoalgesia was also present in the uninjured paw (p<.0001) (Figure 1B). There were no sex differences noted for saline and handled controls (p<.05).
Figure 1. Neonatal injury differentially affects male and female sensory thresholds in adulthood (P60) and during adolescence (P40).
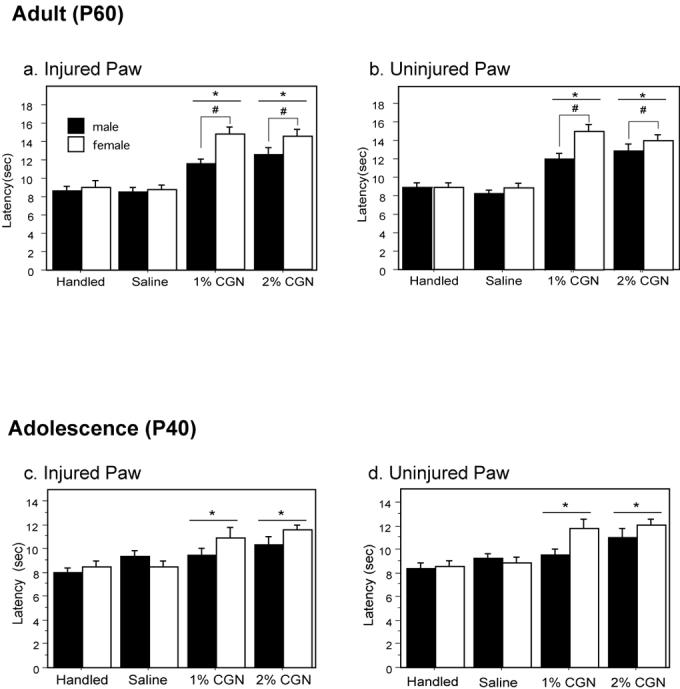
At P60, neonatally injured animals display significantly longer withdrawal latencies compared to saline and handled controls in response to a noxious thermal stimulus applied to the (A) right (injured) paw [F(3,114)=41.76, p<.0001] and (B) (uninjured) paw [F(3,114)=34.68, p<.0001]. There was also a significant main effect of sex with injured females displaying significantly longer latencies in comparison to males: injured paw, F(1,114)=7.34, p=.0078; uninjured paw, F(1,114)=11.31, p=.0011. Significant hypoalgesia was also present at P40 in neonatally injured animals in both the (C) right (injured) paw [F(3,114)=8.17, p< .0001] and (D) contralateral (uninjured) paw [F(3,114)=10.92, p<.0001]. # denotes significant main effect compared to handled controls; * denotes a significant main effect of sex.
Neonatal Injury Alters Sensory Thresholds at P40
The above studies demonstrate that neonatal injury produces decreased responses to noxious thermal stimuli in adulthood and that this effect is exacerbated in females. We next tested whether these sexually dimorphic effects were also evident during the peri-adolescent period (P40). At P40, neonatally injured male and female rats (1% and 2% CGN) displayed significantly longer paw withdrawal latencies in response to a thermal stimulus for the injured paw compared to saline and handled controls (p<.0001) (Figure 1C). Neonatal injury-induced hypoalgesia was also observed in the contralateral paw (p<.0001) (Figure 1D). No significant main effect of sex was noted on any of the withdrawal latencies, although there was a trend for the female contralateral paw (p=.0737).
Neonatal Injury Does Not Affect Body Weight, Paw Diameter, or Estrous Cycle
No significant differences in body weight or paw diameter (right and left hindpaws) were noted in animals exposed to neonatal inflammation (1% and 2% CGN) compared to control animals (saline and handled) at either P40 or P60 (Table 1). No significant differences in estrous cycle were noted in neonatally injured females (1% and 2% CGN) compared to control females (saline and handled) (i.e. all animals displayed normal four day estrous cycles). As we have previously shown that estrous has no effect on baseline pain sensitivity or CFA-induced hyperalgesia (Wang et al. 2006), females were grouped together regardless of estrous stage.
Table 1.
Average Body Weight and Average Paw Diameter. There is no significant difference in the average body weight across neonatal treatment groups on P40 and P60 in males and females. There is also no significant difference in the average paw diameter of the right and left hind paws across neonatal groups on P40 and P60 in males and females.
| MALE | FEMALE | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TREATMENT | P40 | P60 | P40 | P60 | ||||||||
| WT | RP | LP | WT | RP | LP | WT | RP | LP | WT | RP | LP | |
| HANDLED | 298.8 ±36 |
4.88 ±.32 |
4.74 ±.27 |
456.9 ±38 |
5.55 ±.27 |
5.52 ±.28 |
213.8 ±10 |
4.39 ±.20 |
4.46 ±.21 |
272.9 ±19 |
4.67 ±.41 |
4.70 ±.40 |
| SALINE | 297.6 ±11 |
4.76 ±.29 |
4.76 ±.36 |
443.2 ±44 |
5.48 ±.21 |
5.49 ±.21 |
209.7 ±11 |
4.45 ±.14 |
4.36 ±.16 |
265.7 ±16 |
4.71 ±.38 |
4.68 ±.36 |
| 1% CGN | 295.1 ±10 |
4.83 ±.15 |
4.82 ±.15 |
448.6 ±24 |
5.57 ±.14 |
5.57 ±.14 |
206.1 ±10 |
4.33 ±.21 |
4.32 ±.20 |
268.1 ±12 |
4.61 ±.12 |
4.61 ±.14 |
| 2% CGN | 297.5 ±41 |
4.84 ±.30 |
4.77 ±.17 |
462.8 ±30 |
5.60 ±.28 |
5.60 ±.28 |
214.2 ±13 |
4.40 ±.23 |
4.35 ±.19 |
267.3 ±30 |
4.59 ±.15 |
4.56 ±.17 |
Neonatal Injury Has No Impact On Maternal Care
Previous studies have shown that naturally-occurring variations in maternal behavior can have a profound impact on a variety of developmental endpoints, including stress responsiveness, reproductive behavior, pain, and learning and memory (Sternberg 1999; Liu et al. 2000; Johnston and Walker 2003; Sternberg and Ridgway 2003; Weaver et al. 2004). Given the profound and permanent changes induced by alterations in maternal care, daily maternal observations were conducted to determine whether neonatal inflammatory injury alters the display of maternal behavior. There were no significant differences in maternal behavior between injured and non-injured litters in the amount of time the dam spent on/with pups including crouching (nursing posture), lying with pups, and pup retrieval (p=.2231) (Figure 3A). Similarly, no differences were noted in the amount of time the dam spent off/without pups including nesting, eating/drinking, exploring, napping, self-grooming (p=.2087) (Figure 3B); or in the amount of time spent licking/grooming pups (p=.6939) (Figure 3C). This indicates that changes in adult sensory thresholds produced by neonatal injury are not due to differences in maternal behavior directed at injured versus non-injured pups.
Figure 3. The consequences of neonatal injury are critical period dependent.
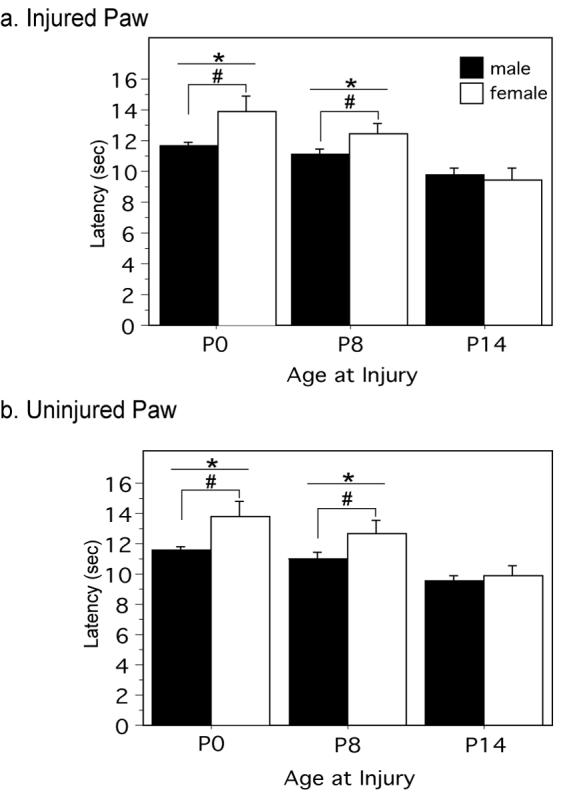
Paw withdrawal latencies for the injured paw are significantly increased on P60 in animals neonatally injured with 1% CGN on P0 and P8 compared to animals injured on P14 [F(2,39)=10.97, p=.0002] . Neonatally injured females (P0 and P8) display significantly longer PWLs compared to neonatally injured males [F(1,39)=3.85, p=.0068]. (B) Similar results were noted for the uninjured paw [F(2,39)=9.94, p=.0003]. PWLs were significantly longer for neonatally injured females (P0 and P8) in comparison to injured males (P0) [F(1,39)=7.09, p=.0014]. N=6-12 rats per group/per sex. * denotes significant main effect of treatment; # denotes significant main effect of sex.
The Long-Term Consequences of Neonatal Injury are Critical Period Dependent
The next experiment was conducted to test whether the long-term consequences of neonatal injury were dependent upon a critical period. Male and female rat pups received a unilateral intraplantar injection of 1% CGN on P0, P8, or P14. Only 1% CGN was used as no significant differences were noted in the previous studies following administration of 1% versus 2% CGN. On P60, paw withdrawal latencies in response to noxious thermal stimulation were determined. Animals that were neonatally injured on P0 and P8 displayed significantly increased PWLs in both the injured (p=.0002) (Figure 4A) and uninjured (p=.0003) (Figure 4B) paws compared to animals that were injured on P14. Furthermore, a significant effect of sex was noted for both paws in animals injured on P0 and P8, with females displaying greater hypoalgesia compared to males (p=.0068, p=.0014). No significant effect of intraplantar carrageenan or sex was noted for animals injured on P14. These results suggest that the impact of neonatal injury is dependent upon a sensitive period, and that noxious insult occurring outside of this critical window does not permanently alter thermal sensory thresholds.
Figure 4. Neonatal injury enhances hyperalgesia following re-inflammation with CFA.
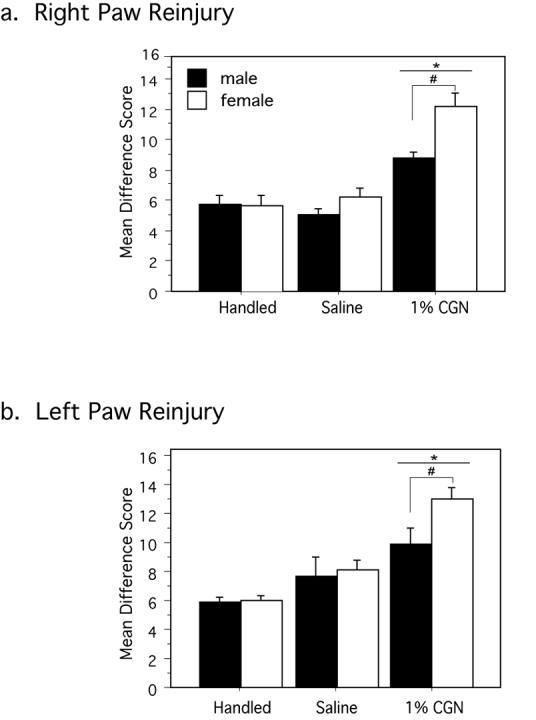
Neonatally injured males and females (1% CGN) had significantly greater difference scores (Bsln PWL – CFA PWL) compared to saline and handled control animals in response to intraplantar CFA in adulthood. This effect was observed in both the (A) right (P0 injured) paw [F(2,85)=43.54, p<.0001] and (B) left (P0 uninjured) paw [F(2,27)=19.08, p<.0001]. Both effects were significantly exacerbated in neonatally injured females compared to neonatally injured males [F(1,85)=3.96, p=.0499] and [F(1,27)=7.50, p=.0108]. N=6-15 rats per group/per sex. * denotes significant main effect of treatment; # denotes significant main effect of sex.
Neonatal Injury Enhances Hyperalgesia Following Re-Inflammation with CFA
The next series of experiments were conducted to test whether neonatally injured animals respond differentially to a subsequent injury in adulthood. Following baseline PWL determination at P60, animals received an injection of Complete Freund's adjuvant (CFA; 1:1 CFA:saline soln; 200ul; Sigma) into the plantar surface of either the right (P0 injured) or left (P0 uninjured) hindpaw. Twenty-four hours following CFA-induced inflammation, PWLs were tested in response to a noxious thermal stimulus.
The effect of neonatal injury on CFA induced hyperalgesia was quite profound. In response to noxious thermal stimulation, the paw withdrawal latencies of CFA treated adult control animals (handled and saline) decreased from approximately 8-9 seconds at baseline to 4-5 seconds following intraplantar CFA (mean difference score of 5.5 sec), a typical hyperalgesic response (Wang et al. 2006). By contrast, latencies for injured animals (1% CGN) decreased significantly from baseline PWLs of 10-12 seconds to 1-3 seconds (Figure 4A). This increased hyperalgesic response following CFA re-injury was significantly greater in neonatally injured females compared to neonatally injured males (mean difference scores of 12 for females versus 9 sec for males). There was no significant effect of neonatal treatment on the degree of edema produced by intraplantar CFA [F(3,118)=1.32, p=.2705].
Intraplantar CFA was administered in a separate group of animals into the left paw to determine whether the increased hyperalgesia following re-injury would be observed following adult re-inflammation of the neonatally uninjured paw. Similar to the previous results, neonatally injured animals (1% CGN) displayed enhanced hyperalgesia following intraplantar CFA compared to saline and handled controls (p=.0176) (Figure 5B). This effect was, again, exacerbated in females compared to males (p=.0108).
Figure 5. Hypoalgesia induced by neonatal injury is significantly attenuated by systemic naloxone.
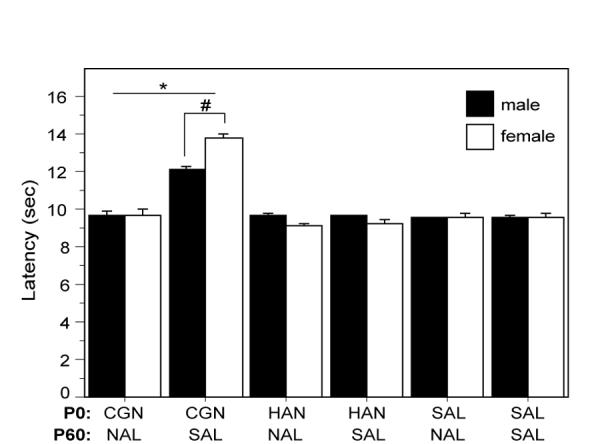
Neonatally injured males and females that received naloxone (CGN/NAL) prior to testing at P60 had significantly lower PWLs than injured animals that received saline control (CGN/SAL) [F(5,38)=52.07, p<.0001]. Administration of naloxone to saline (SAL/NAL) or handled (HAN/NAL) controls had no effect on PWLs. N=6-8 rats per group/per sex. * denotes significant main effect of treatment; # denotes significant main effect of sex.
Neonatal Injury Induced Hypoalgesia is Attenuated By Systemic Naloxone
Neonatal injury results in significant long-term hypoalgesia that is present in both the previously injured and uninjured paw. This bilateral response suggests a global, injury-induced change in basal nociceptive sensitivity. The next series of experiments were conducted to determine whether the observed hypoalgesia was a result of altered endogenous opioid tone. At P60, animals received either systemic administration of the opioid antagonist naloxone hydrochloride (NAL; 1 mg/kg) or equivolume saline (SAL) fifteen minutes prior to testing. As shown in Figure 5, administration of NAL significantly attenuated carrageenan-induced increases in paw withdrawal latencies, with no significant differences noted between neonatally-injured/naloxone treated animals and handled (p=.1576) or saline treated controls (p=.3665). Administration of naloxone alone had no effect on paw withdrawal latencies for P0 saline or handled animals.
DISCUSSION
Our principal findings are as follows: (1) neonatal inflammatory injury produces bilateral basal hypoalgesia that is present in both adolescence and adulthood; (2) neonatally injured animals display enhanced hyperalgesia in response to a subsequent injury in adulthood; (Kamp et al.) the effects of neonatal inflammatory injury on both baseline and re-injury induced changes in nociception are sexually dimorphic, with significantly greater effects present in females compared to males; (4) the long-term consequences of neonatal injury are critical period dependent; (5) injury-induced hypoalgesia is reversed by administration of the opioid antagonist naloxone.
Neonatal Inflammatory Injury Produces Thermal Hypoalgesia in Adulthood
Neonatal injury in rodents produces persistent and dramatic alterations in thermal baseline sensory thresholds (Anand et al. 1999; Bhutta et al. 2001; Lidow 2002; Ren et al. 2004). Previous studies have reported intraplantar carrageenan administered on postnatal day 3 results in thermal and mechanical hypoalgesia in adult male rats (Lidow 2002; Ren et al. 2004). Additionally, long-term visceral hypoalgesia has been reported in animals exposed to carrageenan-induced inflammation as neonates (Wang et al. 2004). Somatic hypoalgesia has also been reported following repeated intraplantar 10% formalin injections in males (Bhutta et al. 2001). Here we demonstrate for the first time that female rats also display thermal hypoalgesia in adulthood following neonatal hind paw inflammation with carrageenan, and this hypoalgesia is significantly greater than that observed in males. Thermal hypoalgesia was manifest at both P40 and P60 and was present in both the injured and uninjured paw.
The global nature of the observed hypoalgesia following neonatal inflammatory injury suggests that the underlying mechanisms are not manifested peripherally at the site of injury, but rather may involve alterations in higher central regulatory systems. Our finding that administration of the opioid antagonist naloxone reverses the injury-induced hypoalgesia supports this theory. However, as there are currently no reliable and reproducible methods for assessing nociception in P0 rat pups, we cannot conclusively state that the observed hypoalgesia was due to the pain associated with inflammation. Indeed, it is also likely that our P0 manipulations induced changes in the developing hypothalamic-pituitary-adrenal axis. Nociception is one component in a broader context of stress reactivity (Anand et al. 1999; Sternberg and Ridgway 2003; Grunau et al. 2005), and experimental studies have shown that exposure to early life stressors such as repetitive neonatal handling can permanently increase nociceptive thresholds in adult rats and decrease the behavioral and physiological responses to stress in adulthood (Pieretti et al. 1991; Coutinho et al. 2002; Sternberg and Ridgway 2003). Interestingly, a recent study demonstrating long-term thermal hypoalgesia in both sham operated and surgically-manipulated mice suggests that the stress of the neonatal procedure, and not necessarily the pain, contributes to the observed hypoalgesia (Sternberg et al. 2005).
Data from human preterm infants also suggests that neonatal exposure to noxious stimuli may alter the responses to subsequent painful or stressful experiences. Ex-preterm infants exposed to four weeks of NICU care display reduced behavioral pain behavior and enhanced cardiovascular responses following heel stick (Johnston and Stevens 1996). In addition, stressful conditions at birth are associated with increased salivary cortisol in response to vaccination at 4 and 6 months of age (Peters et al. 2005). Furthermore, premature infants at 8 and 18 months of age display increased basal levels of stress hormones compared to their full-term counterparts (Grunau et al. 2007).
Administration of the broad-spectrum opioid antagonist naloxone completely reversed the hypoalgesia induced by neonatal injury, suggesting that the pain and stress associated with our neonatal manipulations resulted in a potentiation in descending endogenous opioid tone. Carrageenan-, formalin- and CFA-induced inflammation have all been shown to profoundly enhance pro-dynorphin and proenkephalin biosynthesis in spinal neurons in the dorsal horn (Iadarola et al. 1988; Noguchi et al. 1989). Inflammation–induced changes in endogenous opioid peptide expression and release have also been reported at several supraspinal sites, including the periaqueductal gray (PAG); this increase in opioid peptide expression is associated with hypoalgesia (Williams et al. 1995). Pain-induced changes in opioid peptide expression are also paralleled by an increase in mRNA expression (Iadarola et al. 1988). In the present study, naloxone was administered systemically; therefore, it is not known whether neonatal injury-induced changes in opioid tone are peripherally or centrally mediated. However, studies are currently underway using site-specific injections of naloxone to further identify the loci for injury-induced changes in endogenous opioid tone.
Long-Term Effects of Neonatal Injury are Sexually Dimorphic
The neuroendocrine profile of a newborn rat pup is sexually dimorphic, such that males have higher central levels of estradiol at birth compared to females, and similar differences in hormone levels may also be present in peripheral tissues (Weisz and Ward 1980; Amateau et al. 2004; Balthazart and Ball 2006; Cornil et al. 2006). Estrogens have been reported to exert neuroprotective effects following acute and chronic injuries in the adult CNS (Garcia-Segura et al. 2001; Maggi et al. 2004; Amantea et al. 2005). In the present study, neonatal injury resulted in significantly greater basal hypoalgesia at P60 in females in comparison to males. Indeed, the paw withdrawal latency of females injured with 1% CGN was 3 seconds longer in both the injured and uninjured paws compared to injured males. These results suggest that in males, estrogens may be acting as a neuroprotectant in response to early life injury, thereby leaving female rats with low to non-detectable levels of central estradiol increasingly vulnerable to the effects of neonatal noxious insult. Female rats injured at P14, when estradiol concentrations are comparable in males and females, displayed equivalent levels of baseline hypoalgesia as injured males, further suggesting that sex differences in the neonatal neuroendocrine environment contributed to the observed sexually dimorphic impact of neonatal injury. A potential neuroprotective effect of androgens cannot be ruled out in the present study (Ramsden et al. 2003), and future studies directed at manipulating the neonatal neuroendocrine environment, including masculinizing females or castrating males, are necessary to more specifically implicate gonadal hormones as the primary factor contributing to the observed sex differences in the impact of neonatal inflammatory insult.
Estradiol has been shown to influence the expression of a number of pro-inflammatory and pro-nociceptive agents. For example, prostaglandins (which are pro-inflammatory) are released peripherally in response to injury, and estrogen has been shown to modulate both prostaglandin and COX-1 and COX-2 expression in peripheral tissues (Zhang et al. 1997). Furthermore, peripheral injury also results in increased BDNF that is thought to promote neuronal survival and healing (Price et al. 2005). As estradiol increases BDNF expression centrally, this may also attenuate the adverse effects of peripheral injury (Allen and McCarson 2005).
Re-Injury with CFA Produces Hyperalgesia and the Effect is Sexually Dimorphic
Following re-injury in adulthood with CFA, neonatally injured male and female rats displayed significantly greater hyperalgesia than control animals. Furthermore, neonatally injured females exhibited significantly greater hyperalgesia in the inflamed paw than neonatally injured males, and male and female controls. This effect was observed in both the neonatally injured and uninjured paws, and is consistent with previous studies reporting long-term sensitization of afferent neurons and hyperalgesia following neonatal insult (Reynolds and Fitzgerald 1995; Anand et al. 1999; Al-Chaer et al. 2000).
This increased hyperalgesia following re-injury in adulthood appears disparate with the observed basal hypoalgesia. Our preliminary anatomical studies, however, suggest that neonatal inflammatory injury results in bilateral alterations in primary afferent innervation of the dorsal horn (LaPrairie and Murphy 2005), which may account for our observed hyperalgesia. In particular, neonatal injury increases primary afferent innervation in the L3-L5 spinal cord, as reflected by increased expression of both CGRP and substance P immunoreactivity. As both CGRP and substance P are pro-nociceptive, this increase in primary afferent innervation would be associated with a facilitated response to a noxious stimulus. Our working hypothesis is that the pain associated with intraplantar CGN on P0 results in a compensatory increase in descending inhibitory modulation as a mechanism of pain management. An increase in descending opioid tone is supported by our naloxone data, and would provide a direct mechanism for our observed hypoalgesia at baseline testing. By contrast, reinjury in adulthood with the inflammatory agent CFA results in an enhanced dorsal horn release of CGRP and/or substance P due to increased primary afferent input. Increased release of these pro-nociceptive peptides would be predicted to result in an enhanced hyperalgesic response. Increased primary afferent innervation of the spinal cord may also drive an enhanced descending facilitation in neonatally injured animals
Conclusion
Our findings clearly demonstrate that exposure to a single neonatal inflammatory insult is associated with long-term decreases in nociceptive sensitivity that are significantly exacerbated in females. The presence of a sex difference in the response to early insult may contribute to the higher prevalence, severity, and duration of pain syndromes (i.e. migraine, temporomandibular joint disorder, fibromyalgia and irritable bowel syndrome) that are observed in women.
Figure 2. Neonatal Injury has no impact on maternal care.
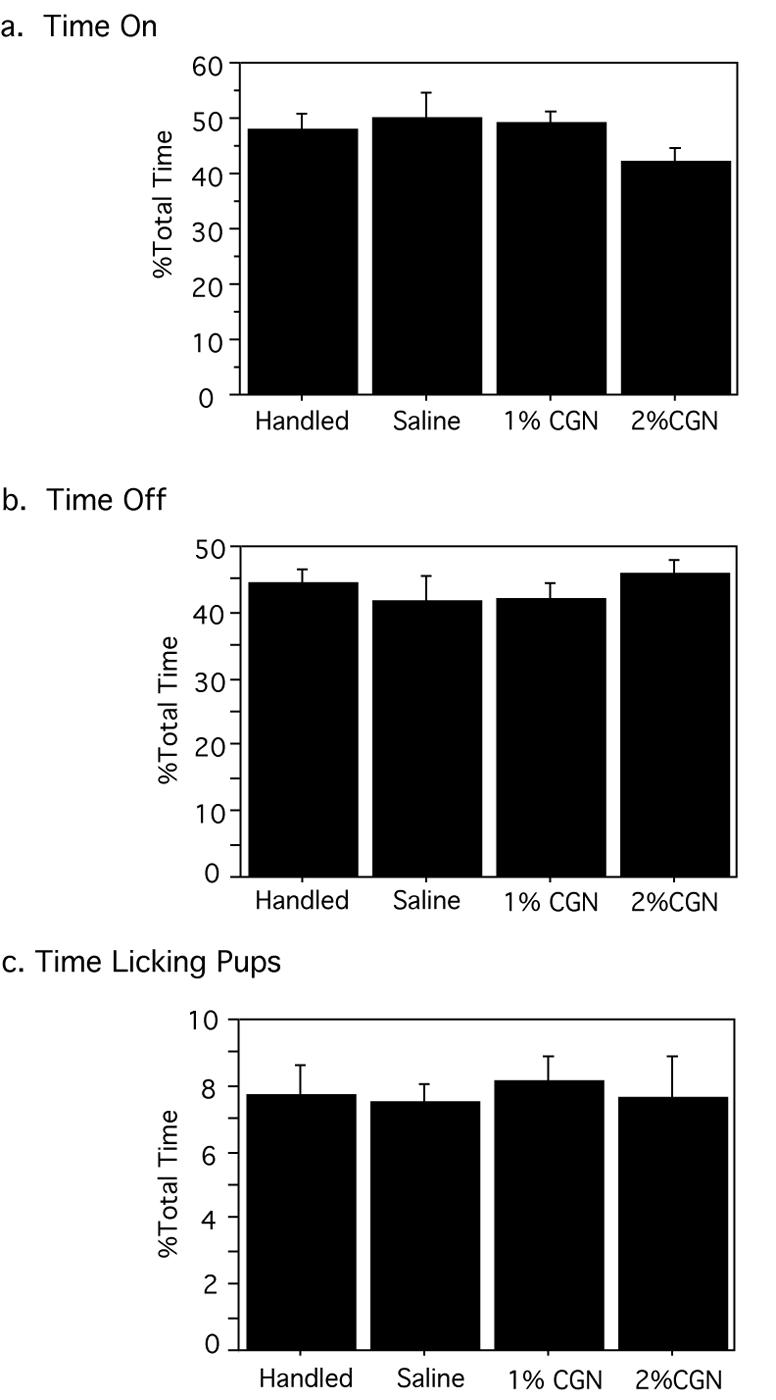
Neonatal injury had no effect on the (A) duration the dam spent on/with her litter [F(3,19)=.783, p=.5507], (B) amount of time the dam spent away from her litter [F(3,19)=.381, p=.7676], (C) duration of maternal licking and grooming behavior [F(3,19)=.128, p=.9425]. N=3-7 litters per group.
Acknowledgements
This work was supported by National Institute of Health grants DA16272 and AR49555 awarded to Anne Z. Murphy, the Center for Behavioral Neuroscience (NSF: IBN 9876754), and the Georgia State University Brains and Behavior Program. The authors are grateful for the constructive comments provided by Michael S. Gold on a previous version of this manuscript.
Footnotes
Competing Interest Statement
The authors declare that they have no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119(5):1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- Allen AL, McCarson KE. Estrogen increases nociception-evoked brain-derived neurotrophic factor gene expression in the female rat. Neuroendocrinology. 2005;81(3):193–199. doi: 10.1159/000087002. [DOI] [PubMed] [Google Scholar]
- Amantea D, Russo R, Bagetta G, Corasaniti MT. From clinical evidence to molecular mechanisms underlying neuroprotection afforded by estrogens. Pharmacol Res. 2005;52(2):119–132. doi: 10.1016/j.phrs.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Amateau SK, Alt JJ, Stamps CL, McCarthy MM. Brain estradiol content in newborn rats: sex differences, regional heterogeneity, and possible de novo synthesis by the female telencephalon. Endocrinology. 2004;145(6):2906–2917. doi: 10.1210/en.2003-1363. [DOI] [PubMed] [Google Scholar]
- Anand KJ. Clinical importance of pain and stress in preterm neonates. Biol Neonate. 1998;73(1):1–9. doi: 10.1159/000013953. [DOI] [PubMed] [Google Scholar]
- Anand KJ. Pain, plasticity, and premature birth: a prescription for permanent suffering? Nat Med. 2000;6(9):971–973. doi: 10.1038/79658. [DOI] [PubMed] [Google Scholar]
- Anand KJ, Coskun V, Thrivikraman KV, Nemeroff CB, Plotsky PM. Long-term behavioral effects of repetitive pain in neonatal rat pups. Physiol Behav. 1999;66(4):627–637. doi: 10.1016/s0031-9384(98)00338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006;29(5):241–249. doi: 10.1016/j.tins.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Bartocci M, Bergqvist LL, Lagercrantz H, Anand KJ. Pain activates cortical areas in the preterm newborn brain. Pain. 2006;122(12):109–117. doi: 10.1016/j.pain.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Bhutta AT, Rovnaghi C, Simpson PM, Gossett JM, Scalzo FM, Anand KJ. Interactions of inflammatory pain and morphine in infant rats: long-term behavioral effects. Physiol Behav. 2001;73(12):51–58. doi: 10.1016/s0031-9384(01)00432-2. [DOI] [PubMed] [Google Scholar]
- Cornil CA, Ball GF, Balthazart J. Functional significance of the rapid regulation of brain estrogen action: where do the estrogens come from? Brain Res. 2006;1126(1):2–26. doi: 10.1016/j.brainres.2006.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho SV, Plotsky PM, Sablad M, Miller JC, Zhou H, Bayati AI, McRoberts JA, Mayer EA. Neonatal maternal separation alters stress-induced responses to viscerosomatic nociceptive stimuli in rat. Am J Physiol Gastrointest Liver Physiol. 2002;282(2):G307–316. doi: 10.1152/ajpgi.00240.2001. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. Painful beginnings. Pain. 2004;110(3):508–509. doi: 10.1016/j.pain.2004.05.022. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Azcoitia I, DonCarlos LL. Neuroprotection by estradiol. Prog Neurobiol. 2001;63(1):29–60. doi: 10.1016/s0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- Grunau RE, Haley DW, Whitfield MF, Weinberg J, Yu W, Thiessen P. Altered basal cortisol levels at 3, 6, 8 and 18 months in infants born at extremely low gestational age. J Pediatr. 2007;150(2):151–156. doi: 10.1016/j.jpeds.2006.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunau RE, Holsti L, Haley DW, Oberlander T, Weinberg J, Solimano A, Whitfield MF, Fitzgerald C, Yu W. Neonatal procedural pain exposure predicts lower cortisol and behavioral reactivity in preterm infants in the NICU. Pain. 2005;113(3):293–300. doi: 10.1016/j.pain.2004.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Terayama R, Dubner R, Ren K. Plasticity in excitatory amino acid receptor-mediated descending pain modulation after inflammation. J Pharmacol Exp Ther. 2002;300(2):513–520. doi: 10.1124/jpet.300.2.513. [DOI] [PubMed] [Google Scholar]
- Iadarola MJ, Douglass J, Civelli O, Naranjo JR. Differential activation of spinal cord dynorphin and enkephalin neurons during hyperalgesia: evidence using cDNA hybridization. Brain Res. 1988;455(2):205–212. doi: 10.1016/0006-8993(88)90078-9. [DOI] [PubMed] [Google Scholar]
- Ji Y, Murphy AZ, Traub RJ. Sex differences in morphine-induced analgesia of visceral pain are supraspinally and peripherally mediated. Am J Physiol Regul Integr Comp Physiol. 2006;291(2):R307–314. doi: 10.1152/ajpregu.00824.2005. [DOI] [PubMed] [Google Scholar]
- Johnston CC, Stevens BJ. Experience in a neonatal intensive care unit affects pain response. Pediatrics. 1996;98(5):925–930. [PubMed] [Google Scholar]
- Johnston CC, Walker CD. The effects of exposure to repeated minor pain during the neonatal period on formalin pain behaviour and thermal withdrawal latencies. Pain Res Manag. 2003;8(4):213–217. doi: 10.1155/2003/305409. [DOI] [PubMed] [Google Scholar]
- Kamp EH, Jones RC, 3rd, Tillman SR, Gebhart GF. Quantitative assessment and characterization of visceral nociception and hyperalgesia in mice. Am J Physiol Gastrointest Liver Physiol. 2003;284(3):G434–444. doi: 10.1152/ajpgi.00324.2002. [DOI] [PubMed] [Google Scholar]
- Komisaruk BR. Antinociceptive effects of vaginal stimulation in rats: neurophysiological and behavioral studies. Brain Res. 1977;137:85–107. doi: 10.1016/0006-8993(77)91014-9. [DOI] [PubMed] [Google Scholar]
- LaPrairie J, Murphy A. Neonatal injury diffrentially affects male and female sensory thresholds and response to re-injury in adulthood. Soc Neuroscience Abst. 2005 [Google Scholar]
- Lidow MS. Long-term effects of neonatal pain on nociceptive systems. Pain. 2002;99(3):377–383. doi: 10.1016/S0304-3959(02)00258-0. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci. 2000;3(8):799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- Maggi A, Ciana P, Belcredito S, Vegeto E. Estrogens in the nervous system: mechanisms and nonreproductive functions. Annu Rev Physiol. 2004;66:291–313. doi: 10.1146/annurev.physiol.66.032802.154945. [DOI] [PubMed] [Google Scholar]
- Noguchi K, Morita Y, Kiyama H, Sato M, Ono K, Tohyama M. Preproenkephalin gene expression in the rat spinal cord after noxious stimuli. Brain Res Mol Brain Res. 1989;5(3):227–234. doi: 10.1016/0169-328x(89)90039-9. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Grunau RE, Whitfield MF, Fitzgerald C, Pitfield S, Saul JP. Biobehavioral pain responses in former extremely low birth weight infants at four months' corrected age. Pediatrics. 2000;105(1):e6. doi: 10.1542/peds.105.1.e6. [DOI] [PubMed] [Google Scholar]
- Peters JW, Schouw R, Anand KJ, van Dijk M, Duivenvoorden HJ, Tibboel D. Does neonatal surgery lead to increased pain sensitivity in later childhood? Pain. 2005;114(3):444–454. doi: 10.1016/j.pain.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Pieretti S, d'Amore A, Loizzo A. Long-term changes induced by developmental handling on pain threshold: effects of morphine and naloxone. Behav Neurosci. 1991;105(1):215–218. doi: 10.1037//0735-7044.105.1.215. [DOI] [PubMed] [Google Scholar]
- Price TJ, Louria MD, Candelario-Soto D, Dussor GO, Jeske NA, Patwardhan AM, Diogenes A, Trott AA, Hargreaves KM, Flores CM. Treatment of trigeminal ganglion neurons in vitro with NGF, GDNF or BDNF: effects on neuronal survival, neurochemical properties and TRPV1-mediated neuropeptide secretion. BMC Neurosci. 2005;6(1):4. doi: 10.1186/1471-2202-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J. Infant pain: does it hurt? Nature. 2006;444(7116):143–145. doi: 10.1038/444143a. [DOI] [PubMed] [Google Scholar]
- Ramsden M, Shin T, Pike C. Androgens modulate neuronal vulnerability to kainate lesion. Neurosci. 2003;122:573–578. doi: 10.1016/j.neuroscience.2003.08.048. [DOI] [PubMed] [Google Scholar]
- Ren K, Anseloni V, Zou SP, Wade EB, Novikova SI, Ennis M, Traub RJ, Gold MS, Dubner R, Lidow MS. Characterization of basal and re-inflammation-associated long-term alteration in pain responsivity following short-lasting neonatal local inflammatory insult. Pain. 2004;110(3):588–596. doi: 10.1016/j.pain.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Reynolds ML, Fitzgerald M. Long-term sensory hyperinnervation following neonatal skin wounds. J Comp Neurol. 1995;358(4):487–498. doi: 10.1002/cne.903580403. [DOI] [PubMed] [Google Scholar]
- Ruda MA, Ling QD, Hohmann AG, Peng YB, Tachibana T. Altered nociceptive neuronal circuits after neonatal peripheral inflammation. Science. 2000;289(5479):628–631. doi: 10.1126/science.289.5479.628. [DOI] [PubMed] [Google Scholar]
- Simons SH, van Dijk M, Anand KS, Roofthooft D, van Lingen RA, Tibboel D. Do we still hurt newborn babies? A prospective study of procedural pain and analgesia in neonates. Arch Pediatr Adolesc Med. 2003;157(11):1058–1064. doi: 10.1001/archpedi.157.11.1058. [DOI] [PubMed] [Google Scholar]
- Slater R, Boyd S, Meek J, Fitzgerald M. Cortical pain responses in the infant brain. Pain. 2006;123(3):332. doi: 10.1016/j.pain.2006.05.009. author reply 332-334. [DOI] [PubMed] [Google Scholar]
- Sternberg WF. Sex differences in the effects of prenatal stress on stress-induced analgesia. Physiol Behav. 1999;68(12):63–72. doi: 10.1016/s0031-9384(99)00164-x. [DOI] [PubMed] [Google Scholar]
- Sternberg WF, Ridgway CG. Effects of gestational stress and neonatal handling on pain, analgesia, and stress behavior of adult mice. Physiol Behav. 2003;78(3):375–383. doi: 10.1016/s0031-9384(03)00015-5. [DOI] [PubMed] [Google Scholar]
- Sternberg WF, Scorr L, Smith LD, Ridgway CG, Stout M. Long-term effects of neonatal surgery on adulthood pain behavior. Pain. 2005;113(3):347–353. doi: 10.1016/j.pain.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Walker SM, Meredith-Middleton J, Cooke-Yarborough C, Fitzgerald M. Neonatal inflammation and primary afferent terminal plasticity in the rat dorsal horn. Pain. 2003;105(12):185–195. doi: 10.1016/s0304-3959(03)00201-x. [DOI] [PubMed] [Google Scholar]
- Wang G, Ji Y, Lidow MS, Traub RJ. Neonatal hind paw injury alters processing of visceral and somatic nociceptive stimuli in the adult rat. J Pain. 2004;5(8):440–449. doi: 10.1016/j.jpain.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Wang X, Traub RJ, Murphy AZ. Persistent pain model reveals sex difference in morphine potency. Am J Physiol Regul Integr Comp Physiol. 2006;291(2):R300–306. doi: 10.1152/ajpregu.00022.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7(8):847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Weisz J, Ward IL. Plasma testosterone and progesterone titers of pregnant rats, their male and female fetuses, and neonatal offspring. Endocrinology. 1980;106(1):306–316. doi: 10.1210/endo-106-1-306. [DOI] [PubMed] [Google Scholar]
- Whitfield MF, Grunau RE. Behavior, pain perception, and the extremely low-birth weight survivor. Clin Perinatol. 2000;27(2):363–379. doi: 10.1016/s0095-5108(05)70026-9. [DOI] [PubMed] [Google Scholar]
- Williams FG, Mullet MA, Beitz AJ. Basal release of Met-enkephalin and neurotensin in the ventrolateral periaqueductal gray matter of the rat: a microdialysis study of antinociceptive circuits. Brain Res. 1995;690:207–216. doi: 10.1016/0006-8993(95)00554-4. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Shaffer A, Portanova J, Seibert K, Isakson PC. Inhibition of cyclooxygenase-2 rapidly reverses inflammatory hyperalgesia and prostaglandin E2 production. J Pharmacol Exp Ther. 1997;283(3):1069–1075. [PubMed] [Google Scholar]


