Abstract
Recent advances in understanding the cellular and molecular mechanisms of atopy have shed light on potential targets for the development of new therapies for allergic diseases. In this issue of the JCI, Seshasayee et al. provide direct in vivo evidence that OX40 has critical roles in allergic inflammation mediated by thymic stromal lymphopoietin (TSLP) (see the related article beginning on page 3868). Blockade of interactions between OX40 on Th2 cells and OX40 ligand (OX40L) on TSLP-activated DCs using an OX40L-specific monoclonal antibody, inhibited Th2 cell–mediated immune responses in both mouse and nonhuman primate models of allergic inflammation. The results point to potential therapeutic approaches to targeting the cellular and molecular mechanism underlying TSLP-mediated allergic inflammation.
TSLP: bridging the epithelial barrier and type 2 immune responses
Asthma and atopic dermatitis (AD) are inflammatory disorders characterized by the infiltration and accumulation of memory-like Th2 cells and eosinophils (1). In addition to the type 2 inflammatory processes, allergic diseases often involve epithelial cell stress and injury that trigger the release of chemokines and growth factors able to support both chronic inflammatory and remodeling responses. The idea that reduced barrier function of the epithelium and altered innate immunity are fundamental to the origin of these diseases is supported by the recent finding that thymic stromal lymphopoietin (TSLP), an IL-7–like cytokine, plays a key role in allergic inflammation at the interface between epithelial cells and DCs (2). TSLP is highly expressed by keratinocytes in skin lesions of patients with AD (3) and by airway epithelial cells of individuals with asthma (4) and can potently activate myeloid DCs by upregulating their surface expression of MHC class II, CD54, CD80, CD83, CD86, and DC-lamp (3). Interestingly, TSLP triggers human myeloid DCs to produce a myriad of chemokines that recruit eosinophils and Th2 cells, but it does not trigger them to produce either Th1-polarizing cytokines of the IL-12 family or the proinflammatory cytokines TNF-α, IL-1β, and IL-6, resulting in a microenvironment permissive for type 2 inflammatory responses (5). TSLP-activated DCs can induce naive T cells to differentiate into inflammatory Th2 cells producing the classical Th2 cytokines IL-4, IL-5, and IL-13, as well as a large amount of TNF-α but little or no IL-10 (6). Moreover, only TSLP-activated DCs can induce in vitro, allergen-specific Th2 memory cells to undergo homeostatic expansion and further Th2 polarization, and to mediate recall responses (7). In support of the findings in humans, mice with conditional overexpression of TSLP in keratinocytes were found to have an AD-like phenotype characterized by scaling lesions accompanied by infiltration of Th2 CD4+ cells in skin and elevated serum IgE level (8). By contrast, mice lacking the TSLP receptor (TSLPR) failed to develop an antigen-specific Th2 inflammatory response in models of asthma (9). Recent studies showed that overexpression of TSLP in mice lacking T cells triggered moderate bronchial or cutaneous allergic inflammation, suggesting that TSLP might directly activate effector cells of the innate immune system (10). Indeed, human TSLP, synergistically with IL-1 and TNF-α, stimulates mast cells to produce high levels of IL-5 and IL-13, as well as the proinflammatory cytokines GM-CSF and IL-6 (11). Thus, TSLP produced by the epithelium can directly activate innate effector mast cells and DCs, which induce adaptive inflammatory Th2 immune responses; TSLP therefore represents a critical factor linking responses at epithelial cell surfaces to allergic type 2 immune responses (Figure 1).
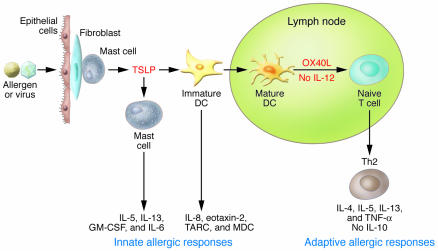
Figure 1. Pathophysiology of TSLP in allergic inflammation.
Insults from allergens or viruses trigger mucosal epithelial cells or skin cells (keratinocytes, flbroblasts, and mast cells) to produce TSLP. TSLP initiates the innate phase of allergic immune responses by activating immature DCs to produce the chemokines IL-8 and eotaxin-2, as well as the Th2-attracting chemokines thymus and activation-regulated chemokine (TARC) and macrophage-derived chemokine (MDC), and by costimulating mast cells to produce IL-5 and IL-13, as well as GM-CSF and IL-6. TSLP-activated DCs mature and migrate into the draining lymph nodes to initiate the adaptive phase of allergic immune responses. TSLP-activated DCs express OX40L, which triggers the differentiation of allergen-speciflc naive CD4+ T cells into inflammatory Th2 cells that produce IL-4, IL-5, IL-13, and TNF-α but not IL-10. Inflammatory Th2 cells then migrate back to the site of inflammation because of the local production of TARC and MDC. The cytokines produced by the inflammatory Th2 cells (IL-4, IL-5, IL-13, and TNF-α) initiate allergic inflammation by triggering IgE and mucus production and eosinophilia. Figure modified with permission from J. Allergy Clin. Immunol. (25).
OX40-OX40L interactions: controlling the fate of CD4+ T cells during allergic inflammation
OX40L, originally termed glycoprotein 34 kDa (GP34), and its cognate receptor OX40 belong to the TNF and TNF receptor superfamilies, respectively (12, 13). OX40 is preferentially expressed by activated CD4+ T cells, whereas OX40L is mainly expressed by APCs, including activated DCs, B cells, macrophages, and Langerhans cells, as well as by T cells and endothelial cells (14, 15). Unlike another costimulatory molecule, CD28, which plays an important role in T cell priming, OX40-OX40L interactions have been shown to be crucial for T cell activation and survival, and for the generation of memory T cells from activated effector T cells (16).
Microarray analyses identified OX40L as the key molecule expressed by TSLP-activated DCs, as it enables them to trigger allergic inflammatory Th2 immune responses (6). Blockade of OX40-OX40L interactions, using a neutralizing antibody specific for OX40L, inhibited the production of Th2 cytokines and TNF-α and enhanced the production of IL-10 by differentiating CD4+ T cells cocultured with TSLP-activated DCs (6). OX40L-induced inflammatory Th2 cell differentiation depends on the absence of IL-12, as OX40L is incapable of triggering inflammatory Th2 cell differentiation in the presence of IL-12 (6). Thus, TSLP-activated DCs can create a Th2-permissive microenvironment by upregulating OX40L expression without producing Th1-polarizing cytokines.
In addition to inducing the differentiation of inflammatory Th2 cells, TSLP-activated DCs can induce the robust expansion of human Th2 memory cells, while maintaining their central memory phenotype and Th2 commitment (7). Th2 memory cells expanded by TSLP-activated DCs undergo further Th2 polarization and express proallergic molecules, such as IL-25R (IL17RB), cystatin A, Charcot-Leyden crystal protein, and prostaglandin D2 synthase (7, 17). Interestingly, OX40L expressed by TSLP-activated DCs also plays an important role in driving the expansion of Th2 memory cells; by binding OX40 on the T cells, it contributes to prolonging the cognate T cell–DC interaction (7). Blockade of OX40-OX40L interactions resulted in arrest at the G0 phase of the cell cycle and limited the proliferation of Th2 memory cells induced by autologous TSLP-activated DCs (7). These data identify plausible explanations for the importance of OX40L during TSLP-mediated allergic inflammation, highlighting its roles in the induction of inflammatory Th2 cells and the maintenance of the Th2 memory cell pool.
In allergen-induced models of allergy, mice lacking OX40 or OX40L exhibit markedly impaired reactivation of Th2 memory cells and Th2 responses, as well as diminished lung inflammation (18, 19). Building on earlier findings in humans and mice (discussed above), Seshasayee et al. have further demonstrated the role of OX40L in TSLP-induced allergic inflammation in the skin of mice and in the lung of mice and nonhuman primates (20). They generated a chimeric hamster-mouse mAb and a fully human mAb specific for mouse and human OX40L, respectively. These invaluable OX40L-specific mAbs proved to be efficacious in inhibiting antigen-driven Th2 inflammation in mouse and nonhuman primate models of asthma. Administration of their OX40L-specific mAbs resulted in substantial reductions in the amount of Th2 cytokines and antigen-specific IgE and IgG1, as well as the loss of infiltrating eosinophils and CD4+ effector/memory T cells. These results demonstrated that in vivo, OX40L is a dominant mediator of TSLP-induced allergic inflammation in the lung and skin of mice. Most importantly, the study by Seshasayee et al. has provided direct evidence that OX40L is required to elicit disease in antigen-driven models of asthma in mice and, in particular, in antigen-driven models of asthma in nonhuman primates, i.e., rhesus monkeys. They further showed that the effects of their mAbs were mediated by blocking OX40-OX40L interactions and depleting OX40L+ DCs. Interestingly, the treatments resulted in only a moderate reduction in the primary effector Th2 inflammatory response, but a marked decrease in reactivation and infiltration of memory CD4+ T cells, production of Th2 cytokines, and antigen-specific serum IgE levels was observed during the recall response to antigen. These results demonstrated that the maintenance and reactivation of Th2 memory cells by OX40L-expressing DCs contributes to the pathogenesis of TSLP-mediated allergic inflammation. In mouse and nonhuman primate models of asthma, the therapeutic efficacy of targeting the regulation and function of pathogenic Th2 memory cells, using OX40L-specific mAbs — as shown in the study by Seshasayee et al. — points to a new therapeutic approach for the prevention and treatment of human allergic diseases.
Future perspectives
Studies using mice lacking OX40 or OX40L have demonstrated that the OX40 signaling pathway plays important roles in controlling the fate and functions of CD4+ T cell not only in TSLP-mediated allergic diseases but also in other models of inflammatory immune disorders, including EAE and animal models of inflammatory bowel disease and graft-versus-host disease (16). These observations suggest that inhibitors targeting OX40 and OX40L in vivo might provide new therapeutic strategies for several inflammatory immunological disorders. Indeed, two different OX40-specific therapeutic interventions have been successful in the treatment of animals with EAE (21, 22). An OX40-specific immunotoxin that depletes autoreactive OX40+ T cells (21) and an OX40L-binding agent that blocks OX40-OX40L interactions (22) have both been reported to successfully reduce the clinical symptoms of EAE in mice. An OX40L-specific mAb has also been employed for the treatment of disease in a mouse model of asthma (22, 23); however, variations in the therapeutic efficacy of this mAb were observed in different strains of mice (16). Therefore, the efficacy of treatment with OX40L-specific mAbs for human allergic asthma might also vary from individual to individual. Whether the effect of blocking OX40-OX40L interactions results in a reduction in the size of other OX40+ memory T cell pools might need further detailed examinations in vivo.
The unexpected findings that point to a role for TSLP in allergic inflammation provide insights for designing new therapeutic approaches for the treatment of human atopic diseases. The finding that TSLP is highly expressed by keratinocytes in the skin and in airway epithelial cells during allergic inflammation raises the question of how expression of TSLP is triggered in these cells. Whether allergen exposure or virus infection can induce TSLP overexpression in the inflammatory tissues remains unclear. The expression of retinoid X receptor (RXR) in keratinocytes might actively suppress TSLP production under normal physiological conditions (24). Further studies on the regulation of this receptor might provide important clues about how allergens or viral infections trigger TSLP production and lead to other therapeutic approaches. Targeting TSLP and the OX40-OX40L interaction can be considered as the first step toward immunological intervention in the treatment of allergic diseases.
Footnotes
Nonstandard abbreviations used: AD, atopic dermatitis; OX40L, OX40 ligand; TSLP, thymic stromal lymphopoietin; TSLPR, TSLP receptor.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 117:3655–3657 (2007). doi:10.1172/JCI34182.
References
- 1.Kay A.B. Allergy and allergic diseases. Second of two parts. N. Engl. J. Med. 2001;344:109–113. doi: 10.1056/NEJM200101113440206. [DOI] [PubMed] [Google Scholar]
- 2.Holgate S.T. The epithelium takes centre stage in asthma and atopic dermatitis. Trends Immunol. 2007;28:248–251. doi: 10.1016/j.it.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Soumelis V., et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 4.Ying S., et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J. Immunol. 2005;174:8183–8190. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y.J. Thymic stromal lymphopoietin: master switch for allergic inflammation. J. Exp. Med. 2006;203:269–273. doi: 10.1084/jem.20051745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ito T., et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J. Exp. Med. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y.H., et al. Maintenance and polarization of human TH2 central memory T cells by thymic stromal lymphopoietin-activated dendritic cells. Immunity. 2006;24:827–838. doi: 10.1016/j.immuni.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 8.Yoo J., et al. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. . J. Exp. Med. 2005;202:541–549. doi: 10.1084/jem.20041503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al Shami A., Spolski R., Kelly J., Keane-Myers A., Leonard W.J. A role for TSLP in the development of inflammation in an asthma model. J. Exp. Med. 2005;202:829–839. doi: 10.1084/jem.20050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou B., et al. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat. Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 11.Allakhverdi Z., et al. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J. Exp. Med. 2007;204:253–258. doi: 10.1084/jem.20062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paterson D.J., et al. Antigens of activated rat T lymphocytes including a molecule of 50,000 Mr detected only on CD4 positive T blasts. Mol. Immunol. 1987;24:1281–1290. doi: 10.1016/0161-5890(87)90122-2. [DOI] [PubMed] [Google Scholar]
- 13.Godfrey W.R., Fagnoni F.F., Harara M.A., Buck D., Engleman E.G. Identification of a human OX-40 ligand, a costimulator of CD4+ T cells with homology to tumor necrosis factor. . J. Exp. Med. 1994;180:757–762. doi: 10.1084/jem.180.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohshima Y., et al. OX40 costimulation enhances interleukin-4 (IL-4) expression at priming and promotes the differentiation of naive human CD4(+) T cells into high IL-4-producing effectors. Blood. 1998;92:3338–3345. [PubMed] [Google Scholar]
- 15.Chen A.I., et al. Ox40-ligand has a critical costimulatory role in dendritic cell:T cell interactions. Immunity. 1999;11:689–698. doi: 10.1016/s1074-7613(00)80143-0. [DOI] [PubMed] [Google Scholar]
- 16.Sugamura K., Ishii N., Weinberg A.D. Therapeutic targeting of the effector T-cell co-stimulatory molecule OX40. Nat. Rev. Immunol. 2004;4:420–431. doi: 10.1038/nri1371. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y.H., et al. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J. Exp. Med. 2007;204:1837–1847. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoshino A., et al. Critical role for OX40 ligand in the development of pathogenic Th2 cells in a murine model of asthma. Eur. J. Immunol. 2003;33:861–869. doi: 10.1002/eji.200323455. [DOI] [PubMed] [Google Scholar]
- 19.Salek-Ardakani S., et al. OX40 (CD134) controls memory T helper 2 cells that drive lung inflammation. J. Exp. Med. 2003;198:315–324. doi: 10.1084/jem.20021937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seshasayee D., et al. In vivo blockade of OX40 ligand inhibits thymic stromal lymphopoietin driven atopic inflammation. J. Clin. Invest. 2007;117:3868–3878. doi: 10.1172/JCI33559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weinberg A.D., et al. Selective depletion of myelin-reactive T cells with the anti-OX-40 antibody ameliorates autoimmune encephalomyelitis. Nat. Med. 1996;2:183–189. doi: 10.1038/nm0296-183. [DOI] [PubMed] [Google Scholar]
- 22.Weinberg A.D., Wegmann K.W., Funatake C., Whitham R.H. Blocking OX-40/OX-40 ligand interaction in vitro and in vivo leads to decreased T cell function and amelioration of experimental allergic encephalomyelitis. J. Immunol. 1999;162:1818–1826. [PubMed] [Google Scholar]
- 23.Arestides R.S., et al. Costimulatory molecule OX40L is critical for both Th1 and Th2 responses in allergic inflammation. Eur. J. Immunol. 2002;32:2874–2880. doi: 10.1002/1521-4141(2002010)32:10<2874::AID-IMMU2874>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 24.Li M., et al. Retinoid X receptor ablation in adult mouse keratinocytes generates an atopic dermatitis triggered by thymic stromal lymphopoietin. Proc. Natl. Acad. Sci. U.S.A. 2005;102:14795–14800. doi: 10.1073/pnas.0507385102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y.J. Thymic stromal lymphopoietin and OX40 ligand pathway in the initiation of dendritic cell–mediated allergic inflammation. J. Allergy Clin. Immunol. . 2007;120:238–244. doi: 10.1016/j.jaci.2007.06.004. [DOI] [PubMed] [Google Scholar]