Abstract
Objective
Major depression is a common concomitant of chronic central nervous system disorders, notably Parkinson’s disease (PD). Repetitive transcranial magnetic stimulation (rTMS) has been investigated as a potential treatment for depression in PD and for the movement disorder of PD, but comprehensive testing in multiple areas of performance has seldom been carried out within a single study. We studied the effect of left dorsolateral prefrontal rTMS on several different functional domains.
Methods
Fourteen PD patients with treatment-resistant depression entered an open, 10-day inpatient study of 10-Hertz rTMS, undergoing extensive psychiatric, neuropsychological, and motor testing from baseline to 6 weeks after treatment. Motor testing included a defined “off” state.
Results
rTMS was well-tolerated. Highly significant improvement in depression scores was seen three days and 3-6 weeks after treatment. Improvement was also found in anxiety, movement scores (especially in the off state), and some neuropsychological measures. We found no evidence of increased risk from rTMS in this population.
Conclusions
Further controlled trials of rTMS in PD appear worthwhile, and should include a defined “off” state.
Significance
TMS may be beneficial for depressed PD patients in multiple functional domains.
Keywords: Transcranial magnetic stimulation, depression, Parkinson disease
Introduction
Repetitive transcranial magnetic stimulation (rTMS) of the left dorsolateral prefrontal cortex (DLPFC) is a promising treatment for refractory depression (Berman et al., 2000; Epstein et al., 1998; George et al., 1997; George et al., 1995; Grunhaus et al., 2003). As rTMS becomes more widely used, determining whether it is effective in the setting of central nervous system (CNS) disorders will be important, because depression is a common concomitant of other (CNS) diseases. Clinical depression occurs in up to 50% of all patients with Parkinson’s disease (PD) (Tandberg et al., 1996), itself one of the most common CNS disorders, with a cumulative lifetime incidence above one percent and prevalence over 3% (Benito-Leon et al., 2003). However, most experimental rTMS protocols have excluded patients with PD and other CNS disorders because of concerns about altered rTMS responsiveness and increased adverse effects.
The conjunction of depression and PD has special interest, because of the possibility that the psychomotor retardation of depression and the bradykinesia of PD are adversely synergistic in producing motor impairment. Electroconvulsive therapy (ECT) has been used intermittently for many years to treat refractory PD in the absence of depression (Jeanneau, 1993; Pridmore et al., 1995; Ward et al., 1980; Wengel et al., 1998). Although the mechanisms of ECT and rTMS are not identical, treating depression in PD might be beneficial for motor dysfunction as well as mood. DLPFC rTMS might help to elucidate such benefits, because its effect is more localized than that of pharmacotherapy or ECT and avoids the primary motor cortex.
A number of TMS studies have addressed the possibility of improvement in multiple domains, testing cognitive function in patients with pure depression and adding movement measures in patients with depression plus PD. Few studies have tested all of these domains in the same patients, and to our knowledge none has focused on the “off” state in PD, when many patients manifest their maximum disability. Indeed, the practicality of such extensive evaluations is uncertain. To assess the feasibility of prolonged inpatient assessments and to provide preliminary results we performed an open trial of rTMS in patients with PD and treatment-resistant depression, including both multimodal testing and defined “off” and “on” states.
Materials and Methods
Patients
Fourteen PD patients (9 male, 5 female, ages 42-78, average 62) gave informed consent to a protocol approved by the Emory University School of Medicine Institutional Review Board. Inclusion criteria were probable PD according to current criteria (Gelb et al., 1999); moderate to severe major depression without psychotic features (defined by DSM-IV criteria); a 17-item Hamilton depression score (HAMD17) ≥ 17 at screening; at least one adequate trial of an antidepressant medication; age 40-80 years; and Folstein mini-mental-status exam (MMSE) of 25 or greater. Exclusions were DSM-IV criteria for organic mood disorder or substance dependence within the last 6 months; other significant central neurological disorders including brain mass, epileptic seizures, stroke, transient ischemic attack within two years, cerebral aneurysm, dementia, and multiple sclerosis; pregnancy; cardiac pacemakers, cochlear implants, or intracranial implants; psychiatric symptoms of significant severity that patients could not tolerate a two-week trial of rTMS or would require psychiatric hospitalization; acute, unstable medical conditions; or requirement for continued treatment with antidepressants, antipsychotics including clozapine and risperidone, benzodiazepines, lithium or anticonvulsants. Zolpidem was acceptable. Other psychoactive medications were tapered off at least two weeks prior to rTMS treatment. One patient was taking selegiline, which may have mild antidepressant effects but was continued for its primary indication of PD.
Test Protocol
Because preliminary discussions with depressed PD patients indicated that many would find it impractical to return for daily rTMS sessions over a period of weeks, treatment was performed in the General Clinical Research Center (GCRC) of Emory University Hospital. Patients were permitted to return home over the weekends between the two treatment weeks and the first post-treatment test session.
The protocol schedule and test abbreviations are outlined in Table 1. The extensive testing required for this study required a total of up to 5 overnight admissions to the GCRC. A defined Parkinsonian “off” state was attained through 10 hour discontinuation of PD medications, beginning at midnight. For defined “on” testing the procedure was to administer the first morning dose at the end of “off” testing, wait 60 minutes, check that the patient had indeed felt the medications become effective, and then begin re-evaluation. Motor performance was assessed by physicians certified in Unified Parkinson’s Disease Rating Scale (UPDRS) testing. UPDRS scores for Activities of Daily Living (ADLs) were not used because of the difficulty comparing ADL ratings made at home and during a 2-week hospital stay. Psychiatric and neuropsychological testing was performed by trained technicians under the supervision of a psychiatrist and a neuropsychologist.
Table 1.
Testing Schedule
| Test Category Timing -------> | Test | Screening | TMS Baseline
|
Post TMS
|
Followup | Final Followup |
|---|---|---|---|---|---|---|
| 3 days pre-TMS
|
3 days after last TMS
|
3 weeks after last TMS
|
6 weeks after last TMS
|
|||
| Psychiatric/ QOL
|
SCID | X
|
|
|
|
|
| HAMD
|
X | X
|
X
|
X
|
X
|
|
|
|
BDI
|
X
|
X
|
X
|
X
|
|
|
|
BPRS
|
|
X
|
X
|
X
|
X
|
|
|
HAMA
|
|
X
|
X | X | X |
|
|
PDQ-39
|
X
|
X
|
X
|
X
|
|
|
|
CGI
|
|
|
X | ||
| Neuropsychological
|
MMSE | X |
|
|
|
|
| RBANS
|
X
|
X
|
X
|
X
|
||
|
|
BTA
|
|
X
|
X
|
X
|
X
|
|
|
DRS
|
|
X
|
X
|
X
|
X
|
| Movement | UPDRS | X | X | X | X | |
| Psychiatric and Quality of Life Scales: | ||||||
| Structured Clinical Interview for DSM-IV (SCID)(First et al., 1995) | ||||||
| Hamilton Depression Rating Scale (HAMD17 and HAMD21)(Hamilton, 1967) | ||||||
| Beck Depression Inventory (BDI)(Beck, 1973) | ||||||
| Brief Psychiatric Rating Scale (BPRS)(Overall and Gorham, 1962) | ||||||
| Hamilton Anxiety Rating Scale (HAMA)(Hamilton, 1959) | ||||||
| Parkinson’s Disease Quality of Life (PDQ-39)(Peto et al., 1998) | ||||||
| Clinical Global Impression (CGI)(Guy, 1976) | ||||||
| Neuropsychological Scales: | ||||||
| Mini-Mental Status Exam (MMSE)(Folstein et al., 1975) | ||||||
| Repeatable Battery for Assessment of Neuropsychological Status (RBANS), (excluding Figure copy and Coding because of possible confounding effects from Parkinsonian motor deficits)(Randolph, 1995) | ||||||
| Brief Test of Attention (BTA)(Schretlen, 1989) | ||||||
| Mattis Dementia Rating Scale (DRS)(Mattis, 1988) | ||||||
| Parkinsonian Scales: | ||||||
| Unified Parkinson’s Disease Rating Scale (UPDRS) (Fahn et al., 1987) |
rTMS Treatment
All rTMS treatment was performed by the PI or under his immediate supervision, using a Cadwell high-speed magnetic stimulator (Cadwell Laboratories, Kennewick, WA, USA) and a custom iron-core coil (Epstein and Davey, 2002). This combination is functionally equivalent to a prototype magnetic stimulator from Neuronetics, Incorporated (Malvern, PA, USA) which is currently being used in multicenter depression trials. The scalp position of lowest motor threshold in the right hand was determined by iterative exploration as previously described, using visual criteria (Epstein et al., 1998); (Pridmore et al., 1998). Resting motor threshold (MT) was then determined at that site as the lowest power setting that produced a visible response in at least 5 of 10 consecutive stimuli (Rossini et al., 1994). The left DLPFC treatment site was determined by measuring 5 cm forward from the point of lowest motor threshold, as described by George and colleagues (George et al., 1995). On the first morning after initial GCRC admission, rTMS treatment was deferred for the test protocol. Otherwise, 1000 fast rTMS pulses were administered morning and afternoon for 10 consecutive weekdays at 10 Hz and 110% of resting motor threshold, totaling 19 treatments of 1000 pulses each. 20 trains of 50 pulses were separated by 25 second rest intervals. No attempt was made to synchronize treatment with medication or with “off” and “on” periods.
Statistical Analysis
Given the limited number of subjects, results were analyzed by matched t-tests. The 17-item Hamilton Depression Score (HAMD17) was pre-selected as the primary outcome measure. Although the other measures were considered exploratory, a Bonferroni correction was noted for assessment of the multiple different scales within the psychiatric and cognitive domains, and for subscores in all domains. Near-significant trends were tabulated for interest. Because some patients returned at either the 3-week or 6-week followup, but not both, these results were averaged for those individuals who returned twice.
Results
All 14 patients completed the 2-week treatment protocol and 12 completed the 3-day follow-up testing. Two participants dropped out of the study immediately after completing the treatment phase. One believed that his condition had deteriorated and elected to pursue ECT therapy. The second stated that he was “too busy” to return for follow-up testing. Only HAMD17 scores were obtained at 3-day followup for these 2 subjects, and are included. Eight subjects returned for additional comprehensive follow-up testing at 3 or 6 weeks post-treatment.
Adverse Events
There were no seizures and no complaints of headache or neurological deterioration. During the course of the GCRC treatment hospitalization 4 patients suffered falls, one had recurrence of paroxysmal atrial fibrillation, and one suffered unilateral hip pain unrelated to any acute injury. None of these occurred in close proximity to TMS or was considered to be a consequence of it.
Psychiatric and QOL Scales
Coordinating the pre- and post-treatment testing visits, the two-week inpatient treatment regimen, and the patients’ social circumstances was unexpectedly difficult, and produced an average delay from screening visit to treatment of almost 10 weeks. Although this delay was not intended, it allowed us the opportunity to evaluate regression to the mean as a possible explanation for improvement. The average HAMD17 score dropped from 21.86 to 19.92 during the period from screening to baseline (Figure 1). Since patients received no other new treatment over this interval, a reasonable first approximation of the regression to the mean effect is the difference of 1.94, or 0.2 points per week (p = 0.09) on 14 observations. By comparison, the drop from baseline to the post-treatment visit is 6.15, or 2.43 points per week, averaging 31% improvement (p = .004, Figure 1.) Only one patient worsened, by a single point, on HAMD17.
Figure 1.
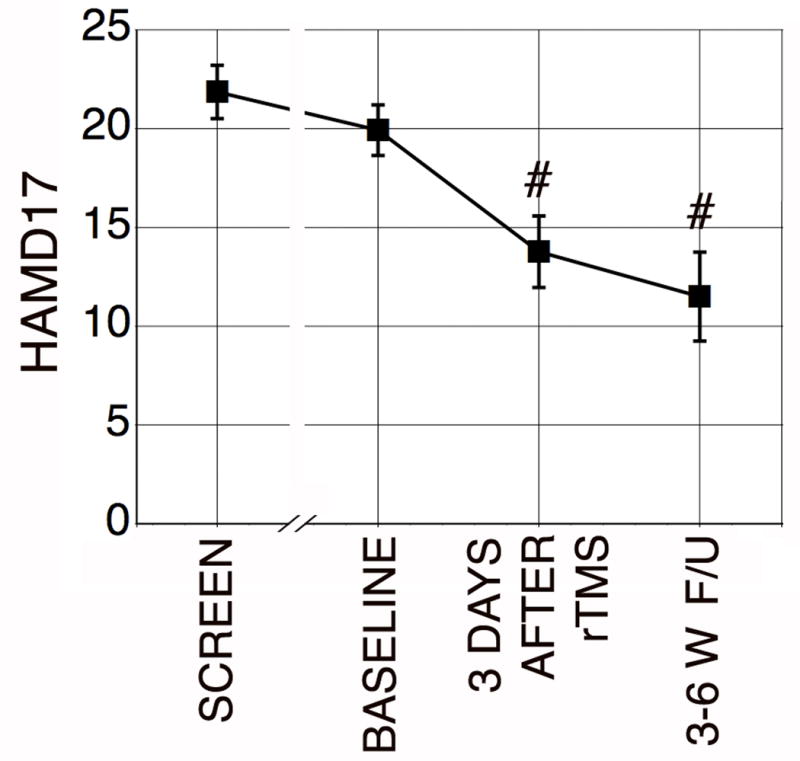
Average values of the 17-Item Hamilton Depression Score (HAMD17) from screening to final followup. #p < .005 compared to Baseline.
Improvement was also seen after treatment in HAMD21, Beck Depression Inventory (BDI), and Hamilton Anxiety Scale (HAMA). These and other significant or near-significant trends are listed in Table 2.
Table 2.
Significant (Bold) and Near-Significant Results
| Test | Before TMS | After TMS | 3-6 week F/U | |||||
|---|---|---|---|---|---|---|---|---|
| Mean/Total | S.D. | Mean/Total | S.D | p | Mean/Total | S.D. | p | |
| HAMD17 | 19.92 | 4.61 | 13.77 | 6.94 | .004* | 11.50 | 5.96 | .002* |
| HAMD21 | 22.60 | 6.04 | 14.80 | 8.18 | .01 | 11.75 | 6.46 | .004* |
| BDI | 28.60 | 8.77 | 21.30 | 7.66 | .001* | NS | NS | NS |
| HAMA | 19.67 | 10.07 | 13.67 | 8.38 | .004* | 9.31 | 6.06 | .002* |
| DRS Total | 129.75 | 12.28 | 135.38 | 9.77 | .01* | NS | NS | NS |
| Conceptualization | 32.33 | 5.74 | 34.89 | 4.94 | .025 | NS | NS | NS |
| Memory | 20.44 | 4.22 | 22.89 | 2.62 | .034 | NS | NS | NS |
| BTA | 12.75 | 5.60 | 11.12 | 5.54 | .08 ⇓ | NS | NS | NS |
| RBANS Recall | 11.00 | 7.09 | 12.7 | 5.56 | .07 | NS | NS | NS |
| UPDRS III Total | 57.36 | 19.2 | 38.91 | 19.5 | .0013* | 47.25 | 12.78 | .09 |
| UPDRS III On | 23.27 | 12.17 | 17.64 | 10.27 | .08 | NS | NS | NS |
| UPDRS III Off | 34.09 | 8.89 | 21.27 | 11.52 | .0002* | 27.38 | 5.29 | .051 |
| H&Y Total | 6.80 | 1.51 | 5.625 | 1.30 | .08 | NS | NS | NS |
| H&Y Worst | 3.55 | .82 | 3.00 | .76 | .03 | NS | NS | NS |
Significant after Bonferroni correction.
Trend towards worsening.
NS = not significant on followup.
The Brief Psychiatric Rating Scale (BPRS) did not change. Neither the total score nor any of eight dimensional sub-scores of the Parkinson’s disease Quality of Life 39-Item questionnaire (PDQ-39) showed significant change. The Clinical Global Inventory (CGI) did not change.
At 3-6 weeks HAMD-17 averaged 42% improvement in the patients who returned for follow-up (Figure 1). HAMD21 and HAMA also remained improved. The standardized effect size for HAMD17 was 0.97 three days after the end of treatment, and 1.50 for the subjects who returned at 3 to 6 weeks. No significant correlation was found between HAMD17 and the findings in other domains.
Neuropsychological Scales
Total Dementia Rating Scale (DRS) scores were improved after two weeks of treatment (Table 2), as were the subscores for Conceptualization and Memory. Improvement in the Recall section of the Repeatable Battery for Assessment of Neuropsychological Status (RBANS) represented a positive trend. No other section of the RBANS showed any change. Conversely, there was a trend towards decline in performance on the Brief Test of Attention (BTA). At 3-6 weeks no measure was different from baseline.
Parkinson’s Disease Scales
Total UPDRS motor scores after treatment were improved. Improvement was most prominent in UPDRS III total (p = .0013) and “off” (p = .0002) scores, but also present for UPDRS V in the modified Hoehn and Yahr (H&Y) “worst” rating (p = .03). Trends towards benefit were noted in UPDRS III “on” and H&Y total.
Discussion
Open rTMS treatment of PD patients with treatment-resistant depression was followed by highly significant improvement in mood scores and anxiety ratings. In participants who returned for evaluation 3-6 weeks post-treatment, improvement in psychiatric measures persisted. The standardized effect sizes of 0.97 and 1.50 for HAMD17 can be cautiously compared to a meta-analysis of left dorsolateral pre-frontal cortex (DLPFC) stimulation for pure depression, which reported a weighted mean effect size of 0.89 (Holtzheimer et al., 2003). (Note that this meta-analysis included only sham-controlled studies.) Additional improvement was found in motor and cognitive measures, but no change occurred in quality of life ratings. There were no adverse effects attributable to rTMS.
The depression results are consistent with the only previous report of rTMS treatment for depression in PD. This double-blind randomized study of rTMS vs fluoxetine (Fregni et al., 2004), found that 15 Hz rTMS over the left DLPFC had antidepressant efficacy comparable to fluoxetine, with fewer adverse effects but no changes in motor performance. Our average mood improvement is similar to that in the prior report. The combined psychiatric outcomes are encouraging, particularly considering that the patients in our study were all treatment resistant; but further confirmation is warranted as neither study included a placebo arm.
Boggio and colleagues found that both rTMS and fluoxetine produced improvement in Stroop test results, in the Hooper visual organization test, and in perseverative errors with the Wisconsin card sorting test (Boggio et al., 2005). Differences in the test batteries between that study and this make the results difficult to compare, but related indices of attention, initiation, and construction in our patients showed no change. Interestingly, however, both studies failed to find a correlation between measures of cognition and mood, reinforcing the earlier conclusion that improvement in these domains could occur independently (Boggio et al., 2005). Improvement in the DRS and the near-significant trend towards improvement in the RBANS were notable for involvement of memory function. Although the DRS may conceivably be vulnerable to practice effects, and the memory findings should be considered quite tentative, it is intriguing that the treatment area in the left DLPFC also plays a prominent role in frontal-hippocampal verbal memory systems (Floel et al., 2004).
The present study is the first to suggest simultaneous benefit from TMS in psychiatric, cognitive, and motor domains for PD patients. The motor improvement is notable for occurring most prominently in a defined ‘off” state and in the “worst” estimate for H&Y, because the “off” condition is when patients are most debilitated and the practical benefit of treatment might be largest. These results are partially consistent with the hypothesis that a focal treatment in the left prefrontal region might improve motor function along with mood. However, the degree of improvement was not correlated, so we could not infer that alleviation of depression might be the cause of improved motor and cognitive performance. Interestingly, both prefrontal TMS and placebo treatments are associated with dopamine release in the striatum, implying that relationships among different neuropsychiatric domains may be difficult to untangle even when benefit can be verified (de la Fuente Fernandez et al, (2001; Keck et al., 2002).
Several other studies of rTMS for motor function in PD have reported positive outcomes lasting for weeks or months, some without specifying medication state at the time of treatment or testing. Mally and colleagues (Mally et al., 2004) conducted an open trial of TMS for motor function in uncomplicated PD, and reported striking and sustained benefit over three years, using low, nonfocal, and infrequent doses of TMS. Several sham-controlled, blinded studies also described improvement in movement measures following TMS of motor regions, DLPFC, or both (Ikeguchi et al., 2003; Lefaucheur et al., 2004; Lomarev et al., 2005). However, Okabe and colleagues (Okabe et al., 2003), using parameters similar to those of Ikeguchi et al plus a more realistic sham, found no benefit. Most recently, Olmo and colleagues (Olmo et al., 2007) reported a double-blind sham-controlled trial of DLPFC rTMS for non-depressed patients with PD. UPDRS III “on” did not change in either group. The difference in our results may have been due simply to testing in the off state, since we also failed to find a difference in UPDRS “on” while treating more patients in an open protocol.
There are important caveats to the present results. Although lack of improvement during the delay from initial screening to treatment argues against simple regression to the mean, placebo effects are common in treatment trials of both depression and PD (Goetz et al., 2000), and the possibility of rater bias could not be prevented. The dropout rate is smaller than reported in some published trials of rTMS for pure depression (Isenberg et al., 2005), but it is possible that patient dropout might have biased the followup results, by favoring those patients who continued to experience improved mood and were more willing to make the effort to return. This hazard appears difficult to avoid if a comprehensive assessment is to be performed. It also remains possible that a subtle adverse synergy exists between depression and PD, and that improvement in other domains is more easily obtained when PD patients are treated for comorbid depression—even if a clear correlation could not be demonstrated in the data. Conversely, more prolonged treatment might have produced greater benefit, as has been observed in outpatient trials involving depression without PD (Avery et al., 2006).
Open studies such as this should always be followed by double-blind trials to confirm the apparent benefits of treatment. As a practical matter, the extensive inpatient assessment protocol was a challenge both for scheduling and for testing many of our subjects. However, the greatest improvement in motor scores occurred off PD medication. Testing during the “off” state may explain the difference between our movement results and some previous studies of TMS in PD. Further studies of TMS in PD appear worthwhile for depression, cognitive, and motor deficits, and should be designed to capture comprehensive measures of movement performance.
Acknowledgments
Supported by R01 AT000610-03 from the National Center for Complementary and Alternative Medicine; NCRR MO1-RR00039 from the National Center for Research Resources for the Emory Hospital General Clinical Research Center; and C2650C for the Veterans Administration Center for Excellence in Rehabilitation, Atlanta.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Avery D, Holtzheimer PE, Fawaz W, Russo J, Neumaier J, Dunner D, Haynor DR, Claypoole KH, Wajdik C, Roy-Byrne P. A controlled study of repetitive transcranial magnetic stimulation in medication-resistant major depression. Biol Psychiatry. 2006;59:187–194. doi: 10.1016/j.biopsych.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Beck AT. The Diagnosis and Management of Depression. Philadelphia: University of Pennsylvania; 1973. [Google Scholar]
- Benito-Leon J, Bermejo-Pareja F, Rodriguez J, Molina J-A, Gabriel R, Morales J-M. Prevalence of PD and Other Types of Parkinsonism in Three Elderly Populations of Central Spain. Mov Disord. 2003;18:267–274. doi: 10.1002/mds.10362. [DOI] [PubMed] [Google Scholar]
- Berman R, Narasimhan M, Sanacora G, Miano AP, Hoffman RE, Hu XS, Charney DS, Boutros NN. A randomized clinical trial of repetitive transcranial magnetic stimulation in the treatment of major depression. Biol Psychiatry. 2000;47:332–337. doi: 10.1016/s0006-3223(99)00243-7. [DOI] [PubMed] [Google Scholar]
- Boggio P, Fregni F, Bermpohl F, Mansur C, Rosa M, Rumi DO, Barbosa ER, Odebrecht Rosa M, Pascual-Leone A, Rigonatti SP, Marcolin MA, Araujo Silva MT. Effect of repetitive TMS and fluoxetine on cognitive function in patients with Parkinson’s disease and concurrent depression. Mov Disord. 2005;20:1178–1184. doi: 10.1002/mds.20508. [DOI] [PubMed] [Google Scholar]
- Epstein C, Davey K. Iron-core coils for transcranial magnetic stimulation. J Clin Neurophysiol. 2002;19:376–381. doi: 10.1097/00004691-200208000-00010. [DOI] [PubMed] [Google Scholar]
- Epstein C, Figiel GS, M WM, Amazon-Leece J, Figiel L. Rapid-rate transcranial magnetic stimulation in young and middle-aged refractory depressed patients. Psychiatric Annals. 1998;28:36–39. doi: 10.1176/jnp.10.1.20. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton R. Members of the UPDRS Development Committee. The UPDRS. In: Fahn S, Marsden C, Calne D, Goldstein M, editors. Recent Developments in Parkinson’s Disease. Vol. 2. Florham Park, NJ: Macmillan Health Care Information; 1987. pp. 153–163.pp. 293–304. [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured clinical interview for DSM-IV axis I disorders - patient edition. 1995 [Google Scholar]
- Floel A, Poeppel D, Buffalo E, Braun A, Wu C, Seo HJ, Stefan K, Knecht S, Cohen LG. Prefrontal cortex asymmetry for memory encoding of words and abstract shapes. Cereb Cortex. 2004:404–409. doi: 10.1093/cercor/bhh002. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatric Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fregni F, Santos C, Myczkowski ML, Rigolino R, Gallucci-Neto J, Barbosa ER, Valente KD, Pascual-Leone A, Marcolin MA. Repetitive transcranial magnetic stimulation is as effective as fluoxetine in the treatment of depression in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2004;75:1171–1174. doi: 10.1136/jnnp.2003.027060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente-Fernandez R, Ruth T, Sossi V, Schulzer M, Calne D, Stoessl A. Expectation and dopamine release: Mechanism of the placebo effect in Parkinson’s disease. Science. 2001;293:1164–1166. doi: 10.1126/science.1060937. [DOI] [PubMed] [Google Scholar]
- Gelb D, Oliver E, Gilman S. Diagnostic Criteria for Parkinson Disease. Arch Neurol. 1999;56:33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- George MS, Wassermann EM, Kimbrell TA, Little JT, Williams WE, Danielson AL, Greenberg BD, Hallett M, Post RM. Mood improvement following daily left prefrontal repetitive transcranial magnetic stimulation in patients with depression: a placebo-controlled crossover trial. Am J Psychiatry. 1997;154:1752–6. doi: 10.1176/ajp.154.12.1752. [DOI] [PubMed] [Google Scholar]
- George MS, Wassermann EM, Williams WA, Callahan A, Ketter TA, Basser PJ, Hallett M, Post RM. Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport. 1995;6:1853–6. doi: 10.1097/00001756-199510020-00008. [DOI] [PubMed] [Google Scholar]
- Goetz C, Leurgans S, Raman R, Stebbins G. Objective changes in motor function during placebo treatment in PD. Neurology. 2000;54:710–714. doi: 10.1212/wnl.54.3.710. [DOI] [PubMed] [Google Scholar]
- Guy W. Alcohol, Drug Abuse and Mental Health Administration 76-338, NIHM. Rockville: US Department of Health Education and Welfare, Public Health Service; 1976. Assessment Manual for Psychopharmacology; pp. 218–222. [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Br J Med Psychiatry. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychology. 1967;6:278–96. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Holtzheimer P, Russo J, Avery D. A meta-analysis of repetitive transcranial magnetic stimulation in the treatment of depression. Psychopharmacol Bull. 2003:37. [PubMed] [Google Scholar]
- Ikeguchi M, Tougea T, Nishiyama Y, Takeuchi H, Kuriyama S, Ohkawa M. Effects of successive repetitive transcranial magnetic stimulation on motor performances and brain perfusion in idiopathic Parkinson’s disease. J Neurol Sci. 2003;209:41–46. doi: 10.1016/s0022-510x(02)00459-8. [DOI] [PubMed] [Google Scholar]
- Isenberg K, Downs D, Pierce K, Svarakic D, Garcia K, Jarvis M, North C, Kormos TC. Low frequency rTMS stimulation of the right frontal cortex is as effective as high frequency rTMS stimulation of the left frontal cortex for antidepressant-free, treatment-resistant depressed patients. Ann Clin Psychiatry. 2005;17:153–159. doi: 10.1080/10401230591002110. [DOI] [PubMed] [Google Scholar]
- Jeanneau A. Electroconvulsive therapy in the treatment of Parkinson disease. Encephale. 1993;19:573–8. [PubMed] [Google Scholar]
- Keck ME, Welt T, M MB, Erhardt A, O F, Toschi N, Holsboer F, Sillaber I. Repetitive transcranial magnetic stimulation increases the release of dopamine in the mesolimbic and mesostriatal system. Neuropharmacology. 2002;43:101–9. doi: 10.1016/s0028-3908(02)00069-2. [DOI] [PubMed] [Google Scholar]
- Grunhaus L, Schreiber S, Dolberg OT, Polak D, Dannon PN. A randomized controlled comparison of electroconvulsive therapy and repetitive transcranial magnetic stimulation in severe and resistant nonpsychotic major depression. Biol Psychiatry. 2003;53:324–331. doi: 10.1016/s0006-3223(02)01499-3. [DOI] [PubMed] [Google Scholar]
- Lefaucheur J-P, Drouota X, Raison FV, Menard-Lefaucheur I, P PC, Nguyen J-P. Improvement of motor performance and modulation of cortical excitability by repetitive transcranial magnetic stimulation of the motor cortex in Parkinson’s disease. Clin Neurophysiol. 2004;115:2530–2541. doi: 10.1016/j.clinph.2004.05.025. [DOI] [PubMed] [Google Scholar]
- Lomarev M, Kanchana S, Bara-Jimenez W, Iyer M, Wassermann E, Hallett M. Placebo-controlled study of rTMS for the treatment of Parkinson’s disease. Mov Disord. 2005;21:325–331. doi: 10.1002/mds.20713. [DOI] [PubMed] [Google Scholar]
- Mally J, Farkas R, Tothfalusi L, Stone T. Long-term follow-up study with repetitive transcranial magnetic stimulation (rTMS) in Parkinson’s disease. Brain Res Bull. 2004;64:259–263. doi: 10.1016/j.brainresbull.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Mattis S. Dementia Rating Scale. Odessa, Florida: Psychological Assessment Resources; 1988. [Google Scholar]
- Okabe S, Ugawa Y, Kanazawa I. 0.2-Hz Repetitive Transcranial Magnetic Stimulation Has No Add-On Effects as Compared to a Realistic Sham Stimulation in Parkinson’s Disease. Mov Disord. 2003;18:382–388. doi: 10.1002/mds.10370. [DOI] [PubMed] [Google Scholar]
- Olmo M, Bello O, Cudiero J. Transcranial magnetic stimulation over dorsolateral prefrontal cortex in Parkinson’s disease. Clin Neurophysiol. 2007;118:131–139. doi: 10.1016/j.clinph.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Overall J, Gorham D. The brief psychiatric rating scale. Psychol Reports. 1962;10:799–812. [Google Scholar]
- Peto V, Jenkinson C, Fitzpatrick R. PDQ-39: a review of the development, validation and application of a Parkinson’s disease quality of life questionnaire and its associated measures. J Neurol. 1998;245(Suppl1):S10–S14. doi: 10.1007/pl00007730. [DOI] [PubMed] [Google Scholar]
- Pridmore S, Fernandes Filho JA, Nahas Z, Liberatos C, George MS. Motor threshold in transcranial magnetic stimulation: a comparison of a neurophysiological method and a visualization of movement method. J ECT. 1998;14:25–7. [PubMed] [Google Scholar]
- Pridmore S, Yeo PT, Pasha MI. Electroconvulsive therapy for the physical signs of Parkinson’s disease without depressive disorder. J Neurol, Neurosurg Psychiatry. 1995;58:641–2. doi: 10.1136/jnnp.58.5.641-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph C. Repeatable Battery for the Assessment of Neuropsychological Status (Manual) San Antonio: Psychological Corporation; 1995. [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijevi MR, Hallett M, Katayama Y, Lücking CH, Maertens de Noordhout AL, Marsden CD, Murray NMF, Rothwell JC, Swash M, Tomberg C. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroenceph Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Schretlen D. Brief test of attention. Lutz, Florida: Psychological Assessment Resources, Inc.; 1989. [Google Scholar]
- Tandberg E, Larsen J, Aarsland D, Cummings J. The occurrence of depression in Parkinson’s disease. A community-based study. Arch Neurol. 1996;53:175–179. doi: 10.1001/archneur.1996.00550020087019. [DOI] [PubMed] [Google Scholar]
- Ward C, Stern GM, Pratt RT, McKenna P. Electroconvulsive therapy in Parkinsonian patients with the ‘on-off’ syndrome. J Neural Transmission. 1980;49:133–5. doi: 10.1007/BF01249195. [DOI] [PubMed] [Google Scholar]
- Wengel SP, Burke WJ, Pfeiffer RF, Roccaforte WH, Paige SR. Maintenance electroconvulsive therapy for intractable Parkinson’s disease. Am J Ger Psychiatry. 1998;6:263–9. [PubMed] [Google Scholar]


