Abstract
Food deprivation triggers a constellation of physiological and behavioral changes including increases in peripherally-produced ghrelin and centrally-produced agouti-related protein (AgRP). Upon refeeding, food intake is increased in most species, however hamsters primarily increase food hoarding. Food deprivation-induced increases in food hoarding by Siberian hamsters are mimicked by peripheral ghrelin and central AgRP injections. Because food deprivation stimulates ghrelin as well as AgRP synthesis/release, food deprivation-induced increases in hoarding may be mediated by melanocortin 3 or 4 receptor (MC3/4-R) antagonism via AgRP, the MC3/4-R inverse agonist. Therefore, we asked: Can a MC3/4-R agonist block food deprivation- or ghrelin-induced increases in foraging, food hoarding and food intake? This was accomplished by injecting melanotan II (MTII), a synthetic MC3/4-R agonist, into the 3rd ventricle in food deprived, fed or peripheral ghrelin injected hamsters and housed in a running wheel-based food delivery foraging system. Three foraging conditions were used: a) no running wheel access, non-contingent food, b) running wheel access, non-contingent or c) a foraging requirement for food (10 revolutions/pellet). Food deprivation was a more potent stimulator of foraging and hoarding than ghrelin. Concurrent injections of MTII completely blocked food deprivation- and ghrelin-induced increases in food intake and attenuated, but did not always completely block, food deprivation- and ghrelin-induced increases in food hoarding. Collectively, these data suggest that the MC3/4-R are involved in ghrelin- and food deprivation-induced increases in food intake, but other neurochemical systems, such as previously demonstrated with neuropeptide Y, also are involved in increases in food hoarding as well as foraging.
Keywords: fasting, foraging, melanotan II, hamster, Siberian hamster, intracerebroventricular
INTRODUCTION
Determining the physiological factors that regulate ingestive behavior is critical to understanding the etiology of obesity. Ingestive behavior, as with other goal-oriented behaviors, occurs in two phases: 1) the actual eating of the food or the consummatory phase and 2) the acquisition and storage of food or the appetitive phase (Craig, 1918). The consummatory aspects of ingestive behavior have received most of the attention in the quest to understand the mechanisms underlying food intake. As for the appetitive phase of ingestive behavior, however, there is comparatively little known about the mechanisms underlying these behaviors, which is surprising, given its pervasive nature across animal taxa (for review see: (Illius et al., 2002)). Food hoarding, the storage of food for later ingestion, has widespread expression among animal species (for review see (Vander Wall, 1990)), but the mechanisms underlying this appetitive ingestive behavior have received little attention compared with food intake (for review see: (Bartness and Day, 2003)). Perhaps the lack of attention to the appetitive phase of ingestive behavior is due to the difficulty in conducting field studies of hoarding or the problem of creating a laboratory-based analog of this behavior.
Siberian hamsters (Phodopus sungorus) and other hamster species (for review see: (Bartness and Demas, 2004)) primarily increase foraging (Day and Bartness, 2003;Bartness and Day, 2003) and food hoarding (Bartness and Clein, 1994;Wood and Bartness, 1996;Bartness, 1997) in response to energetic challenges, rather than food intake as with laboratory rats and mice (for review see: (Bartness and Day, 2003)). Siberian hamsters and other animals that have the capacity to transport significant amounts of food (for review see: (Vander Wall, 1990)) use food hoarding as a crucial part of their ingestive behavioral repertoire in response to many naturally occurring energetic challenges (e.g., pregnancy, lactation (Bartness, 1997;Bartness and Day, 2003); for review see: (Bartness and Day, 2003)).
Another naturally occurring energetic challenge is decreased food availability and in its extreme, food deprivation, a condition that triggers changes in a plethora of peripheral metabolism alterations, peripheral signaling peptides and central neurochemicals (for reviews see: (Newsholme and Leech, 1983;Konturek et al., 2004)). Upon refeeding, there are marked increases in appetitive ingestive behaviors in Siberian hamsters, with relatively minor changes in food intake (Bartness and Clein, 1994;Wood and Bartness, 1996;Bartness, 1997;Day and Bartness, 2003). The exact mechanisms underlying these food deprivation-induced increases in appetitive ingestive behaviors are unknown, but there are increases in peripheral and central peptide synthesis/release implicated in the stimulation of food intake that are associated with food deprivation in these and other animals. For example, food deprivation triggers increases in circulating concentrations of the largely stomach-derived peptide ghrelin in Siberian hamsters (Keen-Rhinehart and Bartness, 2005), as it does in laboratory rats (e.g., (Tschop et al., 2000;Sun et al., 2003)). In addition, peripherally administered ghrelin that creates 24–48 h food deprivation-like plasma active ghrelin concentrations markedly stimulates food hoarding and, to a lesser degree, food intake in Siberian hamsters (Keen-Rhinehart and Bartness, 2005). Food deprivation also increases arcuate nucleus gene expression of the orexigenic peptides neuropeptide Y (NPY) and agouti-related peptide (AgRP) in Siberian hamsters (Mercer et al., 1995;Mercer et al., 2000), as it does in laboratory rats and mice (e.g., (Brady et al., 1990;Kim et al., 1998;Mizuno et al., 1999;Mizuno and Mobbs, 1999)). When AgRP (Day and Bartness, 2004)), NPY (Day et al., 2005)) or a NPY Y1 receptor agonist ([Pro34]NPY; (Day et al., 2005)) are administered centrally to Siberian hamsters, food hoarding strikingly increases whereas food intake minimally or does not increase. Thus, food deprivation increases circulating ghrelin concentrations (Tschop et al., 2000;Sun et al., 2003;Keen-Rhinehart and Bartness, 2005) that, in turn, stimulate NPY/AgRP-producing arcuate neurons (e.g., (Guan et al., 1997;Guan et al., 2003;Kohno et al., 2003;Seoane et al., 2003)) and presumably NPY/AgRP synthesis/release (Wren et al., 2002) finally acting on melanocortin 3 and 4 receptors (MC3/4-R) in the hypothalamic paraventricular nucleus and other areas. Therefore, antagonism of these downstream MC3/4-Rs would appear to at least partly underlie food deprivation- and ghrelin-induced increases in appetitive ingestive behaviors. Therefore, we asked: Can an MC3/4-R agonist (melanotan II [MTII]) block food deprivation- or ghrelin-induced increases in foraging and food hoarding? This was accomplished by attempting to block food deprivation- and peripheral ghrelin-induced increases in foraging and food hoarding by injecting MTII into the third ventricle of food deprived, fed or ghrelin-injected hamsters housed in a running wheelbased food delivery foraging system that is coupled with simulated burrow-housing.
METHODS
Animals
All procedures were approved by the Georgia State University Institutional Animal Care and Use Committee and are in accordance with Public Health Service and United States Department of Agriculture guidelines. Adult male Siberian hamsters, ~3.5 months old and weighing 35–43 g were obtained from our breeding colony. The lineage of this colony has been described recently (Bowers et al., 2004). Hamsters were group-housed and raised in a long-day photoperiod (16:8 light: dark; lights on at 0200h) from birth. Room temperature was maintained at 21±2.0 °C.
Hamsters were acclimated for two weeks in specially designed hoarding apparatuses as previously described (Day and Bartness, 2001;Day et al., 2005) that would serve as their housing for the duration of the experiment.. More specifically, two cages were connected with a convoluted polyvinylchloride tubing system (38.1 mm id. and ~1.52 m long) with corners and straightways for horizontal and vertical climbs. The diet (75 mg pellets: Purified Rodent Diet; Research Diets, New Brunswick, NJ) and tap water were available ad libitum. A running wheel (524 mm circumference) and pellet dispenser were attached to the food cage (top). Wheel revolutions were counted using a magnetic detection system and monitored by a computer based hardware-software program (Med Associates, Lancaster, NH). Hamsters were first trained in these apparatuses (Day et al., 2005;Keen-Rhinehart and Bartness, 2005) and then received a third ventricular cannula both previously described (Day and Bartness, 2004;Day et al., 2005) and described in brief below.
Foraging Training Regimen
We used a wheel-running training regimen that eases the hamsters into their foraging efforts without large changes in body mass or food intake (Day and Bartness, 2001). Specifically, hamsters were given free access to the food pellets for 2 d while they adapted to the running wheel. In addition to the free food, a 75 mg food pellet was dispensed upon completion of every 10 wheel revolutions. On the third day, the free food condition was replaced by a response-contingent condition where only every 10 wheel revolutions triggered the delivery of a food pellet. This condition was in effect for 5 d during which time body mass, food intake, food hoarding, wheel revolutions and pellets earned (foraging) were measured daily. At the end of this acclimation period (7 d total), all animals were removed from the foraging apparatuses and temporarily housed in shoebox cages where the same food pellets were available ad libitum with no foraging requirements. Guide cannulae were then stereotaxically implanted in these hamsters (see below for details). Following a one week post surgical recovery period, all hamsters were returned to the hoarding/foraging apparatus and retrained to the following schedule: 2 d for adaptation with free access to food pellets followed by 5 d at 10 revolutions/pellet. Hamsters remained in the hoarding/foraging apparatus for the remainder of the experiment.
Cannula Implantation
Cannulae were stereotaxically implanted into the third ventricle as described previously (Day and Bartness, 2004). Briefly, the animals were anesthetized with isoflurane and the fur at the top of the head was removed to expose the area to be incised. A hole was trephined at the intersection of bregma and the midsaggital sinus and the guide cannula (26 gauge stainless steel; Plastics One, Roanoke, VA) was lowered using the following stereotaxic coordinates (level skull, anterior-posterior from bregma 0, medial-lateral from midsaggital sinus 0, and dorsal-ventral from the top of the skull −5.0 mm) targeted for placement just above the third ventricle. The guide cannula was secured to the skull using cyanoacrylate ester gel, 3/16 mm jeweler’s screws and dental acrylic. A removable obturator sealed the opening in the guide cannula throughout the experiment except when it was removed for the injections. Hamsters received 0.2 mg/kg buprenorphine at 12 and 24 h post-surgery to minimize discomfort, were given fresh apple bits to encourage food/fluid intake and subsequently were allowed one week to recover fully in the shoebox cage housing before being returned to their simulated burrow housing.
Cannulae Verification
Following the last test, an injection of 0.4 μl of bromophenol blue dye was given to confirm placement of the cannula in the third ventricle. The animals were killed with an overdose of pentobarbital sodium (75 mg/kg), their brains removed and then postfixed in 10% paraformaldehyde for a minimum of two d. Each brain was sliced manually for cannula verification. Cannulae were considered to be located in the third ventricle if the dye was visible in any part of this ventricle. Only the data from animals with confirmed third ventricle cannulae placements were included in the analyses, and there was no incidence of cannula loss during the study.
Intracerebroventricular Injection Protocol
The inner cannula (33 gauge stainless steel, Plastics One, Roanoke, VA) extended 5.5 mm below the top of the skull and all hamsters were injected with a 0.4 μl volume. All injections were given at the beginning of the dark phase of the photoperiod. Animals were lightly restrained by hand during the 30 s injection and the injection needle remained in place ~30 s before withdrawal to minimize injectate reflux, according to our previous procedure (Day and Bartness, 2004;Day et al., 2005;Keen-Rhinehart E. and Bartness, 2007).
Experimental Design
At the end of the 7 d retraining period, the hamsters were separated into three groups (n=8/group) matched for their current body mass and average hoard size across these last 3 d of training while at 10 revolutions/pellet. The three groups consisted of: 10 revolutions/pellet foraging requirement (10 Revolutions/pellet group), no foraging requirement with an active running wheel (Free Wheel; exercise control group) or no foraging requirement with a blocked wheel (Blocked Wheel; sedentary control group) where each of the last two groups had food available non-contingently. For Experiment 1, each group received all drug combinations given in a counterbalanced schedule to control for possible order effects of peptide administration (see below for details). For Experiment 2, each group was observed during a baseline ad libitum, saline-treated period that was followed by 48 h of food deprivation, and behavioral measurements were then performed under either saline or MTII treatment and compared with the baseline measurements (see below for details).
Measurement of Foraging, Food Hoarding and Food Intake
Foraging (pellets earned) was defined as the number of pellets delivered upon completion of the requisite wheel revolutions. Food hoarding (pellets hoarded) was defined as the number of pellets found in the bottom ‘burrow’ cage in addition to those removed from the cheek pouches. For the 10 revolutions/pellet groups, food intake (pellets eaten) was defined as: pellets earned –surplus pellets – hoarded pellets = food intake. For the Free and Blocked Wheel groups, food intake (pellets eaten) was defined as: pellets given – pellets left in the top cage – hoarded pellets = food intake. An electronic balance used to weigh the food pellets was set to ‘parts’ measurement rather then obtaining fractions of a pellet in mg; thus one 75 mg food pellet = 1.
Experiment 1: Does MTII inhibit ghrelin-induced stimulation of appetitive and consummatory ingestive behaviors in Siberian hamsters?
A within-subjects design was chosen to minimize variability; therefore, all animals received all of the following possible injection combinations: i.p. ghrelin (30 μg/kg, Bachem Biosciences, King of Prussia, PA) + MTII (2.5 nmol, Phoenix Pharmaceuticals, Belmont, CA), i.p. saline + icv MTII (2.5 nmol), i.p. ghrelin (30 μg/kg) + icv saline, i.p. saline + icv saline. This dose of ghrelin was chosen because it results in plasma active ghrelin concentrations within the physiological range of food deprivation for 12–48 h in Siberian hamsters (Keen-Rhinehart and Bartness, 2005). Because there were no carry-over effects of ghrelin at this dose for any behavior beyond 7 d (Keen-Rhinehart and Bartness, 2005) a washout period of this length occurred between the counterbalanced injections. The dose of MTII was chosen based on 3rd ventricular injections of this melanocortin receptor agonist that inhibit food intake in this species (Schuhler et al., 2003;Schuhler et al., 2004) as well as a pilot study testing for a MTII dose that inhibited both food intake and food hoarding --the 2.5 nmol dose fulfilled this criterion. Note that because there was no significant changes in body mass for any group after any of the injections, the dose of ghrelin was kept constant.
Experiment 2: Does the MC3/4-R agonist, MTII, inhibit food deprivation-induced stimulation of appetitive and consummatory ingestive behaviors in Siberian hamsters?
Two weeks after the last injection from Experiment 1, animals were divided into two groups, re-balanced for body mass (even though there was no significant changes in body mass throughout the first experiment [data not shown]) as well as for hoarding and were then food deprived for 48 h beginning at lights-out. Half of the animals received icv MTII (2.5 nmol) and the other half received icv saline. In our previous studies of food hoarding, we have used food deprivation ranging from 12 to 56 h (IACUC approved) with the latter length appearing somewhat lengthy or ‘non-physiological’ at first blush. In the utopian conditions of the laboratory, however, Siberian hamsters are almost 50% body fat compared to as low as ~25% in nature (Weiner, 1987); therefore, short fasts in the laboratory of 12–32 h are minimally energetically challenging in these animals and thus stimulation of food hoarding is minimal (Clein and Bartness, unpublished results). Therefore, we selected 48 h food deprivation to trigger the behavior nearly maximally. It also seems reasonable to envision these fast lengths as on a physiological continuum with the inter-meal intervals occurring naturally of much shorter lengths in hamsters (~4 h (Bartness et al., 1986)).
Statistical Analyses
For all measures of food intake, foraging and food hoarding in Experiments 1 and 2, if the saline treated animals did not eat, forage (earn pellets) or hoard at a time interval (i.e., a value of ‘0’), the minimum value (1 pellet) was assigned to avoid a zero in the denominator for calculation of the percent changes from saline for these measures. In Experiment 1, each animal was given saline + saline, ghrelin + saline, MTII + saline, and ghrelin + MTII. The percent saline value was calculated by dividing each animal’s behavioral response to each of the drug conditions by that of the saline + saline control condition multiplied by 100; thus, this yields data for each animal expressed as a percentage of their own saline values. All the individual percent saline values were then averaged to generate a mean across animals for each foraging condition with associated standard errors. In Experiment 2, food intake, foraging and food hoarding were monitored during an ad libitum-feeding, saline injection baseline period and then each animal was food deprived followed by icv injection of either saline or MTII. Similar to Experiment 1, the percent saline value was calculated by dividing each animal’s behavioral response to either food deprivation + saline or food deprivation + MTII by each animal’s ad libitum saline baseline value multiplied by 100; thus, this yields data for each animal expressed as a percentage of their own saline values. Once again, all the individual percent saline values were then averaged to generate a mean across animals for each foraging condition with associated standard errors. The data for both experiments are graphed as the mean percent of saline ± SEM for food intake, food hoarding, foraging (10 revolutions/pellet group) and wheel running (Free Wheel group). Because the data are analyzed for each time interval in order to identify intervals within which any experimental effects occur, rather than cumulatively, no statistical comparisons were made across time (i.e., there was no Time factor analysis). In addition, comparisons among time intervals would be inappropriate, as the intervals are of differing durations. Therefore, the data from one time interval only are compared with data within that time interval. Data were analyzed using a repeated measures two-way ANOVA (Experiment 1: Foraging group × Drug Combination (3 × 4) at each of the 8 time periods; Experiment 2: Foraging group × Drug treatment (3 × 2) at each of the 8 time periods. Bonferroni’s post-hoc tests were used for individual pair-wise comparisons (NCSS v 2000, Kaysville, UT). Differences between means were considered statistically significant if P<0.05. Exact probabilities and test values were omitted for simplicity and clarity of the presentation of the results.
RESULTS
Experiment 1: Does MTII inhibit ghrelin-induced stimulation of appetitive and consummatory ingestive behaviors in Siberian hamsters?
Wheel Running
As seen previously (Keen-Rhinehart and Bartness, 2005), ghrelin did not stimulate wheel running activity when it was uncoupled from foraging (Free Wheel group) suggesting there was no non-specific stimulation or inhibition of locomotor activity by the peptide (data not shown).
Foraging
Ghrelin injections did not significantly stimulate foraging compared with saline (data not shown).
Food Intake
Ghrelin significantly stimulated food intake (range: ~200–450%) at 0–1 h post-injection compared with saline for the Free Wheel and Blocked Wheel groups and at 1–2, 2–4, and 4–24 h for the 10 Revolutions/pellet group (Ps<0.05; Fig. 1). MTII completely blocked ghrelin-induced increases in food intake at 0–1 h in the Blocked Wheel group, 2–4 and 4–24 h in the 10 Revolutions/pellet group (Ps<0.05; Fig. 1), but not at 1–2 h, and did not block food intake in the Free Wheel group at 0–1 h (Fig. 1). MTII alone and MTII + ghrelin treatment further decreased food intake compared with saline treatment at 4–24 h in the Blocked Wheel group, at 2–4 and 4–24 h in the Free Wheel group and at 2–4 h in the 10 Revolutions/pellet group (Ps<0.05; Fig. 1). In addition, MTII alone, but not MTII + ghrelin decreased food intake compared with saline at 1–2 h in the Free Wheel group and at 0–1 h in the 10 Revolutions/pellet group.
Figure 1.
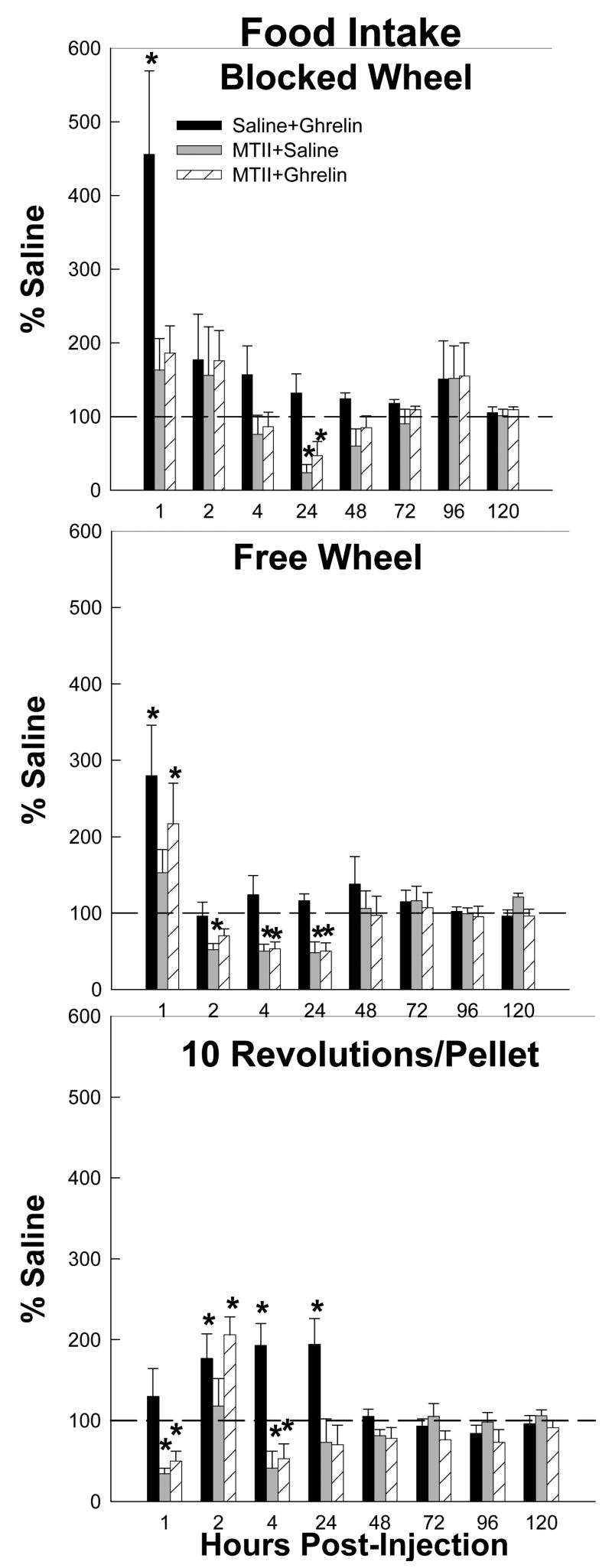
Mean ± SEM of food intake as a percentage of the intracerebroventricularly (icv) and intraperitoneally (ip) saline-injected controls (dashed reference line) for the effects of ip ghrelin treatment with icv saline (black bars), ip saline treatment with icv melanotan II (MTII; gray bars) and ip ghrelin treatment with icv MTII (striped bars) on hamsters without a foraging requirement and a stationary wheel (Blocked Wheel), hamsters with no foraging requirement and a freely moving wheel (Free Wheel) and hamsters with a foraging requirements (10 Revolutions/pellet). *=p<0.05 compared to the saline control condition
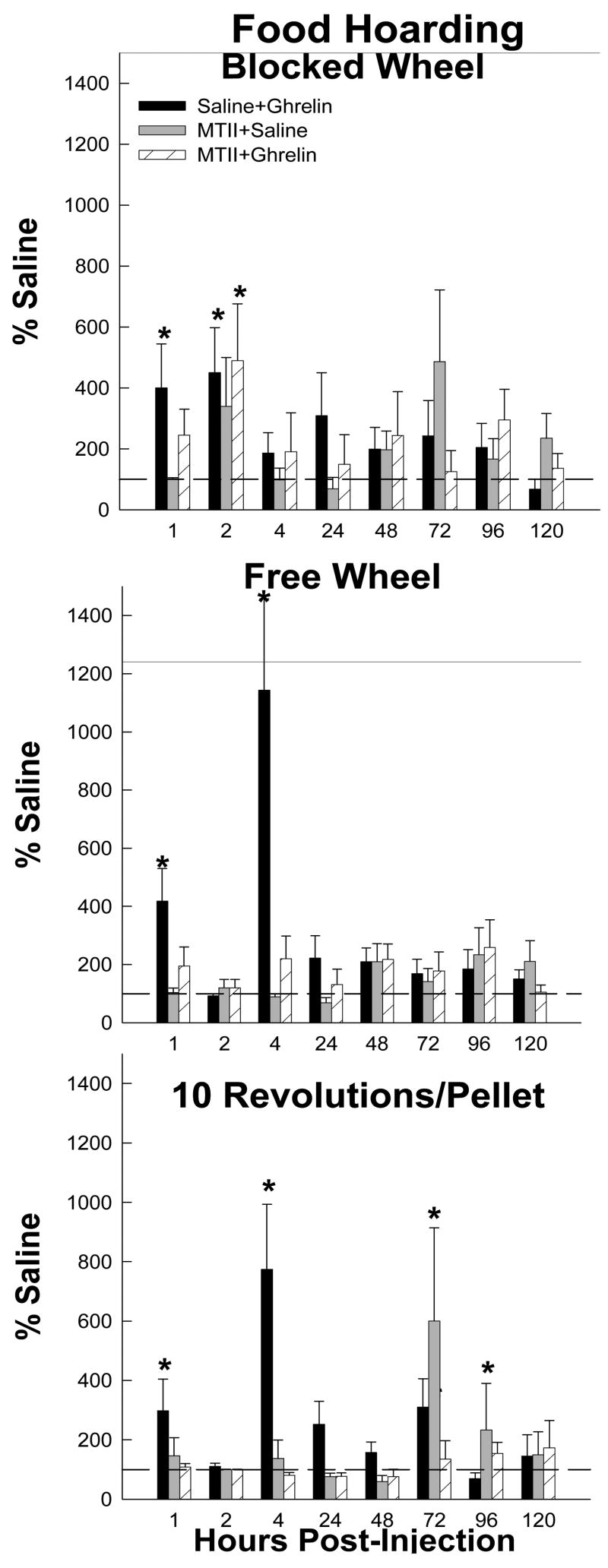
Food Hoarding
Ghrelin significantly increased hoarding (range: ~300–400%) at 0–1 h post-injection for all groups compared with saline injections (Ps<0.05; Fig. 2). Ghrelin also increased hoarding at 1–2 h in the Blocked Wheel group (400%) and at 2–4 h in the Free Wheel group (1100%; Ps<0.05; Fig. 2). In addition, ghrelin significantly increased hoarding in the 10 Revolutions/pellet group at 2–4, 4–24 and 48–72 h post-injection compared with saline (range: ~200–800%, Ps<0.05; Fig. 2).
Figure 2.
Mean ± SEM of food hoarding as a percentage of the intracerebroventricularly (icv) and intraperitoneally (ip) saline-injected controls (dashed reference line) for the effects of ip ghrelin treatment with icv saline (black bars), ip saline treatment with icv melanotan II (MTII; gray bars) and ip ghrelin treatment with icv MTII (striped bars) on hamsters without a foraging requirement and a stationary wheel (Blocked Wheel), hamsters with no foraging requirement and a freely moving wheel (Free Wheel) and hamsters with a foraging requirements (10 Revolutions/pellet). *=p<0.05 compared to the saline control condition
MTII blocked the ghrelin-induced increased hoarding at 0–1 h post injection in the Blocked Wheel group (P<0.05; Fig. 2), but not at 1–2 h. MTII completely blocked ghrelin-induced increased hoarding in the Free Wheel group at 0–1 and 2–4 h (Ps<0.05; Fig. 2). MTII also completely blocked the ghrelin-induced increased food hoarding in 10 Revolutions group at all but 48–72 h post-injection (Ps<0.05; Fig. 2) at which time MTII treatment alone unexpectedly stimulated food hoarding compared with saline (P<0.05; Fig. 2).
Experiment 2: Does the MC3/4-R agonist, MTII, inhibit food deprivation-induced stimulation of appetitive and consummatory ingestive behaviors in Siberian hamsters?
Wheel Running
Neither food deprivation nor MTII + food deprivation had any effect on wheel running (Free Wheel group; data not shown) suggesting there was no non-specific effects on locomotor activity.
Foraging
Food deprivation did not affect foraging, nor did MTII injections or their interactions (data not shown).
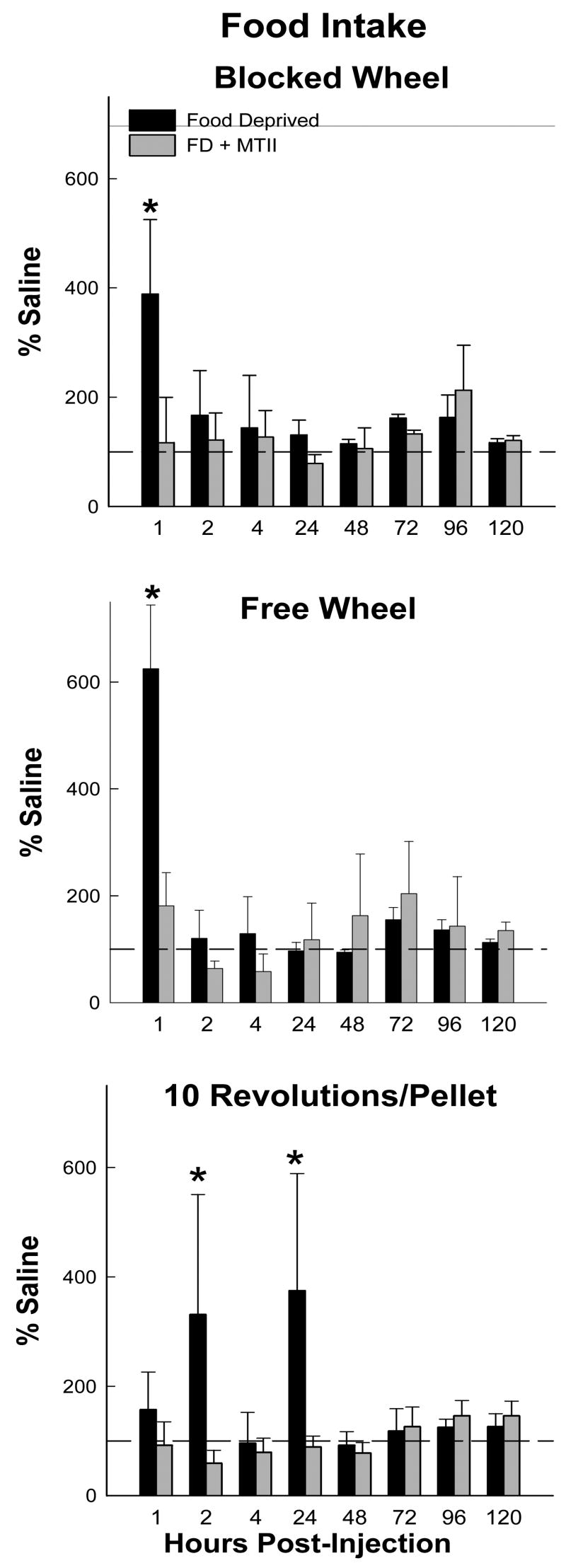
Food Intake
Food deprivation increased food intake at 0–1 h in the Blocked Wheel and Free Wheel groups and at 1–2 and 4–24 h post injection in the foraging hamsters (10 Revolutions group; range: ~300–600%; Ps<0.05; Fig. 3). These food deprivation-induced increases in food intake were smaller than the increases in food hoarding (see below). MTII blocked the food deprivation-induced increases in food intake by all groups at all time points (Fig. 3).
Figure 3.
Mean ± SEM food intake as a percentage of the saline-injected ad libitum fed control condition for the effects of food deprivation with intracerebroventricular (icv) saline injection (black bars) and food deprivation with icv melanotan II (MTII; gray bars) on hamsters without a foraging requirement and a stationary wheel (Blocked Wheel), hamsters with no foraging requirement and a freely moving wheel (Free Wheel) and hamsters with a foraging requirement (10 Revolutions/pellet) *=p<0.05 compared to saline injected ad libitum control condition.
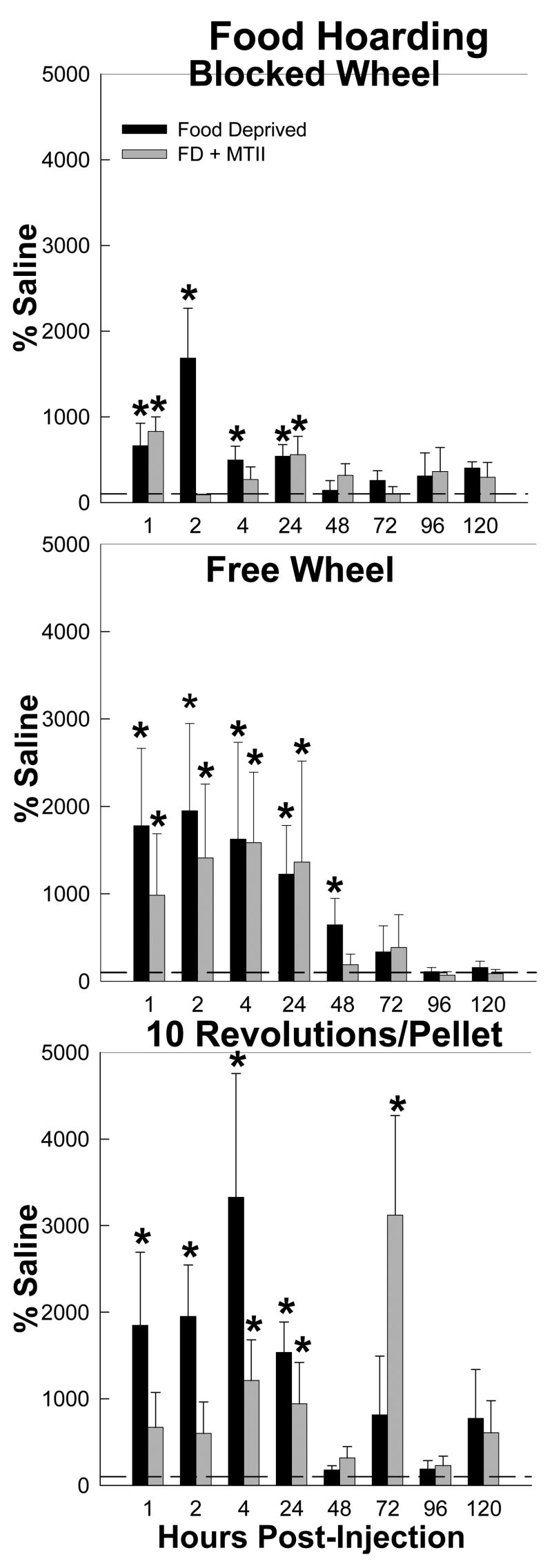
Food Hoarding
Food deprivation stimulated food hoarding in all groups compared with ad libitum-fed hamsters (range: ~1,000–3,200%; Fig. 4). Specifically, the smallest significant increase in food hoarding was ~1,000% by the Blocked Wheel hamsters compared with their ad libitum-fed counterparts at 0–1 h, with significant increases at 1–2 h, 2–4 and 4–24 h (range: ~750–1,500; Ps<0.05; Fig. 4). Food deprivation-induced increased hoarding occurred at all times through 24–48 h by the Free Wheel hamsters (range: ~750–2,000%; P<0.05; Fig. 4). The food deprivation-induced increased hoarding was most pronounced in the foraging hamsters (10 Revolutions group), with at least ~1,500–3,200% increases occurring with refeeding up to 4–24 h with a maximal increase (~3,200%) at 2–4 h (Ps<0.05; Fig. 4).
Figure 4.
Mean ± SEM food hoarding as a percentage of the saline-injected ad libitum fed control condition for the effects of food deprivation with intracerebroventricular (icv) saline injection (black bars) and food deprivation with icv melanotan II (MTII; white bars) on hamsters without a foraging requirement and a stationary wheel (Blocked Wheel), hamsters with no foraging requirement and a freely moving wheel (Free Wheel) and hamsters with a foraging requirements (10 Revolutions/pellet) *=p<0.05 compared to saline injected ad libitum fed control condition.
MTII inhibited food deprivation-induced increased hoarding during at least one time interval for all groups, but its effectiveness in doing so differed across the foraging groups and across time (Fig. 4). Specifically, this MC3/4-R agonist inhibited food deprivation-induced increased hoarding by the Free Wheel group only at 24–48 h (P<0.05; Fig. 4), the Blocked Wheel group at 1–2 and 2–4 h (Ps<0.05; Fig. 4) and the 10 Revolutions/pellet group only at 0–1 and 1–2 h (Ps<0.05; Fig. 4). Surprisingly, MTII increased food hoarding in the food deprived 10 Revolutions/pellet hamsters at 48–72 h (P<0.05; Fig. 4), the same time that it increased food hoarding in Experiment 1 (Fig. 2).
DISCUSSION
This study was predicated on the notion that the food deprivation-induced increases in appetitive (food hoarding) and consummatory (food intake) ingestive behaviors were due to food deprivation-induced increases in circulating ghrelin concentrations that, in turn, stimulated AgRP release and antagonized MC3/4 receptors. This idea is partially, but not wholly, supported by the results of the present study using peripheral ghrelin injections and food deprivation to stimulate the ingestive behaviors, and the MC3/4-R agonist, MTII, to block these responses. Ghrelin-induced increases in food intake appear to act through the MC3/4-Rs in laboratory rats (Kamegai et al., 2000;Kamegai et al., 2001;Nakazato et al., 2001), and in the present study ghrelin-induced increased food intake was blocked 50% of the time across the various foraging conditions. The ghrelin-induced increased food hoarding was more consistently blocked by MTII than the ghrelin-induced increased food intake. Food deprivation-induced increases in food intake, as with ghrelin, appear to act at least partially through MC3/4-R in laboratory rats (e.g., (Kas et al., 2003;de Rijke et al., 2005) and, in the present study, the few times food intake was elevated post food deprivation, MTII consistently blocked this increase. Unlike the ability of MTII to block the effects of ghrelin on food hoarding, MTII was only able to attenuate fasting-induced increases in food hoarding. These data support the idea that food deprivation-induced increases in circulating ghrelin concentrations increase AgRP antagonism of the MC3/4-Rs.
We previously demonstrated that antagonism (inverse agonism) of MC3/4-R by intracerebroventricularly injected AgRP stimulates foraging and especially food hoarding, with a significantly smaller stimulation of food intake (Day and Bartness, 2004). Consistent with the notion of involvement of MC3/4-Rs in these appetitive and consummatory ingestive behaviors is the inhibition of AgRP-triggered increase in food hoarding by prior injection of MTII (Day and Bartness, unpublished observations). Therefore, it might be expected that MC3/4-R agonism would block or at least partially inhibit, food deprivation- and ghrelin-induced increases in these ingestive behaviors in the present experiments; indeed, this was found lending further credence to the involvement of the melanocortin system in the control of food hoarding and food intake.
The effects of ghrelin on food intake were relatively short-lived in the present experiment being only significantly elevated 0–1 h post-injection, except for the hamsters foraging for their food (10 Rev Group), where food intake also was significantly increased 1–2, 2–4 and 4–24 h post injection. These data are consistent with our initial study of ghrelin effects on ingestive behaviors in this species (Keen-Rhinehart and Bartness, 2005) and the relatively early post-injection stimulation of food intake by ghrelin in laboratory rats (Wren et al., 2000;Kamegai et al., 2000;Tschop et al., 2000) and mice (Tschop et al., 2000;Asakawa et al., 2001).
MTII was effective in blocking the sporadic food deprivation-induced increases in food intake for all groups when they occurred. These data support the findings in laboratory rats and mice where this MC3/4-R agonist blocks food deprivation- (Murphy et al., 1998;Vergoni and Bertolini, 2000;Raposinho et al., 2003) and ghrelin- (Shrestha et al., 2004) induced increases in food intake. MTII was partially effective in blocking food deprivation- or ghrelin-induced increased food hoarding and this ability was highly dependent on the foraging effort. Specifically, food deprivation-induced increased food hoarding was abolished for the Blocked Wheel group at two of four intervals where it was increased (2–4 and 4–24 h, but not at 0–1 or 4–24h), at only one of the five intervals where it was increased by Free Wheel hamsters (24–48 h), and at two out of four intervals of increased hoarding by the 10 Revolutions/pellet hamsters (0–1 and 1–2 h, but not at 2–4 or 4–24h). By contrast, MTII was relatively effective in blocking ghrelin-induced increased food hoarding, regardless of the foraging effort. Specifically, MTII failed to block ghrelin-induced increased hoarding at only one of two intervals or increased hoarding for the Blocked Wheel group (1–2 h) and one of four intervals for the 10 Revolution/pellet group (48–72 h). The protracted elevation of food hoarding by the 10 Revolutions group (48–72 h) suggests a post-receptor mechanism, given that we found an absence of increased circulating active ghrelin 4–24 h post injection after administering the same dose of ghrelin in this species previously (Keen-Rhinehart and Bartness, 2005). Because ghrelin activates arcuate AgRP neurons (Lawrence et al., 2002) and increases its gene and protein expression (Kamegai et al., 2000;Kamegai et al., 2001), and because we previously have found that third ventricularly administered AgRP stimulates food hoarding 2–5 d post injection in Siberian hamsters (Day and Bartness, 2004), as it does for food intake in laboratory rats (Hagan et al., 2000;Lu et al., 2001), AgRP is likely to be the mediator of the persisting effects of ghrelin on food hoarding (Keen-Rhinehart and Bartness, 2005).
These interesting, albeit sometimes complicated findings, suggest several points. The foraging effort-dependent effects of peptidergic control of food hoarding is a frequent finding in our studies of food hoarding stimulation by third ventricular injection of NPY or a Y1 or Y5 agonist (Day et al., 2005), or AgRP (Day and Bartness, 2004) or systemic injection of ghrelin (Keen-Rhinehart and Bartness, 2005). The existence of peptide effects on ingestive behavior that are dependent on the presence of an appetitive behavioral component has been reported previously. For example, icv NPY stimulates consumption of sucrose when an animal has to move to a sipper tube to drink (eat), but does not do so when the sucrose is delivered via an intraoral catheter (no appetitive response; (Seeley et al., 1995)). Food intake after food deprivation or ghrelin injections in the present study, and after icv NPY (Day et al., 2005) or AgRP (Day and Bartness, 2004) in our previous studies using this model, are maximal when animals are foraging for their food (10 revolutions/pellet). In laboratory rats, if the appetitive effort (bar pressing requirement) is too energetically demanding, the ability of icv NPY to stimulate food intake is less than when food is freely available (Jewett et al., 1992). These types of effects add a cautionary note to the interpretation of the roles of peptides, and likely other neurochemicals, in ingestive behaviors suggesting their effects are highly modifiable by the testing environment in the laboratory and therefore also likely highly modifiable by the environment in nature. The neural sites where MC3/4-R was acting in the present experiment are unknown and only grossly suggested by the third ventricular injections as being in the periventricular hypothalamic, midbrain and/or brainstem structures.
These studies indicate that both food deprivation- and ghrelin-induced increases in food hoarding can be attenuated by MTII treatment. In addition, when MTII blocked food deprivation- and ghrelin-induced increases in food hoarding in hamsters foraging for their food (10 Revolutions/pellet group), they significantly increased their food hoarding later 48–72 h after refeeding. These results indicate that MTII may not have eliminated the internal stimuli that led to the increased food hoarding by food deprivation or ghrelin injection, but instead delayed the expression of the response until MTII was no longer affecting the relevant circuits underlying food hoarding. This result and interpretation requires further study.
Collectively, the results of this study combined with known relations among food deprivation, ghrelin, NPY/AgRP arcuate nucleus neurons and food hoarding reviewed above suggest the following scenario. First, food deprivation induces increases in plasma active ghrelin (Toshinai et al., 2001;Ariyasu et al., 2001;Keen-Rhinehart and Bartness, 2005) that, in turn, stimulates arcuate nucleus NPY/AgRP synthesizing neurons (Kamegai et al., 2001;Wang et al., 2002;Cowley et al., 2003). Release of AgRP and its action at MC3/4 receptor in the hypothalamus, and perhaps other areas, stimulates circuits underlying the post-food deprivation increases in food hoarding, foraging and food intake in this species (Bartness and Clein, 1994;Wood and Bartness, 1996;Bartness, 1997;Day and Bartness, 2003). The persisting stimulation of food hoarding is likely due to AgRP-activated circuits, given that NPY administration does not produce long-lasting (>24 h) increases in food hoarding (Day et al., 2005). As noted above, the triggering of these appetitive and consummatory behaviors is not this simple, but instead involves a suite of neurochemicals and a network of circuits in addition to peripheral factors, with the present data adding to this complex picture. Specifically, because MTII was only able to attenuate fasting-induced increases in food hoarding and blocked most of ghrelin-induced increases in food hoarding, these data support the notion food deprivation increases ghrelin which increases AgRP antagonism of the MC3/4-R, and that increased ghrelin cannot be considered to have equivalent behavioral/physiological effects on these appetitive ingestive behaviors as food deprivation.
Acknowledgments
The authors thank Raven Jackson, Constance Foster and Teal Pelish for their help with data collection.
Footnotes
This work was supported by the National Science Foundation IBN-9876495 and National Institutes of Health R01 DK078358 to TJB.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S, Shirakami G, Usui T, Shimatsu A, Doi K, Hosoda H, Kojima M, Kangawa K, Nakao K. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab. 2001;86:4753–4758. doi: 10.1210/jcem.86.10.7885. [DOI] [PubMed] [Google Scholar]
- 2.Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, Makino S, Fujimiya M, Niijima A, Fujino MA, Kasuga M. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2001;120:337–345. doi: 10.1053/gast.2001.22158. [DOI] [PubMed] [Google Scholar]
- 3.Bartness TJ. Food hoarding is increased by pregnancy, lactation and food deprivation in Siberian hamsters. Am J Physiol. 1997;272:R118–R125. doi: 10.1152/ajpregu.1997.272.1.R118. [DOI] [PubMed] [Google Scholar]
- 4.Bartness TJ, Clein MR. Effects of food deprivation and restriction, and metabolic blockers on food hoarding in Siberian hamsters. Am J Physiol. 1994;266:R1111–R1117. doi: 10.1152/ajpregu.1994.266.4.R1111. [DOI] [PubMed] [Google Scholar]
- 5.Bartness TJ, Day DE. Food hoarding: a quintessential anticipatory appetitive behavior. Progress in Psychobiology and Physiological Psychology. 2003;18:69–100. [Google Scholar]
- 6.Bartness TJ, Demas GE. Comparative studies of food intake: Lessons from non-traditionally studied species. In: Stricker EM, Woods SC, editors. Food and Fluid Intake. Plenum; New York: 2004. pp. 423–467. [Google Scholar]
- 7.Bartness TJ, Morley JE, Levine AS. Photoperiod-peptide interactions in the energy intake of Siberian hamsters. Peptides. 1986;7:1079–1085. doi: 10.1016/0196-9781(86)90137-3. [DOI] [PubMed] [Google Scholar]
- 8.Bowers RR, Festuccia WTL, Song CK, Shi H, Migliorini RH, Bartness TJ. Sympathetic innervation of white adipose tissue and its regulation of fat cell number. Am J Physiol. 2004;286:R1167–R1175. doi: 10.1152/ajpregu.00558.2003. [DOI] [PubMed] [Google Scholar]
- 9.Brady LS, Smith MA, Gold PW, Herkenham M. Altered expression of hypothalamic neuropeptide mRNAs in food-restricted and food-deprived rats. Neuroendocrinology. 1990;52:441–447. doi: 10.1159/000125626. [DOI] [PubMed] [Google Scholar]
- 10.Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, Horvath TL. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 11.Craig W. Appetites and aversions as constituents of instincts. Biol Bull. 1918;34:91–107. doi: 10.1073/pnas.3.12.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Day DE, Bartness TJ. Agouti-related protein increases food hoarding, but not food intake by Siberian hamsters. Am J Physiol. 2004;286:R38–R45. doi: 10.1152/ajpregu.00284.2003. [DOI] [PubMed] [Google Scholar]
- 13.Day DE, Bartness TJ. Fasting-induced increases in hoarding are dependent on the foraging effort level. Physiol Behav. 2003;78:655–668. doi: 10.1016/s0031-9384(03)00052-0. [DOI] [PubMed] [Google Scholar]
- 14.Day DE, Bartness TJ. Effects of foraging effort on body fat and food hoarding by Siberian hamsters. J Exp Zool. 2001;289:162–171. [PubMed] [Google Scholar]
- 15.Day DE, Keen-Rhinehart E, Bartness TJ. Role of NPY and its receptor subtypes in foraging, food hoarding and food intake by Siberian hamsters. Am J Physiol. 2005;289:R29–R36. doi: 10.1152/ajpregu.00853.2004. [DOI] [PubMed] [Google Scholar]
- 16.de Rijke CE, Hillebrand JJ, Verhagen LA, Roeling TA, Adan RA. Hypothalamic neuropeptide expression following chronic food restriction in sedentary and wheel-running rats. J Mol Endocrinol. 2005;35:381–390. doi: 10.1677/jme.1.01808. [DOI] [PubMed] [Google Scholar]
- 17.Guan JL, Wang QP, Kageyama H, Takenoya F, Kita T, Matsuoka T, Funahashi H, Shioda S. Synaptic interactions between ghrelin- and neuropeptide Y-containing neurons in the rat arcuate nucleus. Peptides. 2003;24:1921–1928. doi: 10.1016/j.peptides.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 18.Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ, Smith RG, Van Der Ploeg LH, Howard AD. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res. 1997;48:23–29. doi: 10.1016/s0169-328x(97)00071-5. [DOI] [PubMed] [Google Scholar]
- 19.Hagan MM, Rushing PA, Pritchard LM, Schwartz MW, Strack AM, Van Der Ploeg LH, Woods SC, Seeley RJ. Long-term orexigenic effects of AgRP-(83---132) involve mechanisms other than melanocortin receptor blockade. Am J Physiol Regul Integr Comp Physiol. 2000;279:R47–R52. doi: 10.1152/ajpregu.2000.279.1.R47. [DOI] [PubMed] [Google Scholar]
- 20.Illius AW, Tolkamp BJ, Yearsley J. The evolution of the control of food intake. Proc Nutr Soc. 2002;61:465–472. doi: 10.1079/pns2002179. [DOI] [PubMed] [Google Scholar]
- 21.Jewett DC, Cleary J, Levine AS, Schaal DW, Thompson T. Effects of neuropeptide Y on food-reinforced behavior in satiated rats. Pharmacol Biochem Behav. 1992;42:207–212. doi: 10.1016/0091-3057(92)90517-j. [DOI] [PubMed] [Google Scholar]
- 22.Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Wakabayashi I. Central effect of ghrelin, an endogenous growth hormone secretagogue, on hypothalamic peptide gene expression. Endocrinology. 2000;141:4797–4800. doi: 10.1210/endo.141.12.7920. [DOI] [PubMed] [Google Scholar]
- 23.Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Wakabayashi I. Chronic central infusion of ghrelin increases hypothalamic neuropeptide Y and agouti-related protein mRNA levels and body weight in rats. Diabetes. 2001;50:2438–2443. doi: 10.2337/diabetes.50.11.2438. [DOI] [PubMed] [Google Scholar]
- 24.Kas MJ, Van Dijk G, Scheurink AJ, Adan RA. Agouti-related protein prevents self-starvation. Mol Psychiatry. 2003;8:235–240. doi: 10.1038/sj.mp.4001206. [DOI] [PubMed] [Google Scholar]
- 25.Keen-Rhinehart E, Bartness TJ. NPY Y1 receptor is involved in ghrelin- and fasting-induced increases in foraging, food hoarding and intake. 2007 doi: 10.1152/ajpregu.00597.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keen-Rhinehart E, Bartness TJ. Peripheral ghrelin injections stimulate food intake, foraging and food hoarding in Siberian hamsters. Am J Physiol. 2005;288:R716–R722. doi: 10.1152/ajpregu.00705.2004. [DOI] [PubMed] [Google Scholar]
- 27.Kim EM, Welch CC, Grace MK, Billington CJ, Levine AS. Effects of palatability-induced hyperphagia and food restriction on mRNA levels of neuropeptide-Y in the arcuate nucleus. Brain Res. 1998;806:117–121. doi: 10.1016/s0006-8993(98)00755-0. [DOI] [PubMed] [Google Scholar]
- 28.Kohno D, Gao HZ, Muroya S, Kikuyama S, Yada T. Ghrelin directly interacts with neuropeptide-Y-containing neurons in the rat arcuate nucleus: Ca2+ signaling via protein kinase A and N-type channel-dependent mechanisms and cross-talk with leptin and orexin. Diabetes. 2003;52:948–956. doi: 10.2337/diabetes.52.4.948. [DOI] [PubMed] [Google Scholar]
- 29.Konturek SJ, Konturek JW, Pawlik T, Brzozowski T. Brain-gut axis and its role in the control of food intake. J Physiol Pharmacol. 2004;55:137–154. [PubMed] [Google Scholar]
- 30.Lawrence CB, Snape AC, Baudoin FM, Luckman SM. Acute central ghrelin and GH secretagogues induce feeding and activate brain appetite centers. Endocrinology. 2002;143:155–162. doi: 10.1210/endo.143.1.8561. [DOI] [PubMed] [Google Scholar]
- 31.Lu XY, Nicholson JR, Akil H, Watson SJ. Time course of short-term and long-term orexigenic effects of Agouti- related protein (86–132) NeuroReport. 2001;12:1281–1284. doi: 10.1097/00001756-200105080-00045. [DOI] [PubMed] [Google Scholar]
- 32.Mercer JG, Lawrence B, Beck B, Burlet A, Atkinson T, Barrett P. Hypothalamic NPY and preproNPY mRNA in Djungarian hamsters: effects of food deprivation and photoperiod. Am J Physiol. 1995;269:R1099–R1106. doi: 10.1152/ajpregu.1995.269.5.R1099. [DOI] [PubMed] [Google Scholar]
- 33.Mercer JG, Moar KM, Ross AW, Morgan PJ. Regulation of leptin receptor, POMC and AGRP gene expression by photoperiod and food deprivation in the hypothalamic arcuate nucleus of the male Siberian hamster (Phodopus sungorus) Appetite. 2000;34:109–111. doi: 10.1006/appe.1999.0301. [DOI] [PubMed] [Google Scholar]
- 34.Mizuno TM, Makimura H, Silverstein J, Roberts JL, Lopingco T, Mobbs CV. Fasting regulates hypothalamic neuropeptide Y, agouti-related peptide, and proopiomelanocortin in diabetic mice independent of changes in leptin or insulin. Endocrinology. 1999;140:4551–4557. doi: 10.1210/endo.140.10.6966. [DOI] [PubMed] [Google Scholar]
- 35.Mizuno TM, Mobbs CV. Hypothalamic agouti-related protein messenger ribonucleic acid is inhibited by leptin and stimulated by fasting. Endocrinology. 1999;140:814–817. doi: 10.1210/endo.140.2.6491. [DOI] [PubMed] [Google Scholar]
- 36.Murphy B, Nunes CN, Ronan JJ, Harper CM, Beall MJ, Hanaway M, Fairhurst AM, Van Der Ploeg LH, MacIntyre DE, Mellin TN. Melanocortin mediated inhibition of feeding behavior in rats. Neuropeptides. 1998;32:491–497. doi: 10.1016/s0143-4179(98)90077-4. [DOI] [PubMed] [Google Scholar]
- 37.Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 38.Newsholme EA, Leech AR. Biochemistry for the Medical Sciences. John Wiley; Chichester: 1983. [Google Scholar]
- 39.Raposinho PD, White RB, Aubert ML. The melanocortin agonist melanotan-II reduces the orexigenic and adipogenic effects of neuropeptide Y (NPY) but does not affect the NPY-driven suppressive effects on the gonadotropic and somatotropic axes in the male rat. J Neuroendocrinol. 2003;15:173–181. doi: 10.1046/j.1365-2826.2003.00962.x. [DOI] [PubMed] [Google Scholar]
- 40.Schuhler S, Horan TL, Hastings MH, Mercer JG, Morgan PJ, Ebling FJ. Decrease of food intake by MC4-R agonist MTII in Siberian hamsters in long and short photoperiods. Am J Physiol. 2003;284:R227–R232. doi: 10.1152/ajpregu.00331.2002. [DOI] [PubMed] [Google Scholar]
- 41.Schuhler S, Horan TL, Hastings MH, Mercer JG, Morgan PJ, Ebling FJ. Feeding and behavioural effects of central administration of the melanocortin 3/4-R antagonist SHU9119 in obese and lean Siberian hamsters. Behav Brain Res. 2004;152:177–185. doi: 10.1016/S0166-4328(03)00260-2. [DOI] [PubMed] [Google Scholar]
- 42.Seeley R, Payne CJ, Woods SC. Neuropeptide Y fails to increase intraoral intake in rats. Am J Physiol. 1995;268:R423–R427. doi: 10.1152/ajpregu.1995.268.2.R423. [DOI] [PubMed] [Google Scholar]
- 43.Seoane LM, Lopez M, Tovar S, Casanueva FF, Senaris R, Dieguez C. Agouti-related peptide, neuropeptide Y, and somatostatin-producing neurons are targets for ghrelin actions in the rat hypothalamus. Endocrinology. 2003;144:544–551. doi: 10.1210/en.2002-220795. [DOI] [PubMed] [Google Scholar]
- 44.Shrestha YB, Wickwire K, Giraudo SQ. Action of MT-II on ghrelin-induced feeding in the paraventricular nucleus of the hypothalamus. NeuroReport. 2004;15:1365–1367. doi: 10.1097/01.wnr.0000127141.62476.d5. [DOI] [PubMed] [Google Scholar]
- 45.Sun Y, Ahmed S, Smith RG. Deletion of ghrelin impairs neither growth nor appetite. Mol Cell Biol. 2003;23:7973–7981. doi: 10.1128/MCB.23.22.7973-7981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toshinai K, Mondal MS, Nakazato M, Date Y, Murakami N, Kojima M, Kangawa K, Matsukura S. Upregulation of ghrelin expression in the stomach upon fasting, insulin- induced hypoglycemia, and leptin administration. Biochem Biophys Res Commun. 2001;281:1220–1225. doi: 10.1006/bbrc.2001.4518. [DOI] [PubMed] [Google Scholar]
- 47.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 48.Vander Wall SB. Food hoarding in animals. Univ Chicago Press; Chicago: 1990. [Google Scholar]
- 49.Vergoni AV, Bertolini A. Role of melanocortins in the central control of feeding. Eur J Pharmacol. 2000;405:25–32. doi: 10.1016/s0014-2999(00)00538-0. [DOI] [PubMed] [Google Scholar]
- 50.Wang L, Saint-Pierre DH, Tache Y. Peripheral ghrelin selectively increases Fos expression in neuropeptide Y - synthesizing neurons in mouse hypothalamic arcuate nucleus. Neurosci Lett. 2002;325:47–51. doi: 10.1016/s0304-3940(02)00241-0. [DOI] [PubMed] [Google Scholar]
- 51.Weiner J. Limits to energy budget and tactics in energy investments during reproduction in the Djungarian hamster (Phodopus sungorus sungorus Pallas 1770) Symp zool Soc Lond. 1987;57:167–187. [Google Scholar]
- 52.Wood AD, Bartness TJ. Food deprivation-induced increases in hoarding by Siberian hamsters are not photoperiod-dependent. Physiol Behav. 1996;60:1137–1145. doi: 10.1016/0031-9384(96)00173-4. [DOI] [PubMed] [Google Scholar]
- 53.Wren AM, Small CJ, Fribbens CV, Neary NM, Ward HL, Seal LJ, Ghatei MA, Bloom SR. The hypothalamic mechanisms of the hypophysiotropic action of ghrelin. Neuroendocrinology. 2002;76:316–324. doi: 10.1159/000066629. [DOI] [PubMed] [Google Scholar]
- 54.Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, Kennedy AR, Roberts GH, Morgan DG, Ghatei MA, Bloom SR. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141:4325–4328. doi: 10.1210/endo.141.11.7873. [DOI] [PubMed] [Google Scholar]