Abstract
HIV-1 uses glycans on gp120 to occlude its highly immunogenic epitopes. To better elucidate escape mechanisms of HIV-1 from carbohydrate-binding agents (CBA) and to understand the impact of CBA-escape on viral immune evasion, we generated and examined the biological properties of HIV-1 resistant to cyanovirin-N (CV-N) or cross-resistant to additional CBAs. Genotypic and phenotypic characterization of resistant env clones indicated that 3-5 high-mannose residues from 289 to 448 in the C2-C4 region of gp120 were mutated and correlated with the resistance levels. The specificity and minimal requirements of deglycosylation for CV-N resistance were further assessed by mutagenesis study. The sensitivity of resistant variants to a range of CBAs, immunoglobulins, sera and monoclonal antibodies (MAb) were investigated. For the first time, our data have collectively defined the high-mannose residues on gp120 affecting CV-N activity, and demonstrated that CBA-escape HIV-1 has increased sensitivity to immunoglobulins and sera from HIV patients, and particularly to V3 loop-directed MAbs. Our study provides a proof-of-concept that targeting HIV-1 glycan shields may represent a novel antiviral strategy.
Keywords: HIV-1, envelope glycoprotein, carbohydrate-binding agent, cyanovirin-N, resistance, antibody, neutralization, V3 loop
INTRODUCTION
The human immunodeficiency virus type 1 (HIV-1) envelope glycoproteins (Env) gp120 and gp41 form a trimeric complex that mediates virus entry into target cells. gp120 is composed of variable regions (V1-V5) and constant regions (C1-C5), and the V3 loop is the major determinant of viral entry. Due to its location on the surface of the virus, gp120 is the principal target for the design of vaccines and antiviral drugs (Moore and Doms, 2003; Pantophlet and Burton, 2006). However, HIV-1 has evolved strategies to evade the host immune system or to escape from drugs. For instance, gp120 is highly glycosylated, with carbohydrates accounting for as much as 50% of its mass. Such carbohydrate moieties are proposed to act as shields to occlude highly immunogenic epitopes on gp120 (Kwong et al., 1998; Wyatt et al., 1998; Wyatt and Sodroski, 1998). Hence, carbohydrate-binding agents (CBAs), specifically targeting HIV-1 glycan shields, may represent a novel antiviral strategy.
A range of CBAs, including cyanovirin-N (CV-N) originally isolated from the cyanobacterium Nostoc ellipsosporum (Boyd et al., 1997), galanthus nivalis agglutinin (GNA) from snowdrop (Van Damme, Allen, and Peumans, 1987) and griffithsin (GRFT) from the red alga Griffithsia sp (Mori et al., 2005), inhibit HIV-1 infection in vitro. Of these, CV-N exhibits remarkable stability and low toxicity (Boyd et al., 1997; Esser et al., 1999), and to date is the only CBA demonstrating efficacy and safety in macaque challenge studies (Tsai et al., 2004; Tsai et al., 2003), making it a promising candidate as a topical microbicide (Shattock and Moore, 2003). In addition, CV-N like agents may have therapeutic potential (Balzarini, 2005; Balzarini, 2006; Botos and Wlodawer, 2005). Previous studies have suggested that CV-N binds to high-mannose residues on gp120 (Bewley, 2001; Bolmstedt et al., 2001; Botos et al., 2002), but the specific binding sites on gp120 required for viral neutralization have not been fully elucidated. Generation of CV-N resistant HIV-1 and understanding the molecular basis for such resistance may provide a powerful approach to define residues on gp120 that affect CV-N antiviral activity. In addition, because CV-N may specifically target viral glycans that largely lie on the immunologically hidden “silent face” of gp120 (Kwong et al., 1998; Wyatt et al., 1998; Wyatt and Sodroski, 1998), HIV-1 escape from CV-N could result in loss of its glycan shields, rendering it more susceptible to antibody neutralization. Thus, elucidating the mechanism of CV-N resistance might generate new insight into the “silent face” and provide novel strategies to enhance the immunogenicity of gp120. To date, the contribution of specific glycans on gp120 to CV-N resistance has not been assessed at the single molecule level (Balzarini et al., 2006; Witvrouw et al., 2005), and the effects of such deglycosylation on antibody neutralization have not been addressed. Therefore, the CV-N specific glycans on gp120 and the impact of CV-N induced deglycosylation on viral immune evasion remain to be fully determined.
In the current study, CV-N resistant HIV-1 strains were generated, and genotypically and phenotypically characterized at both virological and molecular levels. The CV-N specific high-mannose residues on gp120 were systematically investigated using cloned env variants and mutagenesis study. The sensitivity of CV-N resistant variants to a range of antibodies, including immunoglobulins and sera from HIV patients, were also studied.
RESULTS
Phenotype and genotype of CV-N resistant HIV-1
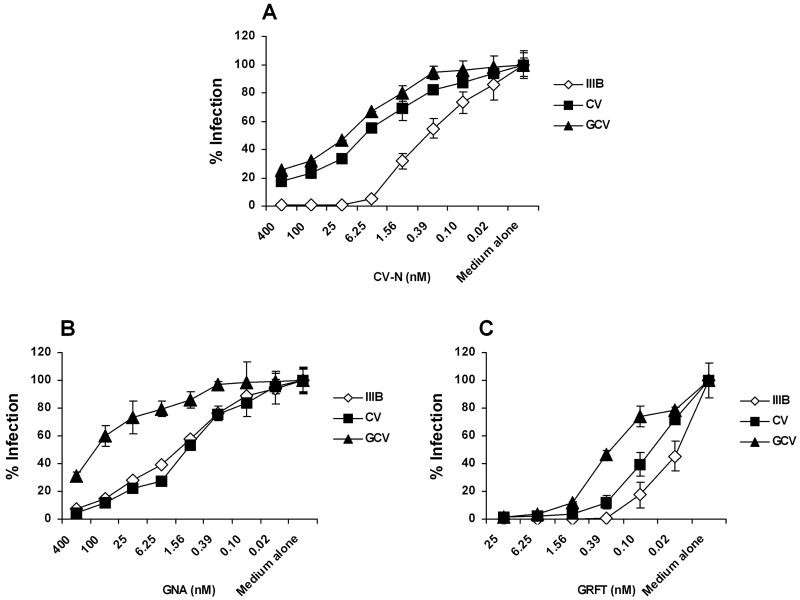
The N-linked glycosylation sites of HIV-1IIIB gp120 have been well-characterized at a biochemical level (Gallaher et al., 1995; Leonard et al., 1990). Therefore HIV-1IIIB was chosen to make resistant variants. Selection for resistance was started from 1 nM of CV-N and selected for more than 25 weeks. Two CV-N resistant isolates, CV and GCV, were generated under escalating selection pressure of CV-N. As shown Fig. 1A, GCV was more resistant to CV-N than CV. In addition, GCV was cross-resistant to the plant lectin GNA (Fig. 1B) and the newly identified red alga lectin GRFT (Fig. 1C), whereas the gp41 fusion inhibitor C52L and CXCR4 inhibitor AMD3100 kept their inhibitory activity against the resistant viral strains (Table 1).
Fig. 1. CV-N resistant viral strains and the cross-resistance to plant and red alga lectins.
Anti-HIV-1 activity was assessed in TZM-bl cells by infecting with HIV-1IIIB, or CV-N resistant isolates CV or GCV in the presence of serially diluted (A) CV-N, (B) GNA and (C) GRFT. The infectivity of each virus in medium alone culture was arbitrarily set to 100%. Data are representative of at least 3 independent experiments, with each determination performed in triplicate (mean ± SD).
Table 1.
Inhibitory activity of CBAs, fusion inhibitors and MAbs to CV-N resistant HIV-1
| IC50 (fold change) | |||
|---|---|---|---|
| Resistant HIV-1IIIB | |||
| Inhibitor | HIV-1IIIB | CV | GCV |
| CV-Na | 0.56 ± 0.36 | 5.71 ± 4.16 (10↑) | 13.72 ± 7.43 (24↑) |
| GNAa | 5.91 ± 2.37 | 3.93 ± 3.17 | 125.28 ± 42.2 (21↑) |
| GRFTa | 0.021 ± 0.011 | 0.058 ± 0.038 | 0.415 ± 0.36 (20↑) |
| C52La | 5.42 ± 1.53 | 8.57 ± 3.42 | 5.29 ± 1.75 |
| AMD3100a | 31.24 ± 11.32 | 45.79 ± 9.89 | 37.15 ± 13.23 |
| MAb 2G12b | 1.97 ± 1.52 | >40c (>20↑) | >40c (>20↑) |
| MAb b12b | 0.114 ± 0.092 | 0.059 ± 0.035 | 0.083 ± 0.075 |
| MAb 17bb | 1.396 ± 0.75 | 0.866 ± 0.23 | 1.459 ± 0.75 |
| MAb 2F5b | 0.371 ± 0.13 | 0.356 ± 0.09 | 0.547 ± 0.15 |
| MAb 447-52Db | 34.08 ± 28.16 | 0.127 ± 0.042 (268↓) | 0.129 ± 0.004 (264↓) |
| MAb 1101b | 2.01 ± 0.42 | 0.201 ± 0.076 (10↓) | 0.251 ± 0.024 (8↓) |
Anti-HIV-1 activity was assessed in TZM-bl cells by infecting with HIV-1IIIB, CV or GCV in the presence of serially diluted inhibitors. IC50 values were calculated by sigmoid-curve fitting in Prism 4 (Graphpad). Data are mean ± SD of 2-5 independent experiments. ↑ increase; ↓ decrease.
Data are expressed in nM.
Data are expressed in μg/ml.
Highest concentration tested.
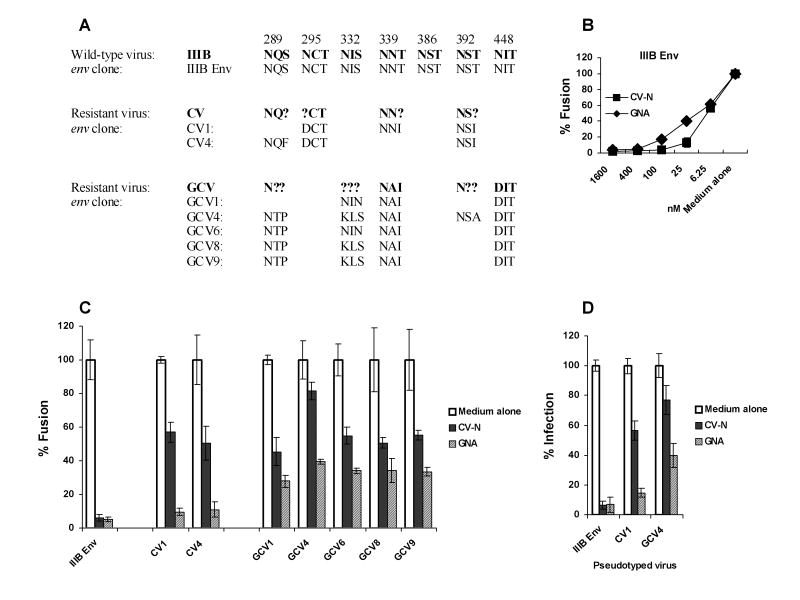
Because CV-N inactivates HIV-1 by binding to Env (Dey et al., 2000; Esser et al., 1999), env genes were amplified by PCR from proviral DNA templates and the PCR products were directly sequenced. A variety of predicted amino acid changes based on their nucleotide sequences were found in gp120 from the CV-N resistant isolates CV and GCV (Fig. 2A). Although there are 7 potential N-glycosylation sites in gp41, none of them changed in resistant viruses (Data not shown). All the mutations exclusively occurred at the N-linked glycosylation sites (N-x-T/S) in the C2-C4 region of gp120, by converting asparagines (N), threonine (T) or serine (S) to another amino acid. CV had 4 glycosylation sites mutated at position 289, 295, 339 and 392, while GCV had 5 at position 289, 332, 339, 392 and 448. Some positions showed ambiguities of the predicted primary structure, which were interpreted to be the result of heterogeneity of integrated proviruses containing both the wild type and the mutated amino acids. HIV-1IIIB gp120 has 24 potential N-glycosylation sites, including 11 high-mannose type structure (Gallaher et al., 1995; Leonard et al., 1990). Of note, the observed deglycosylation residues were all high-mannose type, suggesting the specificity for CV-N binding.
Fig. 2. Genotypic and phenotypic characterization of CV-N resistant env molecular clones.
(A) Alignment of the glycosylation changes in resistant HIV-1 viruses and their env clones. Nonsynonymous sequence polymorphisms in env PCR products, containing both the wild and the mutated amino acids, are indicated by assigning “?” to the position. (B) Fusogenic activity of IIIB Env in the presence of serially diluted CV-N or GNA was determined using the Env mediated cell-cell fusion assay. (C) The fusogenic activity of Env encoded by each env molecular clone in the presence or absence of 100 nM CV-N or 400 nM GNA. The fusogenic activity of each Env in medium alone culture was arbitrarily set to 100%. (D) The infectivity of IIIB Env, CV1 or GCV4 pseudotyped virus in the presence or absence of 100 nM CV-N or 400 nM GNA were assessed in TZM-bl cells. The infectivity of each virus in medium alone culture was arbitrarily set to 100%. Data are representative of 3 independent experiments, with each determination performed in triplicate (mean ± SD).
Genotypic and phenotypic characterization of CV-N resistant env molecular clones
To examine the functional consequences of the mutations in env that occurred during selection, we examined the molecular characteristics of the CV-N resistant viruses by cloning full-length env genes and assessing their phenotypes using our well-established methods (Hu et al., 2000; Hu et al., 2005). Primary env genes were amplified by PCR from proviral DNA templates derived from CV-N resistant viruses and molecularly cloned. env clones were isolated and tested for biological activity by using the Env-mediated cell-cell fusion assay. The representative clones active in fusion assay are shown in Fig. 2. As shown in Fig. 2A, comparison of the nucleotide sequence of env molecular clones with that obtained from the direct analysis of proviral DNA templates confirmed that the former were representative of the predominant viral species in the isolate.
As shown in Fig. 2B, both CV-N and GNA efficiently inhibited the fusogenic activity of IIIB Env in a dose dependent manner, with an EC95 of ~100 nM and ~400 nM, respectively. CV-N at 100 nM and GNA at 400 nM were used for all the following studies unless indicated otherwise. As shown in Fig. 2C, although parental and mutant Env had similar fusogenic activity in the absence of inhibitors, the products of CV and GCV env clones demonstrated a variety of resistance to CV-N. Review of the primary structure of these Env revealed a correlation between the number of depleted glycans and the level of resistance. In addition, the GCV clones also showed 30-40% resistance to GNA (Fig. 2C). Moreover, GCV4, a clone with 5 depleted high-mannose glycans at 289, 332, 339, 392 and 448, showed significant resistance to CV-N and moderate resistance to GNA as demonstrated in fusion and pseudotype infection (Fig. 2C and Fig. 2D). These data together suggest that deglycosylation at high-mannose residues 289 to 448 may be specifically required for HIV-1 to escape from CV-N, whereas GNA and GRFT may bind to additional glycans.
Definition of specific high-mannose residues on gp120 that affect CV-N anti-viral activity
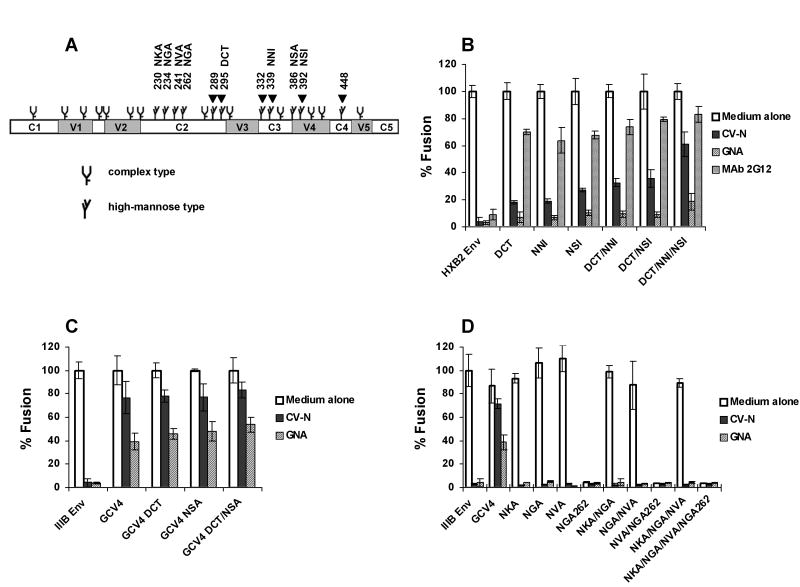
To define the high-mannose residues on gp120 that affect CV-N activity, site-directed mutageneses and subsequent functional studies were carried out. Because env clone CV1 with 3 depleted glycans at 295, 339 and 392 rendered Env moderately resistant to CV-N (Fig. 2), deglycosylated env mutants were made to investigate the minimum number of depleted glycans required for CV-N resistance. Using a well-characterized HXB2 env clone as template, glycans 295, 339 and 392 were mutated to make single, double and triple deglycosylated mutants. The resulting env mutants were designated as DCT, NNI, NSI, DCT/NNI, DCT/NSI and DCT/NNI/NSI, respectively. As shown in Fig. 3B, mutants with single or two deglycosylation mutations showed little or limited resistance to CV-N, while mutant with 3 depleted glycans showed ~60% resistance to CV-N. In contrast, all the mutants were significantly resistant to MAb 2G12 which recognizes high-mannose epitope on gp120, but not to the plant lectin GNA. The former is less surprising as these CV-N mutants lost one or more 2G12 binding glycans on gp120 (Sanders et al., 2002; Scanlan et al., 2002; Trkola et al., 1996), and a 2G12 resistant HIV-1NL4-3 also revealed a depletion of glycan 295 (Huskens et al., 2007).
Fig. 3. Defining high-mannose residues on gp120 that affect CV-N antiviral activity.
(A) The schematic of N-linked glycosylation sites on IIIB and HXB2 gp120 according to Leonard et al (Leonard et al., 1990) and Gallaher et al (Gallaher et al., 1995). The constant regions C1, C2, C3, C4 and C5, and the variable regions V1, V2, V3, V4 and V5 are shown. Arrows indicate sites that were changed in CV-N resistant viruses. The fusogenic activity of (B) HXB2 Env and its mutants, (C) IIIB Env, GCV4 and GCV4 mutants, and (D) IIIB Env and its mutants, in the presence or absence of 100 nM CV-N, 400 nM GNA or 25 μg/ml MAb 2G12. The fusogenic activity of each Env in medium alone culture was arbitrarily set to 100%. Data are representative of 3 independent experiments, with each determination performed in triplicate (mean ± SD).
GCV4 clone with 5 depleted glycans at 289, 332, 339, 392 and 448 (Fig. 2) showed significant resistance to CV-N and moderate resistance to GNA. To examine whether additional deglycosylation could increase resistance, mutants GCV4 DCT, GCV4 NSA and GCV4 DCT/NSA were made using GCV4 as the template to deplete more glycans. As seen in Fig. 3C, no significant enhancement of resistance was observed when mutants with 6 or 7 depleted glycans were tested. Because all the deglycosylation mutations in CV-N resistant viruses were exclusively located within high-mannose residues 289 to 448, we next asked whether deletion of high-mannose glycans 230 to 262 could influence the anti-viral activity of CV-N. The canonical N-x-T/S at position 230, 234, 241 and 262 were replace by N-x-A individually or in combination to create single, double, triple and quadruple mutants (Fig. 3A), named as NKA, NGA, NVA, NGA262, NKA/NGA, NGA/NVA, NVA/NGA262, NKA/NGA/NVA, and NKA/NGA/NVA/NGA262, respectively (Fig. 3D). As shown in Fig. 3D, none of the mutations rendered IIIB Env resistant to CV-N. Of interest, mutants bearing deglycosylated 262 completely lost fusogenic activity, suggesting an essential role of glycan 262 in maintaining gp120 function. Taken together, these data clearly show that high-mannose glycans 289 to 448 are exclusively involved in CV-N anti-HIV activity, whereas GNA may bind to additional glycans outside 289-448.
Sensitivity of resistant viruses to immunoglobilins and sera from HIV+ patients
The N-linked glycan sites on gp120 are highly conserved, having been dubbed on the “silent face” of gp120 (Kwong et al., 1998; Wyatt et al., 1998). Such carbohydrates can render the underlying protein surface invisible to the immune system (Pantophlet and Burton, 2006; Wyatt and Sodroski, 1998). Depletion of glycans could make gp120 more immunogenic or more accessible to antibodies. To test this hypothesis, we evaluated the sensitivity of CBA-escape, CV-N resistant HIV-1 to human immunoglobilins or sera from HIV+ patients. As shown in Fig. 4A, wild-type and CV-N resistant viruses demonstrated similar infectivity in the presence of serially diluted HIV- control human serum. Of great interest, as shown in Fig. 4B, the mutant viruses CV and GCV demonstrated significantly enhanced sensitivity to IgG pooled from HIV+ individuals. This enhancement of sensitivity was also observed in the cell-cell fusion assay, showing that mutant Env was more sensitive to HIV-IgG than the wild-type Env (data not shown). Our findings were further confirmed when we examined HIV serum 1 and HIV serum 2, which were pooled from three or four separate bleeds from the same HIV+ patient, showing that the mutant viruses CV and GCV had significantly increased sensitivity to HIV sera than their parental virus HIV-1IIIB (Fig. 4C and Fig. 4D). Our data suggest that high-mannose depletion in CV-N-escape viruses may increase the number or exposure of available neutralization epitopes on gp120 to antibodies which already exist in sera.
Fig. 4. Sensitivity of CV-N resistant viruses to immunoglobulins and sera from HIV-1 positive individuals.
Anti-HIV-1 activity was assessed in TZM-bl cells by infecting with HIV-1IIIB, CV or GCV in the presence of serially diluted (A) HIV negative serum, (B) HIV-IgG, (C) HIV serum 1 and (D) HIV serum 2. The infectivity of each virus in the absence of indicated serum was arbitrarily set to 100%. Data are representative of 2-5 independent experiments, with each determination performed in triplicate (mean ± SD). * P<0.01, compared with HIV-1IIIB.
Glycan-light viruses induced by resistance to CV-N demonstrate increased sensitivity to V3 loop-directed MAbs
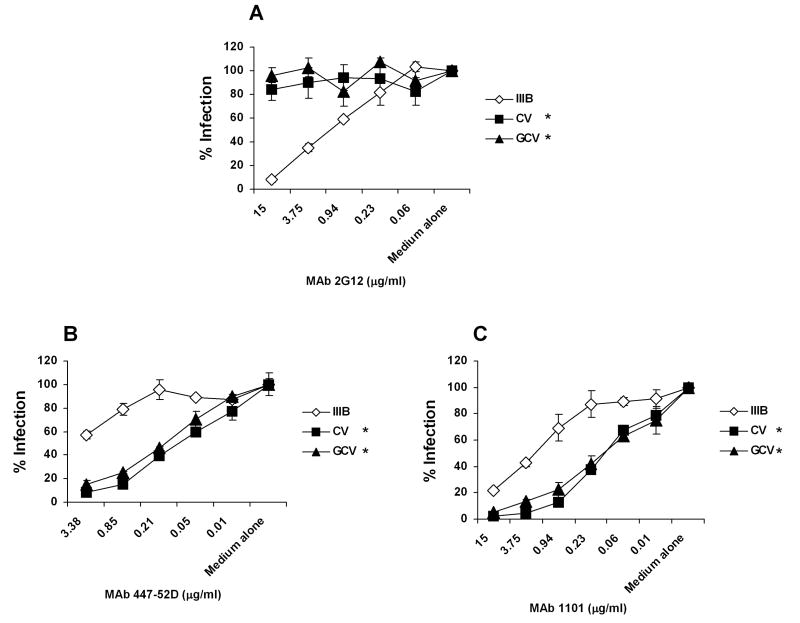
To elucidate the potential exposed neutralizing epitopes induced by resistance to CV-N, we carried out infection experiments using a range of well-characterized MAbs. As shown in Table 1, similar to their parental virus HIV-1IIIB, both CV and GCV maintained sensitivity to MAbs b12 (recognizing CD4 binding-domain on gp120), 17b (against CD4 induced epitope on gp120), and 2F5 (targeting gp41 ectodomain) (Douek, Kwong, and Nabel, 2006). In agreement with our fusion results, CV and GCV were fully resistant to the anti-carbohydrate MAb 2G12 (Fig. 5A and Table 1). Remarkably, CV and GCV were over 200 times more sensitive to MAb 447-52D, which recognizes GPGR in the tip of V3 loop, than the wild-type virus HIV-1IIIB (Fig. 5B and Table 1). CV and GCV also showed 8-10 times increased sensitivity to the murine anti-HIV-1IIIB V3-loop (http://www.immunodx.com) MAb 1101 (Fig. 5C and Table 1). Taken together, our results have provided evidence that targeting glycans on gp120 could force HIV-1 to abandon its glycan shields and make the mutant gp120 more accessible to antibodies and therefore more vulnerable to neutralization.
Fig. 5. CV-N resistant viruses demonstrate increased sensitivity to V3 loop-directed MAbs.
Anti-HIV-1 activity was assessed in TZM-bl cells by infecting with HIV-1IIIB, CV or GCV in the presence of serially diluted (A) MAb 2G12, (B) MAb 447-52D and (C) MAb 1101. The infectivity of each virus in medium alone culture was arbitrarily set to 100%. Data are representative of 3 independent experiments, with each determination performed in triplicate (mean ± SD). * P<0.01, compared with HIV-1IIIB.
DISCUSSION
By genetically and phenotypically characterizing CV-N resistant viral variants and their env clones, we identified changes in specific high-mannose residues in the C2-C4 region of gp120 which resulted in viruses with resistance to CV-N and cross-resistance to the plant lectin GNA and the red algae lectin GRFT. This resistance conversion was further systematically investigated through site-directed mutagenesis studies, revealing that CV-N interacts with gp120 in a specific and high-mannose dependent fashion. Furthermore, our study has demonstrated that the escape viruses with less glycans on gp120, induced by resistance to CV-N, became more sensitive to immunoglobulins and sera from HIV-1 patients, and in particular to the V3-directed MAbs.
All of the carbohydrate moieties on gp120 were found to be N-linked oligosacharides and their sequences at amino acid level are well conserved among different isolates (Botos and Wlodawer, 2005). The 24 potential N-linked glycosylation sites of HIV-1IIIB gp120 are all utilized, including 11 high-mannose type and 13 complex type structure (Gallaher et al., 1995; Leonard et al., 1990). In contrast to mammalian cell surface or serum glycoproteins, which rarely contain terminal high-mannose residues (Weis, Taylor, and Drickamer, 1998), 33% the oligosacharides in HIV-1 gp120 are of the high-mannose type (Mizuochi et al., 1988). HIV-1 uses the terminal high-mannoses to reduce its antigenicity against the immune system (Sanders et al., 2002; Wyatt and Sodroski, 1998). The binding specificity of CV-N to gp120 was previously suggested to be capable of distinguishing high-mannose and complex oligosaccharides (Bewley, 2001; Botos et al., 2002; Shenoy et al., 2001), but the binding sites remained obscure. In the current study, we investigated the mechanism underlying HIV-1 resistance and CV-N antiviral activity at both virological and molecular levels. We chose HIV-1IIIB to make resistant isolates because its mannose types on gp120 were well-characterized at a biochemical level (Gallaher et al., 1995; Leonard et al., 1990). Genetic and phenotypic analyses of CV-N resistant HIV-1 isolates revealed selective mutations of high-mannose residues 289 to 448 in the C2-C4 region of gp120. It is worthy noting that some of the mutant amino acids were not pure and present as a mixture with their wild-type amino acids even after a long-term selection; in agreement with their genotypes, the resistant viral isolates showed sensitivity to CV-N at high concentration. Therefore we investigated the mechanism of resistance at a molecular level using cloned env variants and mutagenesis study. In both Env-mediated fusion and Env-pseudotyped virus infection assays, the number of depleted glycans correlated with the level of resistance. Individual or double elimination of glycans by mutagenesis did not induce CV-N resistance, and only moderate resistance was observed when a minimum of 3 glycans were mutated. Of interest, the GCV isolate with more high-mannose deglycoslations demonstrated significant resistance to GNA and GRFT. Our findings suggest that CV-N, GNA, and GRFT share some common carbohydrate sites on gp120, but their binding sites are not identical.
Deletion of one or two additional glycans in a backbone of Env bearing 5 high-mannose deglycosylation sites from 289 to 448 did not significantly enhance CV-N resistance. Our finding is supported by a stoichiometry study showing an approximate 5:1 stoicchiometry for CV-N binding to soluble form of gp120 (O’Keefe et al., 2000). Of interest, high-mannose residues 230 to 262 were unchanged in the CV-N resistant viruses and seemed to play no role in CV-N anti-HIV activity as evidenced by mutagenesis study. However, depletion of glycan 262 by mutagenesis completely abolished the fusogenic activity of Env, suggesting its significance in maintaining HIV-1 infectivity (Lee et al., 1992). Of note, a variant of HIV-1NL4-3 with glycan depletions at N position 332, 448 and a 13-amno-acid-fragment deletion containing glycans 392, 397 and 406 was recently reported to have resistance to CV-N (Witvrouw et al., 2005), whereas an insolate of HIV-1IIIB showing resistance to CV-N had mutations at N positions 136, 160, 230, 332, 339, 386, 392 and 448 (Balzarini et al., 2006). However, these glycan mutations observed were not solely of the high-mannose type, and were often present as a mixture with wild type or resulted from a fragment deletion containing non-glycosylation residues (Balzarini et al., 2006; Witvrouw et al., 2005). The relative contribution of each individual mutated glycan or amino acid in the previous studies was not assessed at the single molecule level, making it difficult to conclude whether some of the mutations were truly CV-N specific. Nevertheless, the depleted high-mannose glycans in these two reports were largely located within 289-448 with the exception of 230. Given that CV-N is high-mannose specific (Bewley, 2001; Botos et al., 2002; Shenoy et al., 2001), our mutagenesis study suggests that previous reported depletions of high-mannose glycan 230 and complex-type glycans 136 and 160 (Balzarini et al., 2006), as well as complex-type glycans 397 and 406 due to a fragment deletion (Witvrouw et al., 2005), may not be driven by direct CV-N binding. It is unclear whether such mutations had a compensatory role on viral fitness or an indirect resistance effect due to conformational change. We did not observe mutation in the seven potential N-glycosylation sites of gp41 and any other non-glycosylation sites of gp120 and gp41, further strengthening the notion that CV-N specifically interacts with high-mannose moieties. The observed CV-N induced high-mannose deglycosylation in gp120 appeared not to compromise Env expression and/or fusion activity in vitro because parental and mutated viruses or Env clones demonstrated similar infectivity or fusogenic activity in the absence of inhibitors. In addition, consistent results were observed using viruses and Env produced by various cell types, indicating that our findings were not cell type dependent.
It is well documented that HIV-1 uses its glycan shields in virus-infected individuals to avoid neutralization (Wei et al., 2003). The importance of glycan shields for HIV-1 immune evasion was further demonstrated through experiments where deletion of one or several glycosylation sites in gp120 resulted in a higher susceptibility of these viruses to neutralizing antibodies (Kang et al., 2005; Koch et al., 2003; Malenbaum et al., 2000). Moreover, monkeys exposed to SIV with two deglycosylated sites on Env produced a high-titre of neutralizing antibodies (Reitter, Means, and Desrosiers, 1998). Because CBAs, like CV-N, specifically target viral glycans, these types of compounds might be used for therapeutic purpose. In an in vivo environment where there was a consistent pressure of CBAs, HIV-1 would have to abandon its glycan shields to escape from sustained drug pressure. Once glycans are depleted, previously hidden epitopes would be exposed and the virus would become more susceptible to immune pressure (i.e. antibody neutralization). While such an in vivo study could only be conducted if a safe, non-immunological and bioavailable CBA became available, and there have been none to date (Balzarini, 2005; Balzarini, 2006; Botos and Wlodawer, 2005), this could more readily applicable to topical microbicide use. Here it would have the added advantage of rendering resistant virus more susceptible to antibody neutralization, reducing the chance of transmission. Using our CV-N-escape relevant viruses, we were able to test the hypothesis that CBA induced deglycosylation could compromise the mechanisms of HIV-1 immune evasion. Remarkably, both CV-N resistant viral isolates CV and GCV demonstrated significantly enhanced sensitivity to IgG and sera from HIV-1 positive individuals, and in particular to V3-loop directed MAbs 447-52D and 1101, but maintained similar sensitivity to MAbs b12, 17b, and 2F5.
Previous studies have demonstrated that structure-based deglycosylation of HIV-1 gp120, in particular the removal of glycan 301 (Back et al., 1994) in V3 loop, results in increased neutralization sensitivity to CD4 binding site (CD4BS) antibodies, a V3 loop-directed antibody (Koch et al., 2003), and CD4-induced (CD4i) antibodies (Malenbaum et al., 2000). It was suggested that the deletion of glyan 301 at the base of the V3 loop might cause a repositioning of the V1/V2 stem and allow these antibodies to better access the receptor binding sites. The complex-type mannose 301 was intact in our CV-N-escape viruses. Hence, it was not unexpected that the sensitivity of CV-N resistant viruses to CD4BS antibody b12 and CD4i antibody 17b remained unchanged. In contrast, IgG pooled from HIV-infected individuals demonstrated significantly increased activity against the resistant viruses. This finding was reinforced when two additional HIV sera from an individual were tested, showing that CV-N resistant viruses were markedly more sensitive to HIV sera than the wild type virus. Such change in viral neutralization suggests that high-mannose depletion in CV-N-escape viruses may increase the number or exposure of available neutralization epitopes on gp120 to antibodies which already exist in sera. Given that CV-N resistant viruses demonstrated significantly enhanced sensitivity to MAbs 447-2D and 1101 which are directed against V3 loop, deglycosylation in CV-N resistant viruses is highly likely to render the V3 loop of gp120 more accessible to antibodies.
It is reasonable to assume from our in vitro study that, under in vivo CBA pressure, HIV-1 would deplete its glycans to escape and that such deglycosylation would increase the immunogenicity of gp120. Because CBAs, like CV-N, specifically target HIV-1 glycans and thereby compromise the mechanisms of HIV-1 immune evasion, findings in this study may have implications for novel antiviral therapies, microbicides, and vaccine design.
MATERIALS AND METHODS
Entry inhibitors, cells and HIV-1
CV-N (MW 11 kDa) was from Biosyn Inc. GRFT (MW 13 kDa) was provided by Intrucept Biomedicine LLC. GNA (MW 12.5 kDa) was purchased from Sigma. MAb b12, 2G12, 17b, 2F5, HIV immunoglobulin (HIV-IgG), HIV-1 negative control human serum, HIV serum 1 (3 separate bleeds from the same patient), HIV serum 2 (4 separate bleeds from the same patient), MAb 447-52D (tissue culture supernatant, 16.9 μg/ml), AMD3100 and TZM-bl cell line were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIH. C52L was previously described (Veazey et al., 2005). Murine MAb 1101 was purchased from ImmunoDiagnostics Inc. QT6 and Hela cell lines were from the American Type Culture Collection. 293FT was from Invitrogen. C8166 and PM1 cell lines and HIV-1IIIB were obtained from the Centralized Facility for AIDS Reagents, NIBSC, UK. Peripheral blood mononuclear cells (PBMCs) were prepared as described previously (Hu et al., 2004).
Selection of CV-N resistant HIV-1 strains
Selection was started in C8166 cells infected with HIV-1IIIB in the presence of compound at concentration ~ two times of its 50% inhibitory concentration (IC50). Medium in the presence of compound was changed every 3 to 4 days, and the culture was monitored for syncytial formation. When syncytia were observed, the cell-free culture supernatant was collected to infect fresh C8166 cells in the presence of an equal or higher concentration of the compound. Parental and resistant viruses were grown in PM1 or activated PBMCs to prepare virus stocks for antiviral activity study.
Antiviral activity analysis
Anti-HIV-1 activity in TZM-bl cells in the presence of serially diluted entry inhibitors was performed as described (Derdeyn et al., 2000). Infected cells were harvested 48 h post infection, lysed, and assayed using the LucLite Luciferase Assay Kit (Packard) in a Fusion Universal Microplate Analyzer (Packard).
Molecular cloning of env genes
Proviral DNA templates from HIV-1 infected C8166 cells were purified using QIAamp DNA blood mini Kit (Qiagen). Full-length env was amplified using primers Enf (5′-AAAGAGCAGAAGACAGTGGCAATGAGAGTGAAGG) and Enr (5′-CAATCACACTACTTTTTGACCACTTGCCACCCAT), and cloned into the pCDNA3.1/V5/His-TOPO expression vector (Invitrogen) as described previously (Hu et al., 2000; Hu et al., 2005).
Site-directed mutagenesis
env mutants were created by QuickChange Site-Directed Mutagenesis Kit (Stratagene) and the programmed mutations were confirmed by DNA sequencing.
Env-mediated cell-cell fusion assay
The fusogenic activity of the products encoded by the env genes was determined using a cell-cell fusion assay employing a luciferase reporter gene, as described previously (Hu et al., 2000). Briefly, QT6, 293FT or Hela effector cells were prepared by infection with vTF1.1 encoding T7 RNA polymerase for 1 h and followed transfection with pcDNA3 constructs containing env genes. QT6 target cells were transfected with CD4 and CXCR4 in pcDNA3, and constructs encoding luciferase under the control of T7 promotor. The effector and target cell populations were mixed at 16-18 h post-transfection in the presence or absence of entry inhibitors. The luciferase activity of cell lysates was determined approximately 8 h later. Constructs CD4, T7-luciferase, CXCR4, and HXB2 env were previously described (Hu et al., 2000).
Pseudotyped virus infection
Stocks of pseudotyped reporter viruses were prepared by co-transfecting 293FT cells with Env expression vector and plasmid pHIVΔEnv as described previously (Hu et al., 2005). Single cycle infection was assessed in TZM-bl cells in the presence of serially diluted entry inhibitors. The luciferase activity of cell lysates was determined 48 h post-transfection.
DNA sequencing
The nucleotide sequence of the full-length HIV-1 env was determined from amplification products obtained from proviral DNA templates using primers Enf and Enr. The PCR products were excised from agarose gels, purified and concentrated. Primers E20 (5′-GGGCCACACATGCCTGTGTACCCACAG), E110 (5′- CTGTTAAATGGCAGTCTAGCAGAA) E130c (5′-CCTGCCACATGTTTATAATTTGT) were used to prime the sequencing reaction. T7 primer (5′-TAATACGACTCACTATAGGG) residing in the cloning vector, E20, E110 and E130c were used to sequence full-length env clones. Sequencing was performed in Lark (Essex, UK).
Statistical analysis
Statistical analysis was performed using the Student’s t-test. Data were considered to be significant at P < 0.05.
Acknowledgments
We thank Biosyn Inc (Huntingdon Valley, PA), Intrucept Biomedicine LLC (Vacaville, CA) for providing CV-N and GRFT, respectively. The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: TZM-bl from Dr John C. Kappes, Dr. Xiaoyun Wu and Tranzyme Inc; MAb b12 from Dr Dennis Burton and Carlos Barbas; MAb 2G12 and MAb 2F5 from Dr Hermann Katinger; MAb 17b from Dr James E. Robinson; HIV-IG from NABI and NHLBI; HIV Neutralizing Serum 1 and Serum 2, and HIV-1 negative control human serum from Dr Luba Vujcic, FDA, Center for Biologics Evaluation and Research; MAb 447-52D from Dr. Susan Zolla-Pazner; AMD3100 from AnorMED, Inc.
This work was supported, in part, by grants from the National Institute of Health (AI065413 and AI051650), and by the 973 Program (2006CB504200).
Abbreviations
- HIV-1
human immunodeficiency virus type 1
- env
envelope gene
- Env
envelope glycoprotein
- gp120
glycoprotein 120
- V3 loop
third variable loop
- CBA
carbohydrate-binding agent
- CV-N
cyanovirin-N
- GNA
galanthus nivalis agglutinin
- GRFT
griffithsin
- MAb
monoclonal antibody
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Back NK, Smit L, De Jong JJ, Keulen W, Schutten M, Goudsmit J, Tersmette M. An N-glycan within the human immunodeficiency virus type 1 gp120 V3 loop affects virus neutralization. Virology. 1994;199(2):431–8. doi: 10.1006/viro.1994.1141. [DOI] [PubMed] [Google Scholar]
- Balzarini J. Targeting the glycans of gp120: a novel approach aimed at the Achilles heel of HIV. Lancet Infect Dis. 2005;5(11):726–31. doi: 10.1016/S1473-3099(05)70271-1. [DOI] [PubMed] [Google Scholar]
- Balzarini J. Inhibition of HIV entry by carbohydrate-binding proteins. Antiviral Res. 2006;71(23):237–47. doi: 10.1016/j.antiviral.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Balzarini J, Van Laethem K, Peumans WJ, Van Damme EJ, Bolmstedt A, Gago F, Schols D. Mutational pathways, resistance profile, and side effects of cyanovirin relative to human immunodeficiency virus type 1 strains with N-glycan deletions in their gp120 envelopes. J Virol. 2006;80(17):8411–21. doi: 10.1128/JVI.00369-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley CA. Solution structure of a cyanovirin-N:Man alpha 1-2Man alpha complex: structural basis for high-affinity carbohydrate-mediated binding to gp120. Structure. 2001;9(10):931–40. doi: 10.1016/s0969-2126(01)00653-0. [DOI] [PubMed] [Google Scholar]
- Bolmstedt AJ, O’Keefe BR, Shenoy SR, McMahon JB, Boyd MR. Cyanovirin-N defines a new class of antiviral agent targeting N-linked, high-mannose glycans in an oligosaccharide-specific manner. Mol Pharmacol. 2001;59(5):949–54. doi: 10.1124/mol.59.5.949. [DOI] [PubMed] [Google Scholar]
- Botos I, O’Keefe BR, Shenoy SR, Cartner LK, Ratner DM, Seeberger PH, Boyd MR, Wlodawer A. Structures of the complexes of a potent anti-HIV protein cyanovirin-N and high mannose oligosaccharides. J Biol Chem. 2002;277(37):34336–42. doi: 10.1074/jbc.M205909200. [DOI] [PubMed] [Google Scholar]
- Botos I, Wlodawer A. Proteins that bind high-mannose sugars of the HIV envelope. Prog Biophys Mol Biol. 2005;88(2):233–82. doi: 10.1016/j.pbiomolbio.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Boyd MR, Gustafson KR, McMahon JB, Shoemaker RH, O’Keefe BR, Mori T, Gulakowski RJ, Wu L, Rivera MI, Laurencot CM, Currens MJ, Cardellina JH, 2nd, Buckheit RW, Jr, Nara PL, Pannell LK, Sowder RC, 2nd, Henderson LE. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrob Agents Chemother. 1997;41(7):1521–30. doi: 10.1128/aac.41.7.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derdeyn CA, Decker JM, Sfakianos JN, Wu X, O’Brien WA, Ratner L, Kappes JC, Shaw GM, Hunter E. Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gp120. J Virol. 2000;74(18):8358–67. doi: 10.1128/jvi.74.18.8358-8367.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey B, Lerner DL, Lusso P, Boyd MR, Elder JH, Berger EA. Multiple antiviral activities of cyanovirin-N: blocking of human immunodeficiency virus type 1 gp120 interaction with CD4 and coreceptor and inhibition of diverse enveloped viruses. J Virol. 2000;74(10):4562–9. doi: 10.1128/jvi.74.10.4562-4569.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douek DC, Kwong PD, Nabel GJ. The rational design of an AIDS vaccine. Cell. 2006;124(4):677–81. doi: 10.1016/j.cell.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Esser MT, Mori T, Mondor I, Sattentau QJ, Dey B, Berger EA, Boyd MR, Lifson JD. Cyanovirin-N binds to gp120 to interfere with CD4-dependent human immunodeficiency virus type 1 virion binding, fusion, and infectivity but does not affect the CD4 binding site on gp120 or soluble CD4-induced conformational changes in gp120. J Virol. 1999;73(5):4360–71. doi: 10.1128/jvi.73.5.4360-4371.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallaher WR, Ball JM, Garry RF, Martin-Amedee AM, Montelaro RC. A general model for the surface glycoproteins of HIV and other retroviruses. AIDS Res Hum Retroviruses. 1995;11(2):191–202. doi: 10.1089/aid.1995.11.191. [DOI] [PubMed] [Google Scholar]
- Hu Q, Barry AP, Wang Z, Connolly SM, Peiper SC, Greenberg ML. Evolution of the human immunodeficiency virus type 1 envelope during infection reveals molecular corollaries of specificity for coreceptor utilization and AIDS pathogenesis. J Virol. 2000;74(24):11858–72. doi: 10.1128/jvi.74.24.11858-11872.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Frank I, Williams V, Santos JJ, Watts P, Griffin GE, Moore JP, Pope M, Shattock RJ. Blockade of attachment and fusion receptors inhibits HIV-1 infection of human cervical tissue. J Exp Med. 2004;199(8):1065–75. doi: 10.1084/jem.20022212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Napier KB, Trent JO, Wang Z, Taylor S, Griffin GE, Peiper SC, Shattock RJ. Restricted variable residues in the C-terminal segment of HIV-1 V3 loop regulate the molecular anatomy of CCR5 utilization. J Mol Biol. 2005;350(4):699–712. doi: 10.1016/j.jmb.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Huskens D, Van Laethem K, Vermeire K, Balzarini J, Schols D. Resistance of HIV-1 to the broadly HIV-1-neutralizing, anti-carbohydrate antibody 2G12. Virology. 2007;360(2):294–304. doi: 10.1016/j.virol.2006.10.027. [DOI] [PubMed] [Google Scholar]
- Kang SM, Quan FS, Huang C, Guo L, Ye L, Yang C, Compans RW. Modified HIV envelope proteins with enhanced binding to neutralizing monoclonal antibodies. Virology. 2005;331(1):20–32. doi: 10.1016/j.virol.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Koch M, Pancera M, Kwong PD, Kolchinsky P, Grundner C, Wang L, Hendrickson WA, Sodroski J, Wyatt R. Structure-based, targeted deglycosylation of HIV-1 gp120 and effects on neutralization sensitivity and antibody recognition. Virology. 2003;313(2):387–400. doi: 10.1016/s0042-6822(03)00294-0. [DOI] [PubMed] [Google Scholar]
- Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393(6686):648–59. doi: 10.1038/31405. see comments. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WR, Syu WJ, Du B, Matsuda M, Tan S, Wolf A, Essex M, Lee TH. Nonrandom distribution of gp120 N-linked glycosylation sites important for infectivity of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1992;89(6):2213–7. doi: 10.1073/pnas.89.6.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard CK, Spellman MW, Riddle L, Harris RJ, Thomas JN, Gregory TJ. Assignment of intrachain disulfide bonds and characterization of potential glycosylation sites of the type 1 recombinant human immunodeficiency virus envelope glycoprotein (gp120) expressed in Chinese hamster ovary cells. J Biol Chem. 1990;265(18):10373–82. [PubMed] [Google Scholar]
- Malenbaum SE, Yang D, Cavacini L, Posner M, Robinson J, Cheng-Mayer C. The N-terminal V3 loop glycan modulates the interaction of clade A and B human immunodeficiency virus type 1 envelopes with CD4 and chemokine receptors. J Virol. 2000;74(23):11008–16. doi: 10.1128/jvi.74.23.11008-11016.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuochi T, Spellman MW, Larkin M, Solomon J, Basa LJ, Feizi T. Carbohydrate structures of the human-immunodeficiency-virus (HIV) recombinant envelope glycoprotein gp120 produced in Chinese-hamster ovary cells. Biochem J. 1988;254(2):599–603. doi: 10.1042/bj2540599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JP, Doms RW. The entry of entry inhibitors: a fusion of science and medicine. Proc Natl Acad Sci U S A. 2003;100(19):10598–602. doi: 10.1073/pnas.1932511100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, O’Keefe BR, Sowder RC, 2nd, Bringans S, Gardella R, Berg S, Cochran P, Turpin JA, Buckheit RW, Jr, McMahon JB, Boyd MR. Isolation and characterization of griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp. J Biol Chem. 2005;280(10):9345–53. doi: 10.1074/jbc.M411122200. [DOI] [PubMed] [Google Scholar]
- O’Keefe BR, Shenoy SR, Xie D, Zhang W, Muschik JM, Currens MJ, Chaiken I, Boyd MR. Analysis of the interaction between the HIV-inactivating protein cyanovirin-N and soluble forms of the envelope glycoproteins gp120 and gp41. Mol Pharmacol. 2000;58(5):982–92. doi: 10.1124/mol.58.5.982. [DOI] [PubMed] [Google Scholar]
- Pantophlet R, Burton DR. GP120: target for neutralizing HIV-1 antibodies. Annu Rev Immunol. 2006;24:739–69. doi: 10.1146/annurev.immunol.24.021605.090557. [DOI] [PubMed] [Google Scholar]
- Reitter JN, Means RE, Desrosiers RC. A role for carbohydrates in immune evasion in AIDS. Nat Med. 1998;4(6):679–84. doi: 10.1038/nm0698-679. [DOI] [PubMed] [Google Scholar]
- Sanders RW, Venturi M, Schiffner L, Kalyanaraman R, Katinger H, Lloyd KO, Kwong PD, Moore JP. The mannose-dependent epitope for neutralizing antibody 2G12 on human immunodeficiency virus type 1 glycoprotein gp120. J Virol. 2002;76(14):7293–305. doi: 10.1128/JVI.76.14.7293-7305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlan CN, Pantophlet R, Wormald MR, Ollmann Saphire E, Stanfield R, Wilson IA, Katinger H, Dwek RA, Rudd PM, Burton DR. The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of alpha1-->2 mannose residues on the outer face of gp120. J Virol. 2002;76(14):7306–21. doi: 10.1128/JVI.76.14.7306-7321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattock RJ, Moore JP. Inhibiting sexual transmission of HIV-1 infection. Nat Rev Micro. 2003;1(1):25–34. doi: 10.1038/nrmicro729. [DOI] [PubMed] [Google Scholar]
- Shenoy SR, O’Keefe BR, Bolmstedt AJ, Cartner LK, Boyd MR. Selective interactions of the human immunodeficiency virus-inactivating protein cyanovirin-N with high-mannose oligosaccharides on gp120 and other glycoproteins. J Pharmacol Exp Ther. 2001;297(2):704–10. [PubMed] [Google Scholar]
- Trkola A, Purtscher M, Muster T, Ballaun C, Buchacher A, Sullivan N, Srinivasan K, Sodroski J, Moore JP, Katinger H. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J Virol. 1996;70(2):1100–8. doi: 10.1128/jvi.70.2.1100-1108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai CC, Emau P, Jiang Y, Agy MB, Shattock RJ, Schmidt A, Morton WR, Gustafson KR, Boyd MR. Cyanovirin-N inhibits AIDS virus infections in vaginal transmission models. AIDS Res Hum Retroviruses. 2004;20(1):11–8. doi: 10.1089/088922204322749459. [DOI] [PubMed] [Google Scholar]
- Tsai CC, Emau P, Jiang Y, Tian B, Morton WR, Gustafson KR, Boyd MR. Cyanovirin-N gel as a topical microbicide prevents rectal transmission of SHIV89.6P in macaques. AIDS Res Hum Retroviruses. 2003;19(7):535–41. doi: 10.1089/088922203322230897. [DOI] [PubMed] [Google Scholar]
- Van Damme EJM, Allen AK, Peumans WJ. Isolation and characterization of a lectin with exclusive specificity towards mannose from snowdrop (Galanthus nivalis) bulbs. FEBS Lett. 1987;215(1):140–44. [Google Scholar]
- Veazey RS, Klasse PJ, Schader SM, Hu Q, Ketas TJ, Lu M, Marx PA, Dufour J, Colonno RJ, Shattock RJ, Springer MS, Moore JP. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus-cell fusion. Nature. 2005;438(7064):99–102. doi: 10.1038/nature04055. [DOI] [PubMed] [Google Scholar]
- Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS, Komarova NL, Nowak MA, Hahn BH, Kwong PD, Shaw GM. Antibody neutralization and escape by HIV-1. Nature. 2003;422(6929):307–12. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- Weis WI, Taylor ME, Drickamer K. The C-type lectin superfamily in the immune system. Immunol Rev. 1998;163:19–34. doi: 10.1111/j.1600-065x.1998.tb01185.x. [DOI] [PubMed] [Google Scholar]
- Witvrouw M, Fikkert V, Hantson A, Pannecouque C, O’Keefe BR, McMahon J, Stamatatos L, de Clercq E, Bolmstedt A. Resistance of human immunodeficiency virus type 1 to the high-mannose binding agents cyanovirin N and concanavalin A. J Virol. 2005;79(12):7777–84. doi: 10.1128/JVI.79.12.7777-7784.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt R, Kwong PD, Desjardins E, Sweet RW, Robinson J, Hendrickson WA, Sodroski JG. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature. 1998;393(6686):705–11. doi: 10.1038/31514. see comments. [DOI] [PubMed] [Google Scholar]
- Wyatt R, Sodroski J. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science. 1998;280(5371):1884–8. doi: 10.1126/science.280.5371.1884. [DOI] [PubMed] [Google Scholar]