Abstract
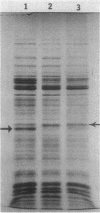
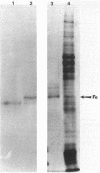
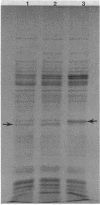
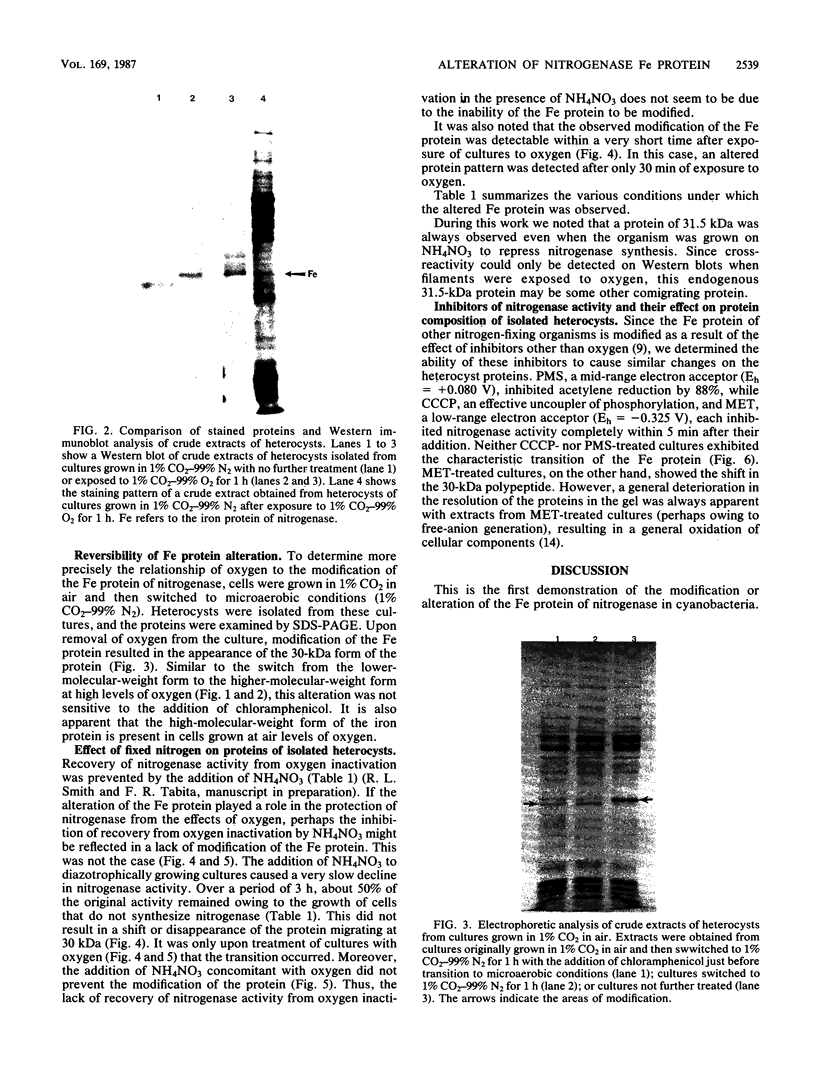
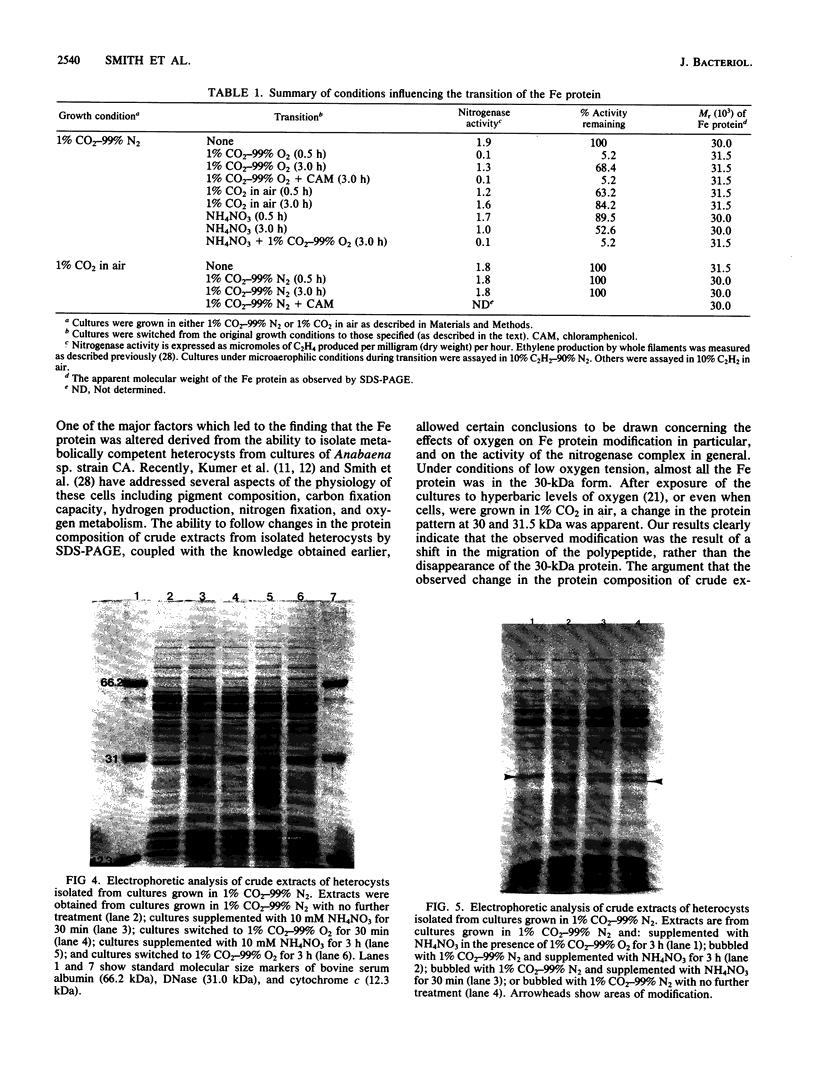
Changes in protein composition were noted when heterocysts of Anabaena sp. strain CA were isolated from filaments grown in 1% CO2-99% N2 and subsequently exposed to oxygen. Immunospecific Western blot analysis showed that the Fe protein of nitrogenase is altered. In cells grown under microaerobic conditions, the Fe protein was found in a form with an apparent molecular weight of 30,000. Exposure to oxygen caused a shift in the migration of this polypeptide to a position corresponding to an apparent molecular weight of 31,500. This modification was reversible upon removal of oxygen from the culture. Chloramphenicol did not inhibit the alteration in either direction. Suppression by ammonium nitrate of the recovery of nitrogenase activity from the effects of oxygen did not prevent the alteration of the protein. Other inhibitors of nitrogenase activity, (metronidazole, carbonyl cyanide m-chlorophenylhydrazone, and phenazine methosulfate) were tested for their effect on Fe protein modification. Alteration of the Fe protein may relate to the protection of nitrogenase from the deleterious effects of oxygen.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Bottomley P. J., Grillo J. F., Van Baalen C., Tabita F. R. Synthesis of nitrogenase and heterocysts by Anabaena sp. CA in the presence of high levels of ammonia. J Bacteriol. 1979 Dec;140(3):938–943. doi: 10.1128/jb.140.3.938-943.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carithers R. P., Yoch D. C., Arnon D. I. Two forms of nitrogenase from the photosynthetic bacterium Rhodospirillum rubrum. J Bacteriol. 1979 Feb;137(2):779–789. doi: 10.1128/jb.137.2.779-789.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotto J. W., Yoch D. C. Regulation of Rhodospirillum rubrum nitrogenase activity. Properties and interconversion of active and inactive Fe protein. J Biol Chem. 1982 Mar 25;257(6):2868–2873. [PubMed] [Google Scholar]
- Kanemoto R. H., Ludden P. W. Effect of ammonia, darkness, and phenazine methosulfate on whole-cell nitrogenase activity and Fe protein modification in Rhodospirillum rubrum. J Bacteriol. 1984 May;158(2):713–720. doi: 10.1128/jb.158.2.713-720.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley B. C., Nicholas D. J. Inhibition of nitrogenase activity by metronidazole in rhodopseudomonas capsulata. Arch Microbiol. 1981 Jul;129(5):344–348. doi: 10.1007/BF00406459. [DOI] [PubMed] [Google Scholar]
- Kumar A., Tabita F. R., Van Baalen C. High endogenous nitrogenase activity in isolated heterocysts of Anabaena sp. strain CA after nitrogen starvation. J Bacteriol. 1983 Aug;155(2):493–497. doi: 10.1128/jb.155.2.493-497.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd D., Pedersen J. Z. Metronidazole radical anion generation in vivo in Trichomonas vaginalis: oxygen quenching is enhanced in a drug-resistant strain. J Gen Microbiol. 1985 Jan;131(1):87–92. doi: 10.1099/00221287-131-1-87. [DOI] [PubMed] [Google Scholar]
- Lowery R. G., Saari L. L., Ludden P. W. Reversible regulation of the nitrogenase iron protein from Rhodospirillum rubrum by ADP-ribosylation in vitro. J Bacteriol. 1986 May;166(2):513–518. doi: 10.1128/jb.166.2.513-518.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludden P. W., Burris R. H. Purification and properties of nitrogenase from Rhodospirillum rubrum, and evidence for phosphate, ribose and an adenine-like unit covalently bound to the iron protein. Biochem J. 1978 Oct 1;175(1):251–259. doi: 10.1042/bj1750251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Mortenson L. E., Thorneley R. N. Structure and function of nitrogenase. Annu Rev Biochem. 1979;48:387–418. doi: 10.1146/annurev.bi.48.070179.002131. [DOI] [PubMed] [Google Scholar]
- Noel D., Nikaido K., Ames G. F. A single amino acid substitution in a histidine-transport protein drastically alters its mobility in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Biochemistry. 1979 Sep 18;18(19):4159–4165. doi: 10.1021/bi00586a017. [DOI] [PubMed] [Google Scholar]
- Paerl H. W., Kellar P. E. Nitrogen-fixing anabaena: physiological adaptations instrumental in maintaining surface blooms. Science. 1979 May 11;204(4393):620–622. doi: 10.1126/science.204.4393.620. [DOI] [PubMed] [Google Scholar]
- Pienkos P. T., Bodmer S., Tabita F. R. Oxygen inactivation and recovery of nitrogenase activity in cyanobacteria. J Bacteriol. 1983 Jan;153(1):182–190. doi: 10.1128/jb.153.1.182-190.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope M. R., Murrell S. A., Ludden P. W. Covalent modification of the iron protein of nitrogenase from Rhodospirillum rubrum by adenosine diphosphoribosylation of a specific arginine residue. Proc Natl Acad Sci U S A. 1985 May;82(10):3173–3177. doi: 10.1073/pnas.82.10.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson R. L., Postgate J. R. Oxygen and hydrogen in biological nitrogen fixation. Annu Rev Microbiol. 1980;34:183–207. doi: 10.1146/annurev.mi.34.100180.001151. [DOI] [PubMed] [Google Scholar]
- Saari L. L., Triplett E. W., Ludden P. W. Purification and properties of the activating enzyme for iron protein of nitrogenase from the photosynthetic bacterium Rhodospirillum rubrum. J Biol Chem. 1984 Dec 25;259(24):15502–15508. [PubMed] [Google Scholar]
- Smith B. E., Lang G. Mössbauer spectroscopy of the nitrogenase proteins from Klebsiella pneumoniae. Structural assignments and mechanistic conclusions. Biochem J. 1974 Feb;137(2):169–180. doi: 10.1042/bj1370169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. L., Kumar D., Zhang X. K., Tabita F. R., Van Baalen C. H2, N2, and O2 metabolism by isolated heterocysts from Anabaena sp. strain CA. J Bacteriol. 1985 May;162(2):565–570. doi: 10.1128/jb.162.2.565-570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoch D. C., Gotto J. W. Effect of light intensity and inhibitors of nitrogen assimilation on NH4+ inhibition of nitrogenase activity in Rhodospirillum rubrum and Anabaena sp. J Bacteriol. 1982 Aug;151(2):800–806. doi: 10.1128/jb.151.2.800-806.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumft W. G., Mortenson L. E. The nitrogen-fixing complex of bacteria. Biochim Biophys Acta. 1975 Mar 31;416(1):1–52. doi: 10.1016/0304-4173(75)90012-9. [DOI] [PubMed] [Google Scholar]