Abstract
OBJECTIVE
To investigate the effect of efforts in the early detection of prostate cancer using prostate-specific antigen (PSA) testing in the USA, by estimating the regional prevalence of androgen deprivation therapy (ADT) among older men in 1993–2000, and correlating the prevalence with early detection and aggressive treatment rates in 1987–91, as some authors predicted that ADT, a treatment traditionally reserved for advanced prostate cancer, would become less common over time as a result of such efforts.
PATIENTS AND METHODS
A sample of 5% of men who were Medicare beneficiaries was used in this prospective population-based cohort study. The main outcome measures were the overall prevalence of ADT (medical and surgical) in the cohort from 1993 to 2000, and correlations between rates of prostate procedures in the 306 USA hospital referral regions in 1987–91 and prevalence of ADT in those regions from 1993 to 2000.
RESULTS
The prevalence of ADT among these men in the USA increased steadily from 1.8% in 1993 to 2.9% in 2000 (P < 0.001). Regions with higher rates of prostate biopsy in 1987–91 had a higher prevalence of ADT in 1993, 1995 and 1997 (P < 0.05). Regions with higher rates of transurethral prostatectomy in 1987–91 had a higher prevalence of ADT in 1993–2000 (P < 0.01). Regions with higher rates of radical prostatectomy in 1987–91 had higher rates of ADT in 1993–99 (P < 0.05).
CONCLUSIONS
Widespread early detection and aggressive treatment for prostate cancer in the USA has been associated with more, not less, ADT among older men over time.
Keywords: prostate cancer, androgen deprivation, epidemiology, prostate-specific antigen
INTRODUCTION
Soon after the PSA test was introduced into the USA in 1987 for the follow-up of men with known prostate cancer, physicians began to use the test for early detection of the disease. By 1992 the incidence of prostate cancer in the USA had approximately doubled, largely as a result of an enormous reservoir of undiagnosed prostate cancer and widespread PSA screening [1]. This dramatic rise in incidence was accompanied by a substantial ‘stage shift’ toward more localized and fewer metastatic cases, along with an absolute decrease in the population-based rates of first presentations of men with metastases, as documented among prostate cancer cases reported to the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) programme [1,2]. Whether the overall incidence of metastatic prostate cancer has decreased is less certain, as patients initially reported as having localized cancer are not re-reported to the SEER programme if they later develop metastases.
Androgen deprivation therapy (ADT) is the traditional first-line treatment for metastatic prostate cancer, achieved either surgically through an orchidectomy or medically, principally through periodic injections of GnRH agonists [3]. Although ADT is an effective treatment for painful bony metastases [4], it also results in many unpleasant side-effects, and a substantial deterioration in overall health status [5–7]. Medical ADT, which is more widely used than surgery, is also extremely expensive, with drug costs of USA $5000–7000 annually [8].
Early in the ‘PSA era’ in the USA the observed stage shift to fewer first presentations with metastatic disease led some experts to hypothesise that one benefit of screening would be less need for ADT in the future as a treatment for advanced prostate cancer [9,10]. Indeed, in our group’s cost-effectiveness analysis of PSA screening for the USA Medicare programme, our ‘base case’ assumptions included savings attributable to early detection reducing the need for eventual treatment of advanced disease with ADT [11].
To determine whether widespread early-detection efforts in the USA have actually decreased rates of ADT among older men, we studied the epidemiology of early-detection efforts and aggressive treatment of prostate cancer among a random sample of men who were Medicare ‘fee-for-service’ beneficiaries in all USA hospital referral regions in the ‘early’ PSA era (1987–91) leading up to the peak incidence in 1992, and the epidemiology of ADT among men in the same regions in the ‘late’ PSA era (1993–2000) after the peak incidence. The USA Medicare programme provides health insurance to almost all Americans aged ≥ 65 years, largely through fee-for-service payments to hospitals, physicians and other providers, but in some case through pre-payments to health maintenance organizations (HMOs). We also used claims data to estimate the proportion of men who began ADT in 1998 and 1999 for different indications.
PATIENTS AND METHODS
We obtained the Medicare Part B (Carrier) standard analytical files (national 5% sample), MedPAR (Part A) and denominator files for the years 1987 through 2000 from the USA Center for Medicare and Medicaid Services, except for the 1992 Part B file, which was not available. The MedPAR files included hospital claims. The Part B files included physician and other supplier claims for services paid by the Part B programme in office, inpatient, outpatient, home and nursing-home settings. Each claim included a unique patient identifier, date of service, a Current Procedural Terminology (CPT) procedure code, and up to four International Center for Disease-9 (ICD-9) diagnosis codes. MedPAR files contained hospital discharge abstracts summarising acute-care stays for fee-for-service Medicare beneficiaries. MedPAR records for each discharge contained hospital use data including up to 10 ICD-9 diagnosis codes and six CPT procedure codes. The denominator file consisted of a unique record for each Medicare beneficiary entitled in that year and contained demographic, programme eligibility and enrolment information, including HMO status.
HMO enrolees do not generate MedPAR or Part B claims and thus we were unable to determine if these patients had a procedure or treatment during their HMO enrolment. Therefore, HMO enrolees were excluded from all analyses beginning with the month of first HMO enrolment. Additionally, we excluded invalid or duplicate claims, and limited analyses to claims for male beneficiaries entitled because they were aged ≥ 65 years, and those living in the USA.
One of the aims was to correlate measures of the early detection of prostate cancer and treatment intensity in the early PSA era with use of ADT in the late PSA era. We used rates of prostate biopsy and TURP as the measures of early detection intensity, and rates of radical prostatectomy (RP) as a measure of treatment intensity. Unlike PSA tests, which were not paid for by the Medicare programme in the early PSA era, biopsies are routinely recorded in Medicare claims data, and biopsy rates reflect the screening intensity with both PSA tests and DREs. Although men may be screened with PSA tests and DREs, a diagnosis of cancer cannot be made without proceeding to biopsy. TURPs are usually performed for BPH, but as 5–15% of these procedures result in the diagnosis of prostate cancer [12,13] they are also effectively prostate cancer screening tests, with a higher cancer detection rate than either PSA tests or a DRE [14].
For the period 1987–91 we used Part B claims to identify the following prostate diagnostic and therapeutic procedures on men: biopsy (CPT codes 55700, 55705), RP (CPT codes 55810, 55812, 55815, 55840, 55842, 55845), TURP (CPT codes 52601, 52612, 52614), and orchidectomy (CPT codes 54520, 56318, 54690). For the period 1993–2000 we used Part B claims to identify ADT: 3.6 mg monthly goserelin (CPT code J9202), 7.5 mg monthly leuprolide (CPT code J9217), and orchidectomy (CPT codes 54520, 56318, 54690). A diagnosis code specifying prostate cancer (ICD-9 codes 185, V1046, 2365) was required for ADT claims. Not all codes are valid in all years. MedPAR files were used to identify beneficiaries who received bilateral orchidectomy (ICD-9 codes 62.4 and 62.41) but did not have a Part B claim. Beneficiary residence postal zip codes were mapped to hospital referral regions (HRRs) following the methods of Wennberg and Cooper [15].
For the outcome cohort 1993–2000: Beneficiaries entered the analysis starting with the first month of fee-for-service Medicare enrolment and remained in the analysis until they joined an HMO, had ≤ 10 months of enrolment in a given year, or died. Numerator events and denominators were restricted to periods of uninterrupted fee-for-service enrolment. Beneficiaries who were discarded from the denominator for a given year for any reason were excluded from all subsequent years.
Biopsy events (1987–91) were restricted to one per beneficiary per day, but a beneficiary could have additional biopsies on different dates. RP, TURP and orchidectomy were restricted to one per beneficiary. If multiple claims were found, the earliest claim date was selected. For medical ADT (1993–2000), a beneficiary was defined as receiving ADT if he had claims for at least three doses of either leuprolide or goserelin, in any sequence, at least 25 days apart, or three or four doses billed on the same day, as that was the mechanism for reimbursement for longer-acting preparations of these agents as they became available during this period.
To calculate incidence rates in 1987–91, the number of biopsies, RPs and TURPs were each summed for the period 1987–91. Denominators in person-years differed for each procedure because, except for biopsy, once a beneficiary had a procedure, they were at no risk of having the same one again, and so the beneficiary was excluded from the denominator in future years. Rates were calculated within HRRs, and indirect standardization was used for age and race using population average rates from 1987–91. Age was divided into five categories of 65–69, 70–74, 75–79, 80–84, ≥ 85 years; race was categorized as black or not black. Indirect standardization was used because >20% of the age by race subclasses within HRRs had no numerator events and >7% had no denominator.
Prevalence was computed within HRRs and year. If the criteria for medical ADT were met, the beneficiary was considered androgen deprived for the year. Indirect standardization for age and race was done using population rates from 1993 to 2000.
To calculate the prevalence of ADT by orchidectomy in 1993–2000, carrier and MedPAR files were used to identify orchidectomies performed in 1987–2000. Any beneficiary receiving an orchidectomy was then considered androgen deprived until death or censoring, i.e. assuming the criteria for the denominator were met, a beneficiary who received an orchidectomy in 1995 would be in the numerator as androgen deprived from 1995 to 2000. Beneficiaries meeting the criteria for both medical and surgical ADT in a given year were considered surgically androgen deprived. Indirect standardization for age and race was done using population rates from 1993 to 2000.
To estimate the proportions of men initiating ADT for different indications in 1998–99, for men newly receiving ADT in 1998–99, the timing of orchidectomy or the first dose of leuprolide or goserelin in relation to the dates of RP (for codes, see above), the initiation of external beam radiotherapy (CPT codes 77400–77416, 77420, 77425, 77430), or brachytherapy (CPT codes 77776–77778) was used to estimate the proportion of men receiving ADT for different indications. The same diagnosis codes were required for radiation therapy as for RP (see above). These men were categorized as receiving ADT for recurrence if there was a claim for one of the three attempted curative treatments >6 months previously, for adjuvant therapy if there was a claim ≤ 6 months previously, and for neoadjuvant therapy if there was a claim following the initiation of ADT. Men with no claims for any of the three treatments before or after the initiation of ADT were considered to have received ADT as primary therapy. Men initiating ADT in 1998–99 were selected to allow the longest possible retrospective time for evidence of previous attempted curative treatment, while still providing a minimum follow-up of 1 year until the end of 2000, to identify men initiating neoadjuvant therapy. Before 1991, diagnostic codes were not available in Medicare claims for radiotherapy, and as radiation could have been given for other indications, only radiotherapy administered specifically for prostate cancer in 1991 or later was included in this analysis. For the retrospective analysis of previous attempted curative treatments, life-table methods were used with the interval in person-months censored before the first month of fee-for-service Medicare eligibility (usually at the 65th birthday), the month of a claim for an attempted curative treatment, or January 1991, whichever was later.
With HRR as the experimental unit, rank correlations were computed between the 1987–91 procedure rates and ADT prevalence for each year from 1993 to 2000. Tests for trends across the years 1993–2000 for ADT by orchidectomy, medicine, and orchidectomy and medicine combined were done by regression analysis (linear) and the Cochran-Armitage test for trend in binomial proportions. All tests indicated the rejection of the null hypothesis of no trend with P < 0.01.
RESULTS
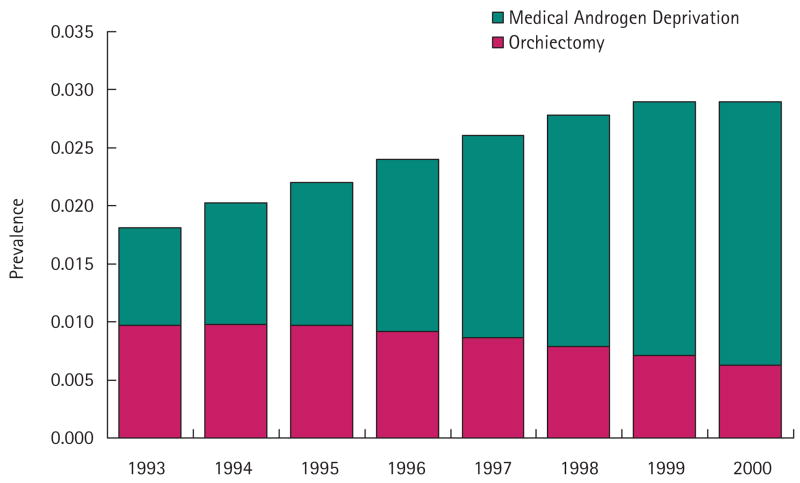
During the late PSA era (1993–2000) the age- and race-adjusted cross-sectional prevalence of ADT according to our definition increased steadily in the cohort from ≈ 1.8% to 2.9% of USA men who were Medicare beneficiaries (P < 0.001; Fig. 1). In 1993, ≈ 53% of men who were androgen deprived had undergone an orchidectomy, while in 2000 the percentage of ADT by orchidectomy decreased to 21%. Table 1 provides the numbers of men undergoing surgical and medical ADT in this 5% sample of USA men over this interval.
FIG. 1.
Cross-sectional prevalence of surgical and medical ADT in men in the USA Medicare population, 1993–2000.
TABLE 1.
Surgical and medical ADT in a 5% sample of men who were USA Medicare beneficiaries, and the correlation coefficients (with P values) of prostate procedures (1987–91) with the prevalence of ADT by year (1993–2000) within 306 HRRs
| Variable | 1993 | 1994 | 1995 | 1996 | 1997 | 1998 | 1999 | 2000 | |
|---|---|---|---|---|---|---|---|---|---|
| Number of men per year | |||||||||
| Surgical only | 4 609 | 4 681 | 4 588 | 4 331 | 3 980 | 3 542 | 3 122 | 2 744 | |
| Medical only | 4 255 | 5 283 | 6 149 | 7 242 | 8 280 | 9 199 | 9 925 | 10 309 | |
| Both | 262 | 241 | 232 | 151 | 119 | 90 | 80 | 63 | |
| Total | 9 126 | 10 205 | 10 969 | 11 724 | 12 379 | 12 831 | 13 127 | 13 116 | |
| Denominator | 530 368 | 521 781 | 507 871 | 490 354 | 469 993 | 451 171 | 439 576 | 435 249 | |
| Prevalence of ADT | |||||||||
| Biopsy rate 1987–91* | 0.14 (0.02) | 0.10 (0.07) | 0.13 (0.03) | 0.11 (0.06) | 0.11 (0.05) | 0.064 (0.27) | 0.023 (0.69) | 0.026 (0.65) | |
| TURP rate 1987–91 | 0.21 (<0.001) | 0.18 (0.002) | 0.18 (0.002) | 0.15 (0.008) | 0.21 (<0.001) | 0.21 (<0.001) | 0.18 (0.002) | 0.16 (0.005) | |
| RP rate 1987–91 | 0.24 (<0.001) | 0.20 (<0.001) | 0.22 (<0.001) | 0.18 (0.001) | 0.15 (0.008) | 0.15 (0.01) | 0.12 (0.03) | 0.086 (0.13) | |
Rates of prostate procedures are age- and race-adjusted.
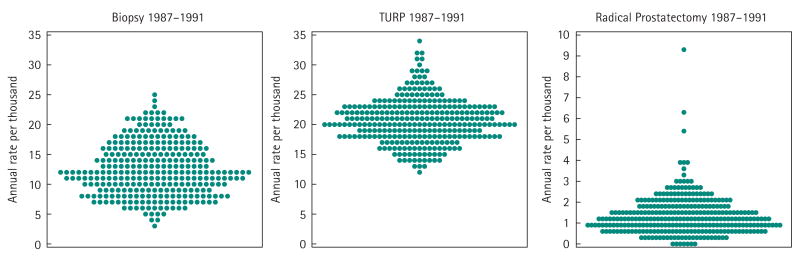
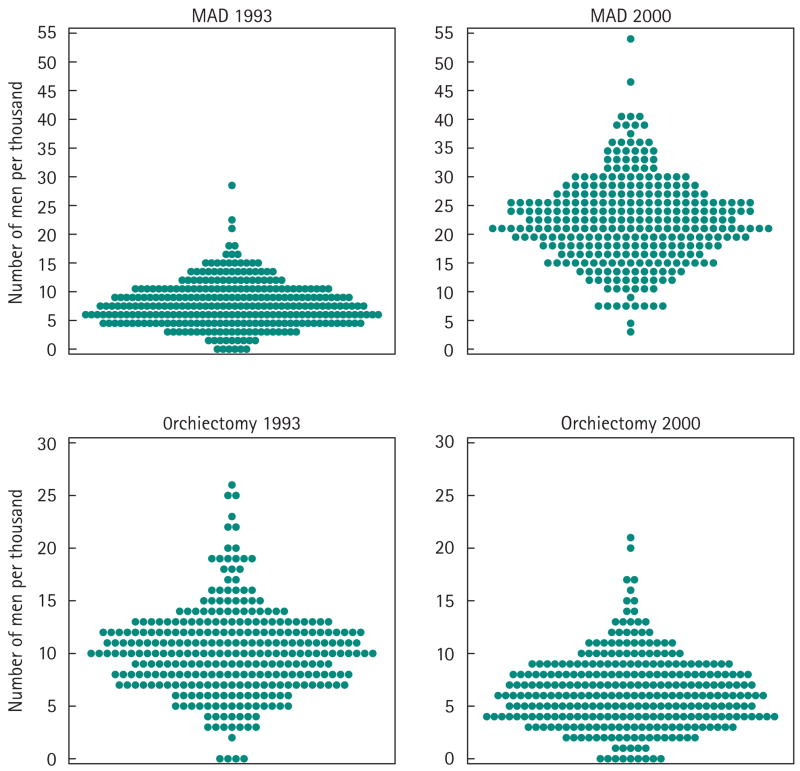
Figure 2 shows the variation in age- and race-adjusted rates of prostate biopsy, TURP and RP among the 306 HRRs in the USA over the early PSA era (1987–91). Figure 3 shows the variation in the proportion of men who were androgen deprived in these same 306 regions in 1993 and 2000; the degree of variation in the intervening years is similar. In these distribution plots, each point represents a region, and the overall pattern gives a sense of the degree of regional variability in the procedure or treatment (note the changes in rates or percentages on the y-axis among the plots).
FIG. 2.
Variation among the rates of age- and race-adjusted rates of prostate biopsy, TURP and RP in the early PSA era (1987–91) among men who were Medicare beneficiaries in 306 USA HRRs. *Scale for Radical Prostatectomy y axis differs to accommodate rate range.
FIG. 3.
Variation among the prevalence of ADT in 1993 and 2000 among men who were Medicare beneficiaries in 306 USA HRRs.
Table 1 also shows the correlations among the 306 HRR rank ordered according to their rates of prostate biopsy, TURP and RP in 1987–90, and their ranks ordered according to the proportion of men who were androgen deprived in each year from 1993 to 2000. Areas with higher rates of prostate biopsy in the early PSA era had significantly higher percentages of men on ADT in 1993, 1995 and 1997, with similar but marginally insignificant correlations in 1994 and 1996. Areas with higher rates of TURP in the early PSA era had significantly higher percentages of men on ADT from 1993 to 2000, and areas with higher RP rates in the early PSA era had higher percentages of men on ADT from 1993 to 1999. Higher rates of these three procedures in the early PSA era were not significantly associated with lower risks of ADT in any follow-up year in the late PSA era.
According to the classification algorithm, of 8282 men in the study population initiating ADT in 1998–99, 29.9% initiated it as neoadjuvant therapy and from the retrospective life-table analysis, 3.1% initiated it as adjuvant therapy and 14.4% for recurrence. The remaining 52.6% were classified as receiving ADT as primary therapy.
DISCUSSION
By contrast with some early expectations that widespread early detection and aggressive treatment of prostate cancer would reduce the future need for ADT in the USA, we found steadily increasing rates of ADT among men in the Medicare population in the late PSA era (1993–2000). Remarkably, ≈ 3% of men in the USA Medicare fee-for-service programme were androgen deprived by 2000. That percentage matches the 3% of men of this age who are eventually expected to die from prostate cancer, according to National Cancer Institute projections [16].
Moreover, regions with higher rates of early detection and aggressive treatment efforts in the early PSA era (1987–91) tended to have a higher, not lower, prevalence of ADT during the late PSA era. These data suggest that the assumptions in our 1997 cost-effectiveness analysis were erroneous [11], and that screening and aggressive treatment increase, rather than decrease, the future risk of ADT.
What explains the rising prevalence of ADT and its association with higher early detection and aggressive treatment efforts early in the PSA era? Our estimates suggest that neoadjuvant therapy, treatment for recurrences, and primary therapy are all common indications for ADT now, late in the PSA era. These observations probably reflect a relaxation in the indications for ADT over time. While ADT was commonly reserved for men with documented metastatic prostate cancer before the PSA era, men are now being treated with ADT for earlier stage disease [17,18]. For example, men who have prostate cancer discovered through screening usually have their PSA levels monitored serially after attempted curative radiotherapy or RP. If the PSA level begins to increase, even minimally, they are increasingly likely to be treated with ADT [19,20], even though the median interval between a rising PSA and documented metastases, at least after surgery, appears to be ≈ 8 years in the absence of such therapy [21]. In addition, men with clinically localized cancer but with clinical characteristics suggesting a higher risk for extracapsular disease are more often being treated with primary ADT [22]. While there is evidence from controlled trials supporting early or neoadjuvant ADT with radiation for clinically advanced prostate cancers in some circumstances [23–26], there is no similar evidence that such therapy for PSA-only recurrences after attempted curative therapy, or as primary therapy for clinically localized tumours, does more good than harm [27].
The relatively high prevalence of ADT among men in the USA Medicare population, at ≈ 3%, might be related to time trends in prostate cancer mortality observed in the USA. Some have opined that the trend toward lower prostate cancer mortality might be related to more intensive screening and aggressive treatment for early-stage disease [28]. However, similar trends are apparent in the UK in the absence of much screening [29]. Furthermore, our group reported that more aggressive screening and treatment in the Seattle-Puget Sound SEER area compared to the Connecticut SEER area during the early PSA era was not associated with lower prostate cancer-specific mortality over 11 years of follow-up [30]. Other experts suggested that more effective treatment for advanced disease, including ADT, might be primarily responsible for these trends [31]. Our data documenting the relatively high prevalence of ADT among older men are consistent with the latter hypothesis.
A limitation of the present analysis of the indications for men starting ADT in 1998–99 is the unavailability of claims for attempted curative treatments before the age of 65 years for any man and before 1991 for all men. These proportions certainly underestimate the true proportion of men treated with ADT for recurrence, and overestimate the true proportion of men treated as primary therapy. As our maximum retrospective interval was only 8 years (1999 to 1991) and the hazard rates for previous attempted curative treatment from the life-table analysis did not appear to decline earlier in the retrospective interval, this underestimate of treatment for recurrence and overestimate of primary treatment might have been substantial.
The widespread use of PSA testing has increased the probability from ≈ 10% to ≈ 18% that men in the USA will eventually have to cope with a prostate cancer diagnosis [16,32], even with incomplete penetration of screening [33,34]. As men weigh the pros and cons of PSA testing, perhaps they should also consider that such testing might increase their risk of eventually requiring ADT with its attendant morbidity and costs, but also with potential, although largely unconfirmed, benefits.
Acknowledgments
Research Support: Funded by Grant No. AG 0189783 from the National Institute of Ageing and Grant No. HS 10278 from the Agency for Health Care Research and Quality. The funders had no other role in the design or conduct of the study, or preparation of the manuscript.
Abbreviations
- ADT
androgen deprivation therapy
- SEER
Surveillance, Epidemiology, and End Results
- HMO
health maintenance organization
- RP
radical prostatectomy
- HRR
hospital referral region
- CPT
Current Procedural Terminology (code)
- ICD
International Center for Disease (code)
Footnotes
CONFLICT OF INTEREST
None declared.
Contributor Information
Michael J. Barry, Medical Practices Evaluation Center, Massachusetts General Hospital, Maine Medical Center, MA, USA
Michael A. Delorenzo, Center for Outcomes Research and Evaluation, Maine Medical Center, MA, USA
Elizabeth S. Walker-Corkery, Medical Practices Evaluation Center, Massachusetts General Hospital, Maine Medical Center, MA, USA
F. Lee Lucas, Center for Outcomes Research and Evaluation, Maine Medical Center, MA, USA.
David C. Wennberg, Center for Outcomes Research and Evaluation, Maine Medical Center, MA, USA
References
- 1.Brawley O. Prostate carcinoma incidence and patient mortality: the effects of screening and early detection. Cancer. 1997;80:1857–63. doi: 10.1002/(sici)1097-0142(19971101)80:9<1857::aid-cncr26>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 2.Farkas A, Schneider D, Perrotti M, Cummings KB, Ward WS. National trends in the epidemiology of prostate cancer, 1973–94: evidence for the effectiveness of prostate-specific antigen screening. Urology. 1998;52:444–9. doi: 10.1016/s0090-4295(98)00242-8. [DOI] [PubMed] [Google Scholar]
- 3.Garnick MB. Hormonal therapy in the management of prostate cancer: from Huggins to the present. Urology. 1997;49(Suppl 3A):5–15. doi: 10.1016/s0090-4295(97)00163-5. [DOI] [PubMed] [Google Scholar]
- 4.Chodak G, Keane T, Klotz L The Hormone Therapy Study Group. Critical evaluation of hormonal therapy for carcinoma of the prostate. Urology. 2002;60:201–8. doi: 10.1016/s0090-4295(02)01677-1. [DOI] [PubMed] [Google Scholar]
- 5.Herr HW, O’Sullivan M. Quality of life of asymptomatic men with nonmetastatic prostate cancer on androgen deprivation therapy. J Urol. 2000;163:1743–6. [PubMed] [Google Scholar]
- 6.Lubeck DP, Grossfeld GD, Carroll PR. The effect of androgen deprivation therapy on health-related quality of life in men with prostate cancer. Urology. 2001;58(Suppl 2):94–100. doi: 10.1016/s0090-4295(01)01250-x. [DOI] [PubMed] [Google Scholar]
- 7.Fowler FJ, Jr, McNaughton Collins M, Walker Corkery E, Elliott DB, Barry MJ. The impact of androgen deprivation on quality of life after radical prostatectomy for prostate carcinoma. Cancer. 2002;95:287–95. doi: 10.1002/cncr.10656. [DOI] [PubMed] [Google Scholar]
- 8.Mariani AJ, Glover M, Arita S. Medical versus surgical androgen suppression therapy for prostate cancer: a 10-year longitudinal cost study. J Urol. 2001;165:104–7. doi: 10.1097/00005392-200101000-00026. [DOI] [PubMed] [Google Scholar]
- 9.Catalona WJ. Screening for prostate cancer: enthusiasm. Urology. 1993;42:113–5. doi: 10.1016/0090-4295(93)90632-k. [DOI] [PubMed] [Google Scholar]
- 10.Andriole GL. The case for prostate cancer screening. Semin Urol. 1993;11:50–3. [PubMed] [Google Scholar]
- 11.Coley CM, Barry MJ, Fleming C, Fahs MC, Mulley AG. Early detection of prostate cancer. Part II: Estimating the risks, benefits and costs. American College of Physicians. Ann Intern Med. 1997;126:468–79. doi: 10.7326/0003-4819-126-6-199703150-00010. [DOI] [PubMed] [Google Scholar]
- 12.Tombal B, De Visccher L, Cosyns J, et al. Assessing the risk of unsuspected prostate cancer in patients with benign prostatic hypertrophy: a 13-year retrospective study of the incidence and natural history of T1a-T1b prostate cancers. BJU Int. 1999;84:1015–20. doi: 10.1046/j.1464-410x.1999.00386.x. [DOI] [PubMed] [Google Scholar]
- 13.Zigeuner RE, Lipsky K, Riedler I, et al. Did the rate of incidental prostate cancer change in the era of PSA testing? A retrospective study of 1127 patients. Urology. 2003;62:451–5. doi: 10.1016/s0090-4295(03)00459-x. [DOI] [PubMed] [Google Scholar]
- 14.Schroder FH, van der Maas P, Beemsterboer P, et al. Evaluation of the digital rectal examination as a screening test for prostate cancer. Rotterdam section of the European Randomized Study of Screening for Prostate Cancer. J Natl Cancer Inst. 1998;90:1817–23. doi: 10.1093/jnci/90.23.1817. [DOI] [PubMed] [Google Scholar]
- 15.Wennberg J, Cooper M. The Dartmouth Atlas of Health Care in the United States. Chicago, IL: American Hospital Publishing, Inc; 1998. [PubMed] [Google Scholar]
- 16.Ries L, Eisner M, Kosary C, et al. SEER Cancer Statistics Review 1975–2001. Bethesda, MD: National Cancer Institute; 2004. [Google Scholar]
- 17.Meng MV, Grossfeld GD, Sadetsky N, Mehta SS, Lubeck DP, Carroll PR. Contemporary patterns of androgen deprivation therapy use for newly diagnosed prostate cancer. Urology. 2002;60(Suppl 3A):7–12. doi: 10.1016/s0090-4295(02)01560-1. [DOI] [PubMed] [Google Scholar]
- 18.Cooperberg MR, Grossfeld GD, Lubeck DP, Carroll PR. National practice patterns and time trends in androgen ablation for prostate cancer. J Natl Cancer Inst. 2003;95:981–9. doi: 10.1093/jnci/95.13.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zietman A, Thakral H, Skowronski U, Shipley W. Freedom from castration: an alternative end point for men with localized prostate cancer treated by external beam radiation therapy. Int J Radiat Oncol Biol Phys. 2002;53:1152–9. doi: 10.1016/s0360-3016(02)02858-4. [DOI] [PubMed] [Google Scholar]
- 20.Mehta SS, Lubeck DP, Sadetsky N, Pasta DJ, Carroll PR. Patterns of secondary cancer treatment for biochemical failure following radical prostatectomy: data from CaPSURE. J Urol. 2004;171:215–9. doi: 10.1097/01.ju.0000100087.83112.23. [DOI] [PubMed] [Google Scholar]
- 21.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–7. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 22.Potosky AL, Knopf K, Clegg LX, et al. Quality-of-life outcomes after primary androgen deprivation therapy: results from the Prostate Cancer Outcomes Study. J Clin Oncol. 2001;19:3750–7. doi: 10.1200/JCO.2001.19.17.3750. [DOI] [PubMed] [Google Scholar]
- 23.The Medical Research Council Prostate Cancer Working Party Investigators Group. Immediate versus deferred treatment for advanced prostatic cancer: initial results of the Medical Research Council trial. Br J Urol. 1997;79:235–46. doi: 10.1046/j.1464-410x.1997.d01-6840.x. [DOI] [PubMed] [Google Scholar]
- 24.Messing EM, Manola J, Sarosdy M, Wilding G, Crawford ED, Trump D. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer. N Engl J Med. 1999;341:1781–8. doi: 10.1056/NEJM199912093412401. [DOI] [PubMed] [Google Scholar]
- 25.Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360:103–6. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 26.D’Amico AV, Manola J, Loffredo M, Renshaw AA, DellaCroce A, Kantoff PW. 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: a randomized controlled trial. JAMA. 2004;292:821–7. doi: 10.1001/jama.292.7.821. [DOI] [PubMed] [Google Scholar]
- 27.Aronson A, Seidenfeld J, Samson D, et al. Relative effectiveness and cost-effectiveness of methods of androgen suppression in the treatment of advanced prostate cancer. Rockville, MD: Agency for Health Care Policy and Research; 1999. [PMC free article] [PubMed] [Google Scholar]
- 28.Oottamasathien S, Crawford ED. Should routine screening for prostate-specific antigen be recommended? Arch Intern Med. 2003;163:661–2. doi: 10.1001/archinte.163.6.661. [DOI] [PubMed] [Google Scholar]
- 29.Oliver SS, Gunnell D, Donovan JL. Comparison of trends in prostate-cancer mortality in England and Wales and the USA. Lancet. 2000;355:1788–9. doi: 10.1016/s0140-6736(00)02269-8. [DOI] [PubMed] [Google Scholar]
- 30.Lu-Yao G, Albertsen PC, Stanford JL, Stukel TA, Walker-Corkery ES, Barry MJ. Natural experiment examining impact of aggressive screening and treatment on prostate cancer mortality in two fixed cohorts from Seattle area and Connecticut. BMJ. 2002;325:740–5. doi: 10.1136/bmj.325.7367.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albertsen PC. The prostate cancer conundrum. J Natl Cancer Inst. 2003;95:930–1. doi: 10.1093/jnci/95.13.930. [DOI] [PubMed] [Google Scholar]
- 32.Merrill RM, Weed DL, Feuer EJ. The lifetime risk of developing prostate cancer in white and black men. Cancer Epidemiol Biomarkers Prev. 1997;6:763–8. [PubMed] [Google Scholar]
- 33.Sirovich BE, Schwartz LM, Woloshin S. Screening men for prostate and colorectal cancer in the United States: does practice reflect the evidence? JAMA. 2003;289:1414–20. doi: 10.1001/jama.289.11.1414. [DOI] [PubMed] [Google Scholar]
- 34.Ross LE, Coates RJ, Breen N, Uhler RJ, Potosky AL, Blackman D. Prostate-specific antigen test use reported in the 2000 National Health Interview Survey. Prev Med. 2004;38:732–44. doi: 10.1016/j.ypmed.2004.01.005. [DOI] [PubMed] [Google Scholar]