Abstract
The introduction of antigen into animals causes antigen-specific T cells to divide and then die. Activated T cell death requires either of the death effector molecules, Bak or Bax. When T cells die, Bak and Bax change their conformations, a phenomenon that is thought to be required for Bak or Bax to drive cell death. Here we show that Bak changes conformation before activated T cells die, as detected by an antibody specific for a peptide near the NH2 terminus of Bak, but Bax does not change its shape markedly until after the cells are dead, as detected by an antibody specific for a peptide near the NH2 terminus of Bax. This latter finding is also true in activated T cells that lack Bak and are therefore dependent on Bax to die. This result suggests that Bax does not have to adopt its final, completely unfolded form until after the cells are dead.
It is by now well known that the death of many types of cells under many circumstances involves members of the Bcl-2 family of proteins (1–3). The Bcl-2 family has been subdivided by structural considerations and known functions into three subcategories (1–3). Some of the proteins, called “protectors,” are almost always antiapoptotic. Other Bcl-2–related proteins, called “messengers,” are thought to monitor the status of various parts of the cell and deliver the signal to the cell to die. A third subcategory of Bcl-2–related proteins is thought to execute cell death, hence the name of this family, “executioners.” Bak and Bax are members of this subcategory (4, 5).
Others have previously shown, however, that one of the two executioners, Bak or Bax, is required for the death of activated T cells (5) and that, in embryonic fibroblasts at least, the action of the messengers requires expression of either Bak or Bax (6). The means whereby the messengers induce the action of the executioners remains unresolved. There is some understanding, however, about the way in which the executioners kill cells. Thus, Bak and Bax change their shape during cell death (7–10), as detected by antibodies against peptides near the NH2 terminus (Nter) of the proteins. Determinants recognized by the antibodies are masked in Bak and Bax in healthy cells, but are revealed when the proteins change their shapes during cell death (9, 11). After changing their shape, Bak and Bax aggregate and may form pores in the outer membrane of mitochondria (12–17), for example, leading to loss of mitochondrial potential, release of cytochrome c, and death of the cell.
Here we examine these events in activated T cells as they die. Resting, activated, and dying T cells all contain about the same amounts of the executioners, Bak or Bax. Most of the Bak in the T cells, regardless of their state, is membrane bound, probably on mitochondria, as reported previously (9). Some of this Bak changes its shape before the T cells die, as detected by a polyclonal anti-BakNter antibody. In contrast, most of the Bax in T cells is cytoplasmic before the T cells die, as detected by a monoclonal anti-BaxNter antibody. Only a small amount of the Bax is bound to membranes (18), and only a small proportion of the Bax changes its shape. This is true even if the T cells lack Bak. Thus, the death of activated T cells requires Bak or Bax, but death is not necessarily preceded by a large change in the location of the executioner protein or by a conversion of the executioner protein to its terminal, fully oligomerized form. Some intermediate form of the executioner proteins may actually drive death of the cell.
RESULTS AND DISCUSSION
Rapid death of activated T cells requires either Bak or Bax
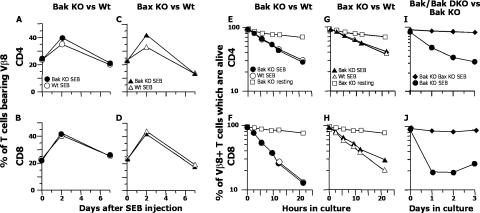
Activated T cells die rapidly in animals after antigen has disappeared (19–21). To find out if this death were dependent on Bak or Bax, Bak KO or Bak KO and control animals were injected with the Vβ8-engaging superantigen, staphylococcal enterotoxin B (SEB), and tested 2 and 7 d later for their numbers of Vβ8+ T cells. Vβ8+ T cells lacking either Bak or Bax expanded and died in vivo in response to SEB with normal kinetics (Fig. 1, A–D). Similar results were observed in vitro. Bak KO or Bax KO and its wild-type littermates were given SEB. 2 d later, their T cells were harvested, cultured for various lengths of time, and monitored for the percentage of activated Vβ8+ T cells that had died (21). As shown in Fig. 1 (E–H), activated CD4+ or CD8+ T cells lacking Bak or Bax died at the same rate as those from wild-type mice, and much more rapidly than resting T cells. To check whether Bak or Bax was needed at all for this in vitro death, we activated T cells lacking both Bak and Bax (produced in chimeric mice) and compared their rates of death with those of T cells lacking only Bak (Fig. 1, I and J). As expected, activated T cells lacking Bak died rapidly. Activated T cells lacking both Bak and Bax, however, died very slowly. Thus, as others have shown (5), either Bak or Bax is required for the rapid death of activated T cells, and the two proteins appear to act interchangeably and with similar kinetics.
Figure 1.
Activated T cell death requires either Bak or Bax. (A–D) BL6, Bak KO, Bax KO, and Bax+/+ littermates of Bax KO mice were injected with 100 ug SEB i.v. At the indicated times, cells were harvested from spleens and the percentage of CD4+ or CD8+ T cells bearing Vβ8x was determined. Closed and open symbols represent the results for KO and wild-type mice, respectively. Results shown are means ± standard deviations of data from three mice at each time point. (E–J) BL6, Bak KO, Bax KO, Bax+/+, and RAG KO mice reconstituted with Bak KO or Bak KO Bax KO bone marrow were given SEB. 63 h later, T cells were purified from their spleens and lymph nodes and cultured. At various times thereafter, the percentages of CD4+ and CD8+ Vβ8+ T cells that were alive, as defined by their light scatter properties (reference 29), were determined. (E and F) •, SEB-injected Bak KO; ◯, SEB-injected BL/6; □, uninjected Bak KO cells. (G and H) ▴, SEB-injected Bax KO; ▵, SEB-injected Bax+/+ littermates; □, uninjected Bax KO cells. (I and J) ♦, SEB-injected Rag KO mice reconstituted with Bak KO Bax KO fetal liver cells; •, SEB-injected Rag KO mice reconstituted with Bak KO fetal liver cells. (E, G, and I) Results for CD4+ T cells. (F, H, and J) Results for CD8+ T cells. Results shown are means ± standard deviations of data from three samples analyzed at each time point in one experiment. Results are also illustrative of two independent experiments for the Bak KO Bax KO T cells and of more than four independent experiments for the Bak KO and Bax KO cells.
Changes in the conformation of Bak, but not Bax, precede activated T cell death
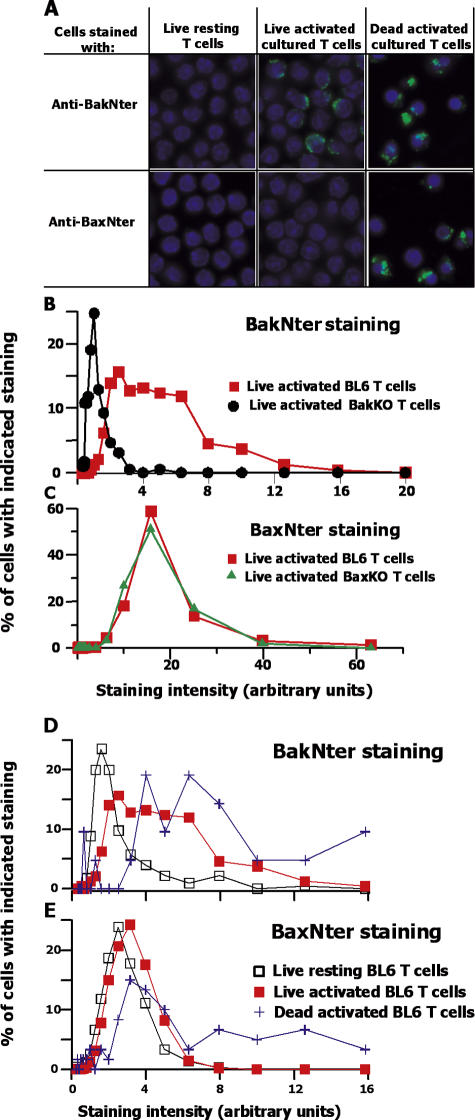
Bak and Bax change their conformation during cell death, measured by the fact that antibodies against peptide sequences near the Nter ends of these proteins (anti-BakNter, anti-BaxNter; references 7–11) do not react with the proteins in healthy cells, but do react when the cells are dead. We used microscopy to follow these changes in conformation of Bak and Bax during T cell death. Healthy resting T cells were obtained from unimmunized mice. Activated cells that were preparing to die and activated dead T cells were obtained by sorting live and dead Vβ8+ cells that had been activated with SEB in vivo, isolated 2–3 d later, and cultured for 12 h, at which time about half of the cells are dead and 20% are destined to die in the next 12 h (Fig. 1). Resting T cells did not stain above the background (determined with Bak KO or Bax KO T cells) with anti-BakNter or anti-BaxNter. Dead activated T cells stained strongly with either antibody. Healthy T cells that were preparing to die stained quite well with anti-BakNter but poorly with anti-BaxNter (Fig. 2 A).
Figure 2.
Before activated T cells die, a considerable amount of Bak, but not Bax, changes its shape. BL6 mice were injected with 100 ug SEB. 2 d later, T cells were isolated from their spleens and lymph nodes and cultured for 7 h. The cells were then sorted based on PI staining to obtain Vβ8+ live and dead populations, and stained for NterBak or NterBax. (A) Typical fields of the different types of cells are shown. (B–E) Individual cells were ringed and analyzed for their extent of NterBak (B and D) or NterBax (C and E) staining and surface area. The level of staining of each cell was corrected for background levels of staining, with the following calculation: [(the amount of staining with the particular antibody/surface area) − (background staining/surface area)] × surface area. The cells were then assigned to bins based on their level of staining with each antibody. Results shown are as follows: (the number of cells in each bin/total number of cells analyzed) × 100. Results represent analyses of at least 226 live cells and 21 dead cells of each type. The experiments shown in B and D and in C and E were performed at different times. Settings and conditions were slightly different between the two experiments shown, which led to differences in the level of anti-Bax staining of activated cells.
To quantitate these results, we measured the intensity of staining of individual cells with anti-BakNter or anti-BaxNter. Anti-BakNter had a low level of background staining on Bak KO–activated T cells. Wild-type resting T cells stained at about the same level as Bak KO T cells, indicating that no Bak was in the unfolded state in these cells. Live activated T cells that had been cultured to bring them to the brink of death, on the other hand, stained quite well with the anti-BakNter, and dead activated T cells stained even more intensely (Fig. 2, B and D).
Anti-BaxNter had some background staining on Bax KO–activated T cells (Fig. 2 C), and, again, levels of staining with this antibody of wild-type resting T cells were not above the background. As before, anti-BaxNter staining of activated live T cells, cultured to bring them close to death, was only barely brighter than that of resting T cells or Bax KO T cells. Dead activated T cells, on the other hand, stained well with anti-BaxNter (Fig. 2, C and E).
Thus, quite a lot of Bak changes its shape in activated T cells before they die, but most of the Bax does not expose its Nter sequence until after the cells are dead.
Bim acts upstream of Bak or Bax
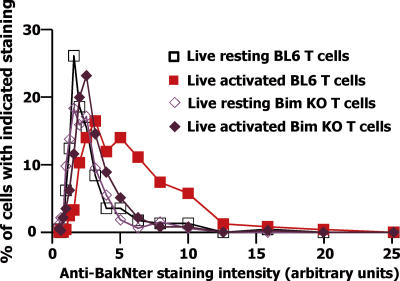
We and others have reported that activated T cells lacking Bim (Bim KO) die slowly (22–24). To find out whether Bim acts before or after Bak or Bax in the events leading to T cell death, we studied whether Bim was needed for Bak or Bax to change its shape as T cells prepare to die. T cells were harvested from SEB-immunized Bim KO and wild-type mice and cultured for a short time. The cells were then sorted to purify the cells bearing Vβ8, stained with anti-BakNter or anti-BaxNter, and analyzed as described above.
As before, living activated wild-type cells contained a considerable amount of Bak that had changed its shape and was now detectable with the anti-BakNter antibody. However, the change of Bak was barely detectable in cells lacking Bim (Fig. 3). Bax did not detectably change its shape at all in Bim KO T cells (not depicted). Thus, in activated T cells, Bim is involved in the change in configuration of Bak, which precedes cell death.
Figure 3.
The change in shape of Bak during T cell death requires Bim. Bim KO and Bim+/+ littermates were immunized with SEB as described in the legend to Fig. 2. Live Vβ8x+ T cells were purified by cell sorting, stained with anti-NterBak, and analyzed as described in the legend to Fig. 2. Results shown are the percentages of cells of each type that fell into each bin, calculated as described in the legend to Fig. 2. Anti-NterBak staining of at least 226 cells of each type was analyzed.
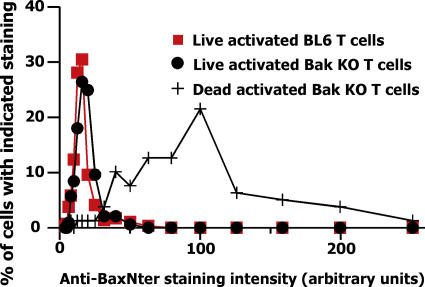
Absence of Bak does not affect the kinetics with which Bax changes its shape
These results suggested that Bak might be more crucial than Bax to the death of activated T cells. However, as shown in Fig. 1, activated T cells that lack Bak die at the same rate as wild-type cells (Fig. 1). Therefore, we predicted that in the absence of Bak, Bax might change its shape more extensively. To test this, activated T cells from C57BL/6 (BL6) and Bak KO mice were cultured, sorted for live and dead cells based on propidium iodide (PI) staining, and stained with anti-BaxNter. The intensity of anti-BaxNter staining/cell was estimated as before. The BL6 cells stained poorly with anti-BaxNter (Figs. 2, C and E, and 4). The dead activated Bak KO T cells stained very well with anti-BaxNter. Surprisingly, however, the staining of live activated Bak KO T cells with anti-BaxNter was barely above that of the BL6 cells (Fig. 4). These results were confirmed by attempts to use the anti-BaxNter to immunoprecipitate Bax from Bak KO or BL6 resting, activated, or activated cells that were preparing to die. Under no circumstances could we see evidence of precipitation with the antibody (not depicted).
Figure 4.
The extent to which Bax changes its shape during cell death is only slightly affected by lack of Bak. Cells were prepared, stained, and analyzed as described in the legend to Fig. 2. Results shown are the percentages of cells of each type that fell into each bin, calculated as described in the legend to Fig. 2. Bax staining in at least 292 live cells of either type and 79 dead cells was analyzed.
These results show that the kinetics with which Bax changes its conformation are not affected very much by the absence of Bak from the cells, and suggest that just a small amount of conversion, if any, of Bax to its supposed death-dealing, fully converted form is sufficient to drive cell death.
Bax does not change its location when T cells are activated
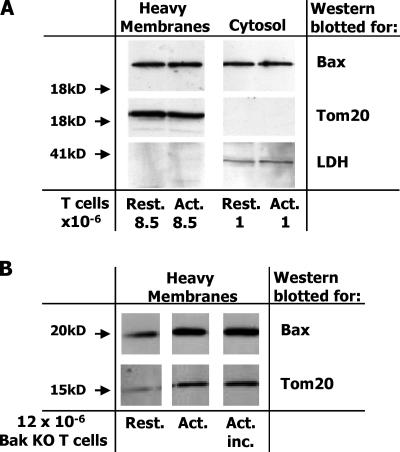
The executioners, Bak and Bax, are thought to act by disrupting the integrity of intracellular membranes, particularly those of the mitochondria (12–17). Because most of the Bax in healthy cells is cytoplasmic (18), we thought that Bax might translocate to membranes as T cells prepared to die and that such movement might be particularly marked in cells lacking Bak. To check this, T cells were isolated from normal or 3 d SEB-primed BL6 mice. The cells were lysed by passage through a homogenizer. The lysates were cleared of intact cells and nuclei by a low speed spin. Heavy membranes, which include mitochondria, were then prepared with an intermediate speed spin. The heavy membrane and supernatant fractions were then Western blotted.
Control proteins were found in the expected fraction, Tom20 was found in the heavy membrane fraction, and lactate dehydrogenase was found in the supernatant. Resting and activated T cells contained about the same amount of Bax. Only ∼10% of the Bax in the cell was in the heavy membrane fraction, regardless of whether or not the T cells had been activated (Fig. 5 A and Fig. S1, which is available at http://www.jem.org/cgi/content/full/jem.20051736/DC1).
Figure 5.
The amount of Bax bound to heavy membranes does not change before T cells die. (A) Vβ8x T cells were activated in BL6 mice with SEB. 3 d later, T cells were isolated from the animals and cultured for 7 h. Live Vβ8x T cells were isolated by cell sorting and homogenized without detergent. Homogenates were made from resting T cells and heavy membrane and cytosol fractions prepared from each homogenate, run on SDS PAGE, and Western blotted for the indicated proteins. Cell equivalents run in each lane are shown at the bottom of the panel. (B) BAK KO mice were immunized with 100 ug SEB. 3 d later, spleen and lymph node T cells were isolated from these and other unimmunized (Rest.) BAK KO mice. Half of the T cells from the immunized BAK KO mice were incubated for 5 h to bring them closer to death (Act. inc.). The other half were kept on ice (Act.). Live cells were then purified from all the samples, and heavy membrane and cytosol fractions were prepared, run on SDS PAGE, and Western blotted as described in A. Blots shown are representative of at least three independent experiments.
Similar experiments were performed with cells from Bak KO mice. In this case, there was somewhat more Bax/106 cells in the heavy membrane fractions from activated T cells than in the fractions from resting T cells, a result we observed intermittently in the activated T cell preparations. The increase in Bax was paralleled by a similar increase in the amount of Tom20 in the heavy membrane fractions, such that the ratio of Bax/Tom20 remained constant within the different types of cells, suggesting that the result is due to an overall increase in the numbers of mitochondria in activated versus resting T cells. In spite of this, the amounts of Bax and Tom20 in the heavy membrane fractions were unaffected by incubation of the activated T cells to bring them closer to death (Fig. 5 B and Fig. S2, which is available at http://www.jem.org/cgi/content/full/jem.20051736/DC1).
These experiments showed that the amount of Bax associated with heavy membranes changes only slightly when cells are activated, and that the concentration of Bax/heavy membrane probably remains constant regardless of the activation state or Bak content of the cell. Moreover, Bax does not change its conformation, as judged by its anti-Nter monoclonal antibody, before the T cells die. We believe these results have three interpretations. First, Bax may move to membranes, change its shape, as defined by the monoclonal anti-BaxNter antibody, and deliver its lethal hit to the Bak KO–activated T cell exactly at the moment the T cell dies. This is a possibility that we certainly cannot dismiss. However, in the microscopy experiments described here, T cell death was defined by permeability to PI or Ficoll/diatrizoate, which is loss of plasma membrane integrity. This is a relatively late event in T cell death. T cells expose phosphatidyl serine and thus bind annexin V, and lose their mitochondrial function before their plasma membranes become permeable. Second, Bax may not have to change its levels on heavy membranes or change its shape to deliver its lethal hit to T cells. The native form of Bax, defined by nuclear magnetic resonance experiments (25) may, under some circumstances, be able to do the job. Finally, Bax may have several conformations. One is folded as discerned in the nuclear magnetic resonance analyses (25) and is harmless to the cell. A second conformation may be analogous to the form of Bak detected by the polyclonal anti–Bak21-35 antisera. We suggest that this conformation of Bax does not react with the anti–Bax12-24 monoclonal antibody, and it is this form of Bax and the analogous form of Bak that actually kill the cell. A third conformation of Bax may be represented by completely unfolded, aggregated protein. This form reacts with the anti–BaxNter12-24 monoclonal antibody and appears only after the cell is dead.
We prefer the last of these hypotheses because we think it unlikely that Bak and Bax act in such different ways to kill cells. An additional thought in favor of the last hypothesis is the fact that the anti–BakNter23-35 antisera and anti-BaxNter monoclonal antibody do not react with exactly analogous stretches of the two proteins. The determinant recognized by the anti-BakNter antisera actually lies a little COOH-terminal of the determinant recognized by the anti–BaxNter12-24 monoclonal antibody. Therefore, it is possible that the intermediate death-dealing form of the two proteins has a partial shape change near the end of its first α helix, exposing the determinant recognized by anti–Bak21-35. Only after unfolding of the proteins is complete do their NH2 termini become completely accessible to antibodies such as the anti–BaxNter12-24.
In summary, these experiments support the idea that either Bak or Bax is needed for the death of activated T cells, with either acting downstream of Bim. However, under circumstances in which death is entirely dependent on Bax, as in Bak KO T cells, dramatic changes in the amount and location of Bax and complete unfolding of the protein are not needed. Perhaps some intermediate form of the protein is the actual executioner of T cell death.
MATERIALS AND METHODS
Mice.
BL6 mice were purchased from The Jackson Laboratory. VβDO animals, expressing a transgene for a Vβ8.2+ T cell receptor β chain (26), were bred in the Biological Resource Center at the National Jewish Medical and Research Center. Bak KO (4), Bax KO (27), and Bim KO (22) mice were bred from animals provided by T. Lindsten and C. Thompson (University of Pennsylvania, Philadelphia, PA), S. Korsmeyer (Harvard University, Boston, MA), and P. Bouillet and A. Strasser (Walter and Eliza Hall Institute, Melbourne, Australia). Because we have been unable to produce live Bak KO Bax KO double KO mice in our facility, double KO T cells were obtained from chimeric mice prepared as follows. Bak KO Bax KO fetuses were obtained at day 15 of pregnancy from Bak KO Bax+/− females mated with Bak KO Bax+/− males. Fetal livers were harvested from the mice, and cell suspensions from the birds were frozen in 90% FBS, 10% DMSO. Embryos were typed by PCR for Bax genotype. Fetal liver cells from the appropriate mice were transferred into lethally irradiated BL6 recipients. T cells were activated and harvested from these mice 8 wk later.
Vβ8+ T cells were activated in vivo as described previously (21) by i.v. injection of 100 ug SEB 2–3 d before harvest of the cells.
All animals were maintained in the Biological Resource Center of the National Jewish Medical and Research Center under specific pathogen-free conditions and in accordance with federal guidelines approved by the National Jewish Institutional Animal Care and Use Committee.
T cell preparation, staining, and isolation by sorting.
T cells were purified by passage over nylon wool columns. In some cases, they were cultured for 5–12 h in complete culture medium at 37°C. Live cells were purified on lymphocyte separation medium (Ficoll/diatrizoate; MP Biomedicals) step gradients or by sorting. To isolate live and dead cells, cells were cultured, stained with anti-Vβ8x and PI as described previously, and sorted into live and dead populations based on PI staining (28). The cells were then stained with anti-BakNter or anti-BaxNter and DAPI and analyzed as described in Results.
Immunofluorescence microscopy.
Lymph node cells were harvested from BL6, VβDO, Bak KO, or Bax KO mice that had been injected with 150 μg SEB 2 d previously. T cells were purified by passage through nylon wool columns, and Vβ8+ T cells were purified by flow cytometry (except for cells from VβDO animals, for which purification was not necessary). Some of the activated T cells were cultured for 5–12 h to bring them closer to death. In this case, live and dead cells were subsequently purified by cell sorting. Resting T cells were harvested from similar unmanipulated mice and purified simply by passage through nylon wool columns. Aliquots of the activated T cells were cultured for 18 h to confirm their rapid death in vitro. Resting, activated, and activated plus cultured T cells were then loaded onto coverslips pretreated with 100 μg/ml poly-l-lysine and incubated at 37°C for 10 min. Immobilized cells were fixed with prewarmed 3% paraformaldehyde at 37°C for 15 min and permeabilized with 0.2% CHAPS (3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate; Pierce Chemical Co.). CHAPS was used to avoid untoward induction of conformational changes in Bak or Bax (11). Permeabilized cells were blocked with DMEM plus 5% FBS for 30 min and incubated with anti-BaxNter (catalog no. 06-499; Upstate Biotechnology) or anti-BakNter (catalog no. 06-536; Upstate Biotechnology) for 2 h, and then with Alexa Fluor 488– or Alexa Fluor 647–conjugated anti–rabbit IgG for 1 h. A wash of six changes of PBS was applied after each antibody incubation. Finally, the cells were stained with 1 μM Hoechst 33342 for 5 min and mounted onto slides. Visual data were acquired by examining slides under a Leica DMXRA epifluorescence microscope equipped with a SensiCam CCD camera (PCO CCD Imaging) and analyzed with SlideBook software (Intelligent Imaging Innovations).
Subcellular fractionation by differential sedimentation and analysis by Western blotting.
T cells were resuspended at a concentration of 4.5 × 107 cells/ml in ice-cold mitochondrial buffer (200 mM mannitol, 70 mM sucrose, 10 mM Hepes, 1 mM EDTA, 1 mM EGTA, pH 7.5; reference 7) supplemented with 0.1 mM PMSF, 1 mM DTT, and the protease inhibitor cocktail, Complete Mini (Roche Diagnostics). Homogenization was performed in a ball-bearing homogenizer (H&Y Enterprise) with 20 strokes. Cell lysates were centrifuged twice at 950 g for 15 min to pellet out the nuclei and intact cells. The supernatants were then centrifuged at 10,000 g for 25 min to acquire the mitochondria-enriched heavy membrane fraction. Proteins were separated by SDS-PAGE on Criterion 10–20% gradient gels (Bio-Rad Laboratories) and transferred to Hybond ECL nitrocellulose membranes (GE Healthcare) by a semidry method. Membranes were blocked at room temperature for 1–2 h with BLOTTO (5% milk and 1% FBS in Tris-buffered saline [TBS] plus 0.05% Tween-20), incubated with the primary antibody for 4 h, washed with several changes of TBS-T (TBS plus 0.05% Tween-20), and incubated with secondary antibodies for 3 h. Bound secondary antibodies were visualized by ECL detection reagents (GE Healthcare). The primary antibodies used were rabbit anti-Bak pAb (Upstate Biotechnology), goat anti–lactate dehydrogenase pAb (Rockland Immunochemicals), rabbit anti-Bax N-20 pAb, and anti-TOM20 pAb (Santa Cruz Biotechnology, Inc.).
Online supplemental material.
Figs. S1 and S2 show the complete gel bands for the Western blots shown in Fig. 5 (A and B, respectively) and are available at http://www.jem.org/cgi/content/full/jem.20051736/DC1.
Supplemental Material
Acknowledgments
The authors would like to thank Drs. Richard Youle, Andreas Strasser, Philippe Bouillet, Tullia Lindsten, Craig Thompson, and the late Stanley Korsmeyer for their generous gifts of monoclonal antibody–secreting cells and mice.
This work was supported in part by United States Public Health Service grants AI-17134, AI-18785, AI-22295, and AI-52225.
The authors have no conflicting financial interests.
References
- 1.Cory, S., and J.M. Adams. 2002. The Bcl2 family: regulators of the cellular life-or-death switch. Nat. Rev. Cancer. 2:647–656. [DOI] [PubMed] [Google Scholar]
- 2.Opferman, J.T., and S.J. Korsmeyer. 2003. Apoptosis in the development and maintenance of the immune system. Nat. Immunol. 4:410–415. [DOI] [PubMed] [Google Scholar]
- 3.Green, D.R., and G. Kroemer. 2004. The pathophysiology of mitochondrial cell death. Science. 305:626–629. [DOI] [PubMed] [Google Scholar]
- 4.Lindsten, T., A.J. Ross, A. King, W.X. Zong, J.C. Rathmell, H.A. Shiels, E. Ulrich, K.G. Waymire, P. Mahar, K. Frauwirth, et al. 2000. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol. Cell. 6:1389–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rathmell, J.C., T. Lindsten, W.X. Zong, R.M. Cinalli, and C.B. Thompson. 2002. Deficiency in Bak and Bax perturbs thymic selection and lymphoid homeostasis. Nat. Immunol. 3:932–939. [DOI] [PubMed] [Google Scholar]
- 6.Cheng, E.H., M.C. Wei, S. Weiler, R.A. Flavell, T.W. Mak, T. Lindsten, and S.J. Korsmeyer. 2001. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol. Cell. 8:705–711. [DOI] [PubMed] [Google Scholar]
- 7.Gross, A., J. Jockel, M.C. Wei, and S.J. Korsmeyer. 1998. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 17:3878–3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu, Y.T., and R.J. Youle. 1998. Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J. Biol. Chem. 273:10777–10783. [DOI] [PubMed] [Google Scholar]
- 9.Griffiths, G.J., B.M. Corfe, P. Savory, S. Leech, M.D. Esposti, J.A. Hickman, and C. Dive. 2001. Cellular damage signals promote sequential changes at the N-terminus and BH-1 domain of the pro-apoptotic protein Bak. Oncogene. 20:7668–7676. [DOI] [PubMed] [Google Scholar]
- 10.Schinzel, A., T. Kaufmann, M. Schuler, J. Martinalbo, D. Grubb, and C. Borner. 2004. Conformational control of Bax localization and apoptotic activity by Pro168. J. Cell Biol. 164:1021–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu, Y.T., and R.J. Youle. 1997. Nonionic detergents induce dimerization among members of the Bcl-2 family. J. Biol. Chem. 272:13829–13834. [DOI] [PubMed] [Google Scholar]
- 12.Antonsson, B., F. Conti, A. Ciavatta, S. Montessuit, S. Lewis, I. Martinou, L. Bernasconi, A. Bernard, J.J. Mermod, G. Mazzei, et al. 1997. Inhibition of Bax channel-forming activity by Bcl-2. Science. 277:370–372. [DOI] [PubMed] [Google Scholar]
- 13.Schlesinger, P.H., A. Gross, X.M. Yin, K. Yamamoto, M. Saito, G. Waksman, and S.J. Korsmeyer. 1997. Comparison of the ion channel characteristics of proapoptotic BAX and antiapoptotic BCL-2. Proc. Natl. Acad. Sci. USA. 94:11357–11362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marzo, I., C. Brenner, N. Zamzami, J.M. Jurgensmeier, S.A. Susin, H.L. Vieira, M.C. Prevost, Z. Xie, S. Matsuyama, J.C. Reed, and G. Kroemer. 1998. Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis. Science. 281:2027–2031. [DOI] [PubMed] [Google Scholar]
- 15.Nouraini, S., E. Six, S. Matsuyama, S. Krajewski, and J.C. Reed. 2000. The putative pore-forming domain of Bax regulates mitochondrial localization and interaction with Bcl-X(L). Mol. Cell. Biol. 20:1604–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuwana, T., M.R. Mackey, G. Perkins, M.H. Ellisman, M. Latterich, R. Schneiter, D.R. Green, and D.D. Newmeyer. 2002. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 111:331–342. [DOI] [PubMed] [Google Scholar]
- 17.Annis, M.G., E.L. Soucie, P.J. Dlugosz, J.A. Cruz-Aguado, L.Z. Penn, B. Leber, and D.W. Andrews. 2005. Bax forms multispanning monomers that oligomerize to permeabilize membranes during apoptosis. EMBO J. 24:2096–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nechushtan, A., C.L. Smith, Y.T. Hsu, and R.J. Youle. 1999. Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J. 18:2330–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kearney, E.R., K.A. Pape, D.Y. Loh, and M.K. Jenkins. 1994. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1:327–339. [DOI] [PubMed] [Google Scholar]
- 20.Webb, S.R., and N.R. Gascoigne. 1994. T-cell activation by superantigens. Curr. Opin. Immunol. 6:467–475. [DOI] [PubMed] [Google Scholar]
- 21.Vella, A.T., J.E. McCormack, P.S. Linsley, J.W. Kappler, and P. Marrack. 1995. Lipopolysaccharide interferes with the induction of peripheral T cell death. Immunity. 2:261–270. [DOI] [PubMed] [Google Scholar]
- 22.Bouillet, P., J.F. Purton, D.I. Godfrey, L.C. Zhang, L. Coultas, H. Puthalakath, M. Pellegrini, S. Cory, J.M. Adams, and A. Strasser. 2002. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 415:922–926. [DOI] [PubMed] [Google Scholar]
- 23.Davey, G.M., C. Kurts, J.F. Miller, P. Bouillet, A. Strasser, A.G. Brooks, F.R. Carbone, and W.R. Heath. 2002. Peripheral deletion of autoreactive CD8 T cells by cross presentation of self-antigen occurs by a Bcl-2–inhibitable pathway mediated by Bim. J. Exp. Med. 196:947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hildeman, D.A., Y. Zhu, T.C. Mitchell, P. Bouillet, A. Strasser, J. Kappler, and P. Marrack. 2002. Activated T cell death in vivo mediated by proapoptotic bcl-2 family member bim. Immunity. 16:759–767. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki, M., R.J. Youle, and N. Tjandra. 2000. Structure of Bax: coregulation of dimer formation and intracellular localization. Cell. 103:645–654. [DOI] [PubMed] [Google Scholar]
- 26.Fenton, R.G., P. Marrack, J.W. Kappler, O. Kanagawa, and J.G. Seidman. 1988. Isotypic exclusion of gamma delta T cell receptors in transgenic mice bearing a rearranged beta-chain gene. Science. 241:1089–1092. [DOI] [PubMed] [Google Scholar]
- 27.Knudson, C.M., K.S. Tung, W.G. Tourtellotte, G.A. Brown, and S.J. Korsmeyer. 1995. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science. 270:96–99. [DOI] [PubMed] [Google Scholar]
- 28.Zhu, Y., B.J. Swanson, M. Wang, D.A. Hildeman, B.C. Schaefer, X. Liu, H. Suzuki, K. Mihara, J. Kappler, and P. Marrack. 2004. Constitutive association of the proapoptotic protein Bim with Bcl-2-related proteins on mitochondria in T cells. Proc. Natl. Acad. Sci. USA. 101:7681–7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hildeman, D.A., T. Mitchell, T.K. Teague, P. Henson, B.J. Day, J. Kappler, and P.C. Marrack. 1999. Reactive oxygen species regulate activation-induced T cell apoptosis. Immunity. 10:735–744. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.