Abstract
The prohormone convertase SPC2 (PC2) participates in the processing of proinsulin, proglucagon, and a variety of other neuroendocrine precursors, acting either alone or in conjunction with the structurally related dense-core granule convertase SPC3 (PC3/PC1). We have generated a strain of mice lacking active SPC2 by introducing the neomycin resistance gene (Neor) into the third exon of the mSPC2 gene. This gene insertion results in the synthesis of an exon 3-deleted form of SPC2 that does not undergo autoactivation and is not secreted. The homozygous mutant mice appear to be normal at birth. However, they exhibit a small decrease in rate of growth. They also have chronic fasting hypoglycemia and a reduced rise in blood glucose levels during an intraperitoneal glucose tolerance test, which is consistent with a deficiency of circulating glucagon. The processing of proglucagon, prosomatostatin, and proinsulin in the alpha, delta, and beta cells, respectively, of the pancreatic islets is severely impaired. The islets in mutant mice at 3 months of age show marked hyperplasia of alpha and delta cells and a relative diminution of beta cells. SPC2-defective mice offer many possibilities for further delineating neuroendocrine precursor processing mechanisms and for exploring more fully the physiological roles of many neuropeptides and peptide hormones.
Keywords: proprotein convertase, neuroendocrine precursor, gene disruption
SPC2 and SPC3 are members of a larger mammalian family of serine proteases that are related to the yeast convertase kexin and to the bacterial protease subtilisin (1–5). SPC2 and SPC3 are expressed almost exclusively in neuroendocrine cells throughout the brain, gut, pancreatic islets, and other endocrine tissues of the body (6–9). Their acidic pH optima, requirement for calcium ions, and ability to be sorted into dense-core vesicles of the regulated secretory pathway are consistent with their putative role as the major endoproteases responsible for the maturation of a large number of hormone and neuropeptide precursors throughout the organism (10–15).
In the islets of Langerhans, both SPC2 and SPC3 are expressed in the beta cells and are believed to act together to process proinsulin into insulin by cleavage at the Lys–Arg and Arg–Arg sites that flank the C peptide, followed by excision of these basic residues from the products by the exopeptidase carboxypeptidase E (7, 16–18). SPC2 is also expressed at high levels in the islet alpha cells, where it selectively processes proglucagon to liberate only glucagon as the major active hormone from this large multihormone precursor (19). On the other hand, in the neuroendocrine L cells of the gut, proglucagon is processed at different sites by SPC3 to liberate mainly two glucagon-like peptides—GLP1 and GLP2 (20–22). GLP17–37 enhances insulin secretion from the beta cells in response to glucose, whereas glucagon counters the hypoglycemic action of insulin by triggering hepatic glycogenolysis and enhancing hepatic gluconeogenesis to raise blood glucose levels (23–25). Thus, under some circumstances, SPC2 and SPC3 may act together to promote precursor maturation (e.g., in the case of proinsulin; refs. 3 and 7), whereas in other instances these enzymes are expressed differentially in certain cell populations, leading to tissue-specific processing of multifunctional precursors such as proglucagon (19, 20) and proopiomelanocortin, the precursor of corticotropin, melanocyte-stimulating hormone, and β-endorphin (10, 26, 27). The widespread expression of these two convertases in the central nervous system is consistent with suggestions that they play important roles in processing a wide variety of neuropeptide precursors (28–30).
Here we report the effects of disruption of the mSPC2 gene in the processing of three important prohormones of the islets of Langerhans: proinsulin, proglucagon, and prosomatostatin. The results unequivocally establish SPC2 as a major convertase in neuroendocrine and hormone precursor processing and support the hypothesis that SPC2 and SPC3 represent the core set of secretory granule processing endoproteases in neuroendocrine cells (10).
MATERIALS AND METHODS
Sources.
The 129Sv mouse genomic library and expression vector pCMV6C were provided by G. I. Bell (University of Chicago). All restriction enzymes were purchased from New England Biolabs. The R1 ES cell line was kindly provided by L. Degenstein (University of Chicago). Reagents for ES cell culture were purchased from GIBCO/BRL. C57BL/6J female mice used for mating with chimeric mice were obtained from the Charles River Breeding Laboratories. The care of all animals used in these studies was in accordance with the National Institutes of Health and institutional guidelines.
Strategy for SPC2 Gene Disruption.
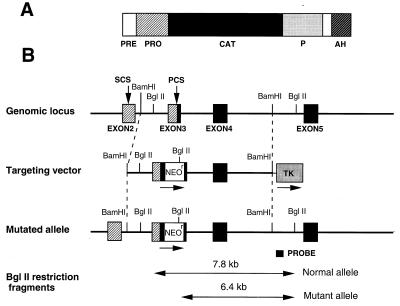
An 18-kb fragment of the mouse SPC2 gene was isolated from a mouse 129Sv genomic library and sequenced. A 11-kb BamHI fragment was subcloned into pGEM11Z (Promega), and neomycin resistance cassette (Neor) was inserted into the unique KpnI site of exon 3. The thymidine kinase gene was then placed at the 3′ end of the plasmid. Linearized plasmid DNA (30 μg) was electroporated (0.25 V, 960 μF) into R1 ES cells (1.8 × 106 cells per ml). Colonies resistant to G418 (400 μg/ml) and gancyclovir (GANC) (2 μM) were selected, and PCR and Southern blot analysis following digestion by BglII were performed to confirm homologous recombination. Among the G418 and GANC resistance colonies five independent ES cell clones were selected and then injected into C57BL/6J blastocysts to produce chimeric males, as described (31); one line yielded germ-line-transmitting chimeras. Heterozygotes were then bred and homozygous offspring were identified by Southern blot analysis and/or PCR of genomic DNA samples from tails of 3-week-old pups. For performing PCR, one set of oligonucleotides (5′ primer: CGC TGC AAC AAG AAG GAT T; 3′ primer: TAG AGA AAC TTA CCA GGT ACC) amplified a 117-bp product corresponding to the wild-type SPC2 allele. Another set of oligonucleotides (5′ primer: CGC TGC AAC AAG AAG GAT T; 3′ primer: CCA CTT GTG TAG CGC CAA GT) amplified a 180-bp product corresponding to the allele at the junction between exon 3 and Neor. PCR was performed using AmpliTaq DNA polymerase (Perkin–Elmer). PCR conditions were: 94°C for 1 min, 50°C for 1 min, 72°C for 1 min, 35 cycles. For Southern blot analysis, DNA (7 μg) was digested with BglII, subjected to electrophoresis on 0.8% agarose gels, transferred to nitrocellulose membranes, and hybridized with the probe shown in Fig. 1 (1.4-kb SPC2 genomic DNA fragment prepared by digestion with EcoRI and XbaI) labeled with nick translation kit (Amersham). Filters were hybridized at 42°C and washed at 55 to 60°C and followed by autoradiography at −80°C.
Figure 1.
Gene targeting scheme for disruption of the mouse SPC2 locus. (A) Schematic representation of the mouse SPC2 protein. The signal sequence (PRE), proregion (PRO), catalytic domain (CAT), P domain (P), and predicted amphipathic helical region (AH) are shaded as indicated. (B) Strategy for replacement of a segment of the SPC2 gene by homologous recombination. The exon–intron junctions in the mouse gene corresponded to those in the human gene (38). The primary cleavage site for activation (PCS) and a secondary cleavage site (SCS) are indicated by vertical arrows.
Biosynthesis of Mutant proSPC2.
To investigate the synthesis, possible activation, and secretion of mutant SPC2, an expression vector encoding mutant SPC2 (SPC2ΔExon 3) was constructed and stably transfected into HEK293 cells and AtT-20 cells. To delete exon 3, a 250-bp fragment of SPC2 was prepared by PCR using a sense primer TGC TCT AGA CAC TCC CAA AGA AGG ATG GAG and an antisense primer AAG GGT ACC ACC TGG GGT CTC TCT CTA GCT GC to generate an XbaI site in the 5′ upstream region of the initiation start codon and a KpnI site near the 3′ end of exon 3. The PCR fragment was digested by XbaI and KpnI and ligated into pCMV6C-SPC2, which was digested by the same restriction enzymes, resulting in plasmid pCMV6C-SPC2ΔExon 3. HEK293 cells and AtT-20 cells were stably transfected with wild-type SPC2 or SPC2ΔExon 3.
Immunohistochemistry.
Samples of pancreas tissue from 3-month-old wild-type and SPC−/− mice were fixed in 10% formalin. Four-micron sections were mounted on slides, baked at 60°C for 1 hr, cleared in xylene, and hydrated through a descending alcohol series to distilled water. For somatostatin immunostaining, sections were placed in 0.01 M citrate buffer, pH 7.0, and heated for 2 min on high, followed by 13 min at 20% power in a Samsung 1.5-ft3, 900-W microwave. Endogenous peroxidase activity was blocked by treating all sections with 3% H2O2 in methanol for 20 min. Sections were then incubated overnight at 4°C with rabbit polyclonal antibodies diluted as follows: 1:20 for antisomatostatin (BioGenex Laboratories, San Ramon, CA); 1:100 for antiglucagon (Dako); 1:160 for anti-insulin (BioGenex); or normal rabbit Ig as a control (Ventana Medical Systems, Tucson, AZ). Staining was performed on a Ventana GenII system (Ventana Medical Systems) using an indirect strepavidin/biotin–horseradish peroxidase conjugate with diaminobenzidine as a substrate. Immunostained sections were counterstained with hematoxylin.
Pancreatic Extracts.
Mice were anesthetized with ether and killed by decapitation. The pancreas was removed, weighed, and then homogenized in 3 vol of acid–ethanol (95% ethanol, 0.1 M HCl). The partially purified extract was then applied to a 1 × 50-cm Bio-gel P-30 column eluted with 3 M acetic acid (32). Fractions were dried and redissolved in immunoassay buffer (0.05 M Tris⋅HCl/0.1 M NaCl/2.5 mg/ml BSA, pH 7.6).
Glucose Tolerance Testing.
Mice were anesthetized with pentobarbital, and 2 mg/g of d-glucose was injected intraperitoneally. Blood samples were taken from the tail vein at 0, 30, 60, 90, and 120 min after glucose injection. Data were obtained from 8 wild-type mice and 10 SPC2−/− mice. Whole-blood glucose was measured by the glucose oxidase method with Glucometer Elite (Bayer, Wuppertal, Germany).
RESULTS
Expression of a Deleted Form of proSPC2.
To gain more information regarding the role and importance of SPC2, we sought to abrogate its function by introducing the Neor gene into the third exon of the mouse SPC2 gene (Fig. 1) via homologous recombination. This disruption strategy resulted in altered splicing of transcripts. Reverse transcription–PCR on islet and brain mRNA indicated that exon 3 was absent from SPC2 transcripts in SPC2−/− mice, but such altered transcripts were not detectable in normal islet or brain RNA samples (data not shown). Western blot analysis using antisera to the C terminus of SPC2 revealed the presence of a protein of 72 kDa, the size expected for an SPC2 precursor lacking the 38 residues (amino acids 94–131) encoded by exon 3 (Fig. 2). As expected, no mature SPC2 protein was detected because exon 3 contains the activation site at the junction between the propeptide and the mature enzyme (see Fig. 1). Expression of cDNAs encoding the normal and mutant protein in HEK293 and AtT-20 cell lines confirmed the lack of processing of the mutant precursor to a mature protein (Fig. 3). The mutant protein was not secreted (Fig. 3), and there was no evidence of SPC2 activity, which is consistent with the likelihood that it was unable to fold normally and exit the endoplasmic reticulum.
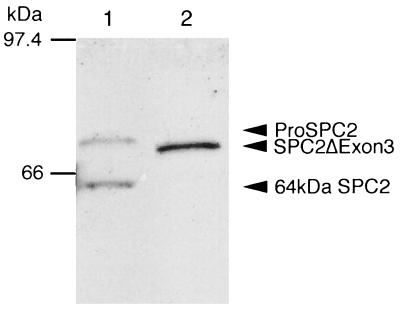
Figure 2.
Western blot analysis of islets for SPC2 expression. In wild-type islets, 75-kDa proSPC2 and 64-kDa mature SPC2 were demonstrated (lane 1), whereas in SPC2−/− islets, only the mutant SPC2ΔExon3 protein of 72 kDa was demonstrated (lane 2). Fifty islets were isolated from 3-month-old SPC2−/− or SPC2+/+ mice, and the proteins were resolved on 7.5% SDS/PAGE, transferred to Immunobilon-P (Amersham), incubated with antiserum PEP4 (immunogenic peptide: residues 611–635 of mouse SPC2; diluted 1:10,000), and detected by ECL (Amersham).
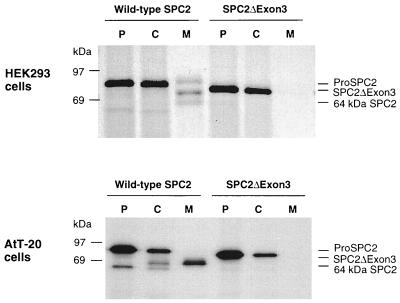
Figure 3.
Biosynthesis of normal and mutant SPC2 in cultured cells. HEK293 cells or AtT-20 cells were stably transfected with constructs encoding wild-type SPC2 or mutant SPC2 (SPC2ΔExon3) (39). Cells were labeled with [35S]methionine [1 mCi/ml; 1,000 Ci/mmol; 1 Ci = 37 GBq] for 30 min and then chase-incubated in nonradioactive medium for 3 hr at 37°C. At the termination of the incubation, media were collected and cells were lysed with a N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid/Mannitol buffer (40). Cell extracts and media samples were immunoprecipitated with SPC2 C-terminal antiserum and then analyzed by SDS/PAGE followed by fluorography. The mutant protein was not cleaved or secreted, in contrast to wild-type SPC2. To investigate whether the lack of processing activity of SPC2ΔExon3 in HEK293 cells was due to the lack of 7B2, a neuroendocrinal-specific protein that is involved in the transport and activation of SPC2 (41, 42), wild-type SPC2 and SPC2ΔExon3 were also stably transfected into AtT-20 cells, a corticotrope cell line that endogenously expresses 7B2. Pulse–chase metabolic labeling yielded similar results in this endocrine cell line. SPC2ΔExon3 appeared to be unstable and to undergo more rapid intracellular degradation without detectable secretion. P, pulse cell lysates; C, chase cell lysates; M, chase media.
When sections of pancreas were immunostained with antibodies to the C terminus of SPC2, both wild-type and (−/−) islets were stained. However, when immunostaining was carried out with antibodies to the N terminus of mature SPC2 (encoded by exon 3), only normal islets were stained (data not shown).
Phenotype of SPC2 Mutant Mice.
The frequency of each genotype was 64 (19.8%) homozygous, 83 (25.6%) wild-type, and 177 (54.6%) heterozygous out of 324 offspring of heterozygous matings. SPC2−/− mice were normal at birth, grew at only slightly subnormal rates (5–18% below wild-type mice in body weight), but showed no gross disturbances in body proportions, adiposity, or major organs. There was no obvious abnormality in suckling, grooming, and rearing behavior. However, although SPC2−/− male mice and female mice mated normally and had a normal average litter size, the number of consecutive litters from SPC2−/− female mice were less than those from SPC2+/− or wild-type female mice. Moreover, SPC2−/− female mice sometimes gave birth to dead pups.
Impaired Islet Prohormone Processing in SPC2 Mutant Mice.
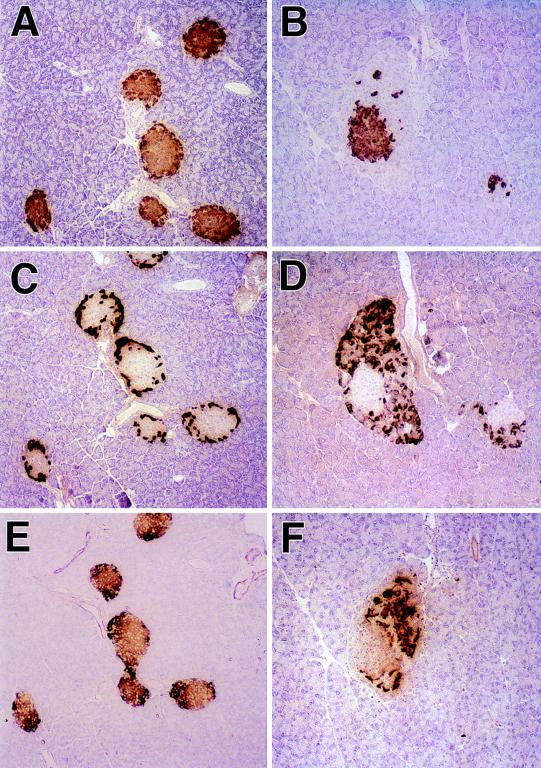
The lack of active SPC2 activity in the SPC2−/− mice was indicated by the morphological appearance of the islets of Langerhans (Fig. 4). Whereas glucagon-positive alpha cells make up only a small percentage of cells in the periphery of normal mouse islets (Fig. 4C), in the SPC2−/− mice there was a dramatic increase in the relative volume of these cells (Table 1). When stained with a glucagon antiserum that crossreacts with proglucagon, the mutant islets were surrounded by a very thick mantle of glucagon-positive alpha cells intermixed with increased numbers of somatostatin positive delta cells (Fig. 4D and F). The hyperplasia of these cells appears to be accompanied by some reduction in the numbers of beta cells (Fig. 4A and B). Electron microscopy showed that both the beta and alpha cells contain large numbers of immature-appearing secretory granules that have a homogeneous density without the characteristic halo of the mature, fully processed granules. These findings strongly suggest that proinsulin, proglucagon, and prosomatostatin processing are all affected by the lack of active SPC2. This possibility was confirmed by radioimmunological analyses (RIAs) for insulin, glucagon, and somatostatin components in acid–ethanol extracts of pancreas after size fractionation by gel chromatography. The results of insulin assays are summarized in Table 2, and these indicate a severe but incomplete block in processing (i.e., levels of 30–35% proinsulin-like components in SPC2−/− extracts vs. 6% or less in wild-type extracts). Pulse–chase labeling studies with isolated islets also confirmed the delayed conversion of proinsulin to insulin (unpublished results). Notable also is the decreased total amounts of insulin-related components consistent with a reduction in total beta cell mass in these animals (Table 2).
Figure 4.
Immunohistochemical staining of islets of normal (A, C, and E) and mutant (B, D, and F) mice. A and B show islets stained with an insulin antibody that crossreacts with proinsulin. C and D show islets stained with a glucagon antibody that crossreacts with proglucagon, and E and F show islets stained with somatostatin antibody that crossreacts with prosomatostatin. A positive reaction is indicated by the cytoplasmic accumulation of brown reaction product. (Magnification = ×145.)
Table 1.
Volume density (Vv × 102) of (pro)glucagon-containing (alpha) cells in the islets of wild-type (wt) and SPC2−/− mice
| Genotype | Volume density | Number of islets evaluated |
|---|---|---|
| Wt 1 | 9.9 ± 2.4 | n = 20 |
| Wt 2 | 7.8 ± 2.1 | n = 20 |
| SPC2−/− 1 | 51.2 ± 3.4 | n = 20 |
| SPC2−/− 2 | 60.1 ± 3.8 | n = 20 |
Pancreata were fixed in Bouin’s fluid, dehydrated with ethanol, and embedded. Five-micron-thick sections were incubated for 2 h at 24°C with rabbit N-terminal antiglucagon serum (K6248 from L. Heding, Denmark; 1:100) and then with goat anti-rabbit IgG conjugated to fluorescein isothiocyanate for 1 h. Sections that were counterstained with 0.01% Evans blue were observed for fluorescence with a Leitz Orthoplan microscope. Morphometric analysis was performed on the first 20 islets encountered in each animal. The volume density (Vv) of the glucagon cells within the islets was determined by the point-counting method of Weibel (47), modified as described (48). Data are mean ± SEM.
Table 2.
Pancreatic proinsulin and insulin content
| Genotype | Pancreatic proinsulin | Pancreatic insulin | Proinsulin/insulin |
|---|---|---|---|
| Wild type | 0.30 | 5.00 | 0.06 |
| SPC2−/− | 0.71 | 1.30 | 0.35 |
Whole pancreata from wild type or SPC2−/− mice (3 months old) were extracted with acid ethanol and fractionated by gel filtration for radioimmunoassay (32). Results are given as nmol per pancreas.
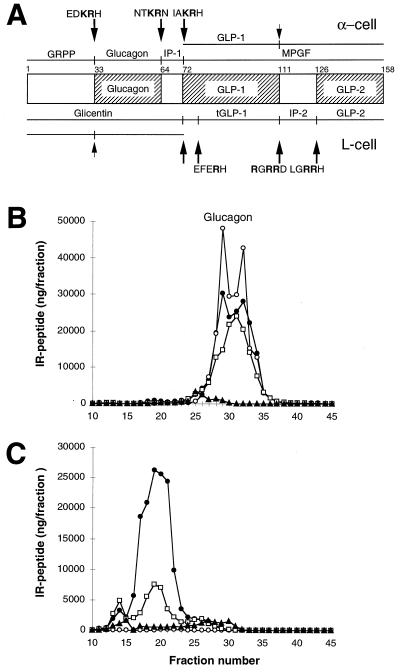
The results of RIAs for glucagon- and proglucagon-related components in pancreas are shown in Fig. 5. Using antisera specific for molecules having the mature C terminus of glucagon, the controls showed the expected high proportion of immunoreactive material with the size characteristics of mature glucagon (Fig. 5B). On the other hand, the SPC2−/− extracts contained no detectable mature glucagon but had high levels of larger components, including mainly intact and only partially processed large proglucagon fragments (Fig. 5C). These findings indicate that, in the absence of SPC2, active glucagon is not produced and proglucagon processing is severely blocked. The protease(s) in the mutant alpha cells that partially processes proglucagon is not yet known but could include small amounts of SPC3 and/or other convertases. Examination of plasma from SPC2−/− mice after Bio-gel P30 fractionation confirmed the presence of large amounts of proglucagon-related components and a lack of any detectable mature hormone (data not shown). Taken altogether these findings lead to the conclusion that active glucagon cannot be produced in the absence of SPC2, whereas some processing of proinsulin to insulin is possible due to the normal coexpression of SPC3 with SPC2 in the beta cells. The latter findings confirm that SPC3 can cleave both sites in proinsulin to yield insulin (33).
Figure 5.
Differential processing of proglucagon in the normal pancreas (α-cell) and the intestine (L-cell), and results of radioimmunoassay of glucagon in pancreas extracts. (A) Schematic representation of the structure and processing of proglucagon (20, 24, 25). At the top are the peptides resulting from proglucagon processing at the sites indicated in the pancreatic alpha cell. At the bottom are the peptides resulting from processing at the indicated sites in the intestinal L cell. These peptides include the glicentin-related polypeptide (GRPP, [proglucagon-(1–30)]), glucagon [proglucagon-(33–61)], the intervening peptide 1 (IP-1, [proglucagon-(64–69)]), the major proglucagon fragment (MPGF, [proglucagon-(72–158)]), GLP-1 [proglucagon-(72–107)], glicentin [proglucagon-(1–69)], truncated GLP-1 (tGLP-1, [proglucagon-(78–107)]), intervening peptide 2 (IP-2, [proglucagon-(111–122)]), GLP-2 [proglucagon-(126–158)], and oxyntomodulin [proglucagon-(33–69)]. The cleavage sites are indicated by arrows, with the sequence surrounding the site shown above or below the arrow. (Note that all cleavages are to the right of residues shown in bold.) Partially processed sites are indicated by small arrows. B and C show gel filtration profiles of extracts of wild-type (B) and SPC2−/− (C) pancreas analyzed using antibodies against four different regions of proglucagon. Antiserum 4304 (•) recognizes the 6–15 sequence of glucagon; 4305 (○) recognizes only the free, unextended form of glucagon; 4830 (□) was raised against the N terminus of glucagon, but shows some crossreaction with N-terminally extended forms of glucagon; 2135 (▴) recognizes a C-terminal immunodeterminant of GLP-1 and also reacts with MPGF (43).
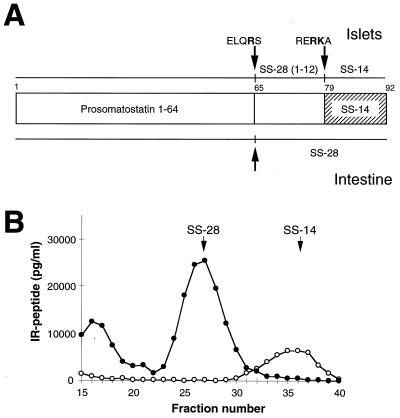
Somatostatin assays of pancreatic extracts revealed that although SS-14 is the major somatostatin-related peptide in normal pancreas (Fig. 6 A and B), it was completely absent from extracts derived from SPC2−/− mice (Fig. 6B). Instead, SS-28 is seen to be present as a major end-product of prosomatostatin processing in the SPC2−/− extracts. These results indicate that cleavage at the single Arg64 residue occurs normally, probably catalyzed by another convertase, whereas cleavage at the Arg–Lys pair (positions 77 and 78 in prosomatostatin) to yield SS-14 (and SS-281–14) requires SPC2. Although SPC3 can also cleave at this position (34), only SPC2 can be detected in delta cells by immunostaining (49).
Figure 6.
Radioimmunoassay of somatostatin-related peptides in pancreas extracts. (A) Schematic depiction of islet vs. intestinal processing of prosomatostatin (44). Indicated peptides are prosomatostatin 1–64, somatostatin 28 [SS-28, prosomatostatin-(65–92)], somatostatin 28(1–12) [prosomatostatin-(65–76)], and somatostatin 14 [SS-14, prosomatostatin-(79–92)]. The cleaved sites are indicated by arrows, with the sequence surrounding the site shown above or below the arrow. (B) Gel filtration of extracts of wild-type and SPC2−/− pancreas analyzed by radioimmunoassay for somatostatin (RIK 8001; Peninsula Laboratories). This somatostatin antibody crossreacts with both SS-28 and prosomatostatin. ○ indicate wild-type extract and • indicate SPC2−/− pancreas extract. The arrows show the elution positions of SS-28 and SS-14.
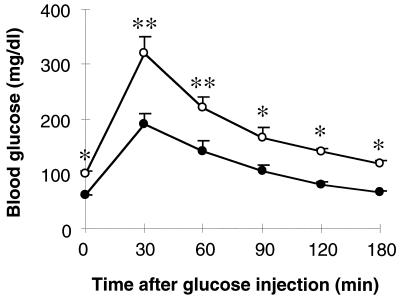
Fasting blood sugar levels were significantly reduced in SPC2−/− mice, and the rise in blood glucose levels in response to intraperitoneal glucose injection was markedly reduced (Fig. 7), which is consistent with the lack of detectable circulating glucagon. Circulating insulin levels were also low and accompanied by elevated amounts of proinsulin. These findings are similar to those reported recently by Brand et al. (35) from short-term glucagon immunoneutralization experiments in normal rabbits. Acute blockage of circulating glucagon by high-affinity monoclonal antibodies produced a reduction in blood glucose levels similar to that observed in the SPC2−/− mice, and this was accompanied by a marked reduction in circulating levels of insulin. From these results and others, Brand et al. (35) suggested that glucagon normally exerts a tonic hyperglycemic influence that is balanced by insulin. Our findings agree with that model and indicate that protracted glucagon deficiency is associated with chronic mild hypoglycemia, flattened glucose-tolerance curves, reduced insulin production (and beta cell mass), and very marked compensatory alpha cell hyperplasia. Observations on pancreatic islet morphology of newborn SPC2−/− mice indicate no such disturbed islet morphology and thus are consistent with the interpretation that the alpha cell hyperplasia is a response to chronic severe glucagon deficiency rather than being the result of disturbed developmental processes.
Figure 7.
Glucose tolerance tests. Glucose tolerance tests were carried out on 8-week-old wild-type (○) and SPC2−/− (•) mice after fasting for 20 hr. Results are mean ± SEM. ∗, P < 0.05; ∗∗, P < 0.01 (Student’s t test).
DISCUSSION
It is well known that the beta cells of the islets of Langerhans can undergo regeneration and hyperplasia in response to functional stresses, as in partial pancreatectomy or in states of obesity and/or insulin resistance (36). A number of factors have been identified that stimulate beta cell proliferation, preeminent among which is hyperglycemia (37). Therefore, perhaps it is not surprising that the beta cell population appears to be decreased in the SPC2−/− mice, in view of the reduced stimulus to both insulin secretion and beta cell proliferation that must arise due to the chronically low blood glucose levels.
On the other hand, the striking hyperplasia of the alpha and delta cells we describe here is, to our knowledge, unprecedented in either experimental or clinical experience. Alpha cell hyperfunction and hyperplasia accompany diabetes, especially when it is poorly controlled, and evidence suggests that insulin is necessary for the suppression of glucagon secretion that normally occurs in hyperglycemic states (23). This being so, it might be speculated that in SPC2−/− mice, decreased levels of active insulin in the circulation, in conjunction with the relatively low blood glucose levels, tend to relieve tonic alpha cell inhibition, leading to enhanced secretory activity. But then, when only inactive precursor forms of glucagon are released, a metabolic negative feedback loop that normally inhibits alpha cell proliferation may be interrupted. In addition, other regulatory circuits may act to control the alpha cell mass, such as direct inhibitory feedback by glucagon itself or other endocrine inhibitory influences. Somatostatin is a possible candidate because the somatostatin-producing delta cells and the alpha cells lie in close proximity in the periphery of the islets (23). The lack of islet SS-14 arising from the SPC2 deficiency (Fig. 6) may thus contribute to the alpha (and delta) cell hyperplasia. In addition to the foregoing possibilities, positively acting growth factors of some kind may also be released in increased amounts from target tissues such as liver in response to the lack of circulating glucagon. Further studies to evaluate these various possibilities and to accurately quantify these dynamic changes in islet cell populations are in progress.
Although the observations on the effects of SPC2 deficiency reported here are confined to the hormones of the islets of Langerhans, we anticipate that similar defects in processing will be found in numerous other neuroendocrine systems in SPC2−/− mice. Indeed, preliminary observations on the levels of Leu and Met enkephalins in the brains of SPC2−/− mice indicate marked decreases, which is consistent with impaired processing of proenkephalins (ref. 45; I. Lindberg, personal communication). König et al. (46) have recently reported that mice with disruptions in the preproenkephalin gene lack enkephalins in the brain and exhibit significant behavioral abnormalities and differences in supraspinal pain responses. It clearly will be of interest to assess the behavioral alterations in the SPC2−/− mice in greater detail.
Acknowledgments
We thank A. Kuznetsov, T. Montag, G. Zagaja, and D. Baunoch for pancreatic immunohistochemistry; H. Swift for electron microscopy; G. I. Bell for helpful discussions; K. Siegrist and L. Degenstein for advice and assistance with injections; and K. Bisqup, D. Ostrega, S. Martin, J. LaMendola, G. Chiu, P. Gardner, and J. Stein for technical assistance. We thank Florence Rozenfeld for expert assistance in preparing this report. This work was supported by grants from the National Institutes of Health (DK13914 and DK20595), by the Howard Hughes Medical Institute, and by the Swiss National Research Foundation (L.O.).
References
- 1.Steiner D F, Smeekens S P, Ohagi S, Chan S J. J Biol Chem. 1992;267:23435–23438. [PubMed] [Google Scholar]
- 2.Seidah N G, Chrétien M. Trends Endocrinol Metab. 1992;3:133–140. doi: 10.1016/1043-2760(92)90102-7. [DOI] [PubMed] [Google Scholar]
- 3.Bailyes E M, Bennett D L, Hutton J C. Enzyme (Basel) 1991;45:301–313. doi: 10.1159/000468903. [DOI] [PubMed] [Google Scholar]
- 4.Seidah N G, Chrétien M, Day R. Biochimie. 1994;76:197–209. doi: 10.1016/0300-9084(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 5.Hook V Y H, Azaryan A V, Hwang S-R, Tezapsidis N. FASEB J. 1994;8:1269–1278. doi: 10.1096/fasebj.8.15.8001739. [DOI] [PubMed] [Google Scholar]
- 6.Schäfer M K-H, Day R, Cullinan W E, Chrétien M, Seidah N G, Watson S J. J Neurosci. 1993;13:1258–1279. doi: 10.1523/JNEUROSCI.13-03-01258.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smeekens S P, Montag A G, Thomas G, Albiges-Rizo C, Carroll R, Benig M, Phillips L A, Martin S, Ohagi S, Gardner P, Swift H H, Steiner D F. Proc Natl Acad Sci USA. 1992;89:8822–8826. doi: 10.1073/pnas.89.18.8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macro J A, Dimaline R, Dockray G J. Gastrointest Liver Physiol. 1996;33:G87–G93. doi: 10.1152/ajpgi.1996.270.1.G87. [DOI] [PubMed] [Google Scholar]
- 9.Scopsi L, Gullo M, Rilke F, Martin S, Steiner D F. J Clin Endocrinol Metab. 1995;80:294–301. doi: 10.1210/jcem.80.1.7829629. [DOI] [PubMed] [Google Scholar]
- 10.Thomas L, Leduc R, Thorne B A, Smeekens S P, Steiner D F, Thomas G. Proc Natl Acad Sci USA. 1991;88:5297–5301. doi: 10.1073/pnas.88.12.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Y, Lindberg I. J Biol Chem. 1993;268:5615–5623. [PubMed] [Google Scholar]
- 12.Rufaut N W, Brennan S O, Hakes D J, Dixon J E, Birch N P. J Biol Chem. 1993;268:20291–20298. [PubMed] [Google Scholar]
- 13.Zhou A, Bloomquist B T, Mains R E. J Biol Chem. 1992;268:1763–1769. [PubMed] [Google Scholar]
- 14.Shennan K I J, Taylor N A, Jermany J L, Matthews G, Docherty K. J Biol Chem. 1995;270:1402–1407. doi: 10.1074/jbc.270.3.1402. [DOI] [PubMed] [Google Scholar]
- 15.Paquet L, Zhou A, Chang E Y, Mains R E. Mol Cell Endocrinol. 1996;120:161–168. doi: 10.1016/0303-7207(96)03834-8. [DOI] [PubMed] [Google Scholar]
- 16.Fricker L D, Evans C J, Esch F S, Herbert E. Nature (London) 1986;323:461–464. doi: 10.1038/323461a0. [DOI] [PubMed] [Google Scholar]
- 17.Steiner D F, Bell G I, Tager H S. In: Endocrinology. 3rd Ed. DeGroot L, editor. Philadelphia: Saunders; 1995. , Chapter 76, pp. 1296–1328. [Google Scholar]
- 18.Naggert J K, Fricker L D, Varlamov O, Nishina P M, Rouillé Y, Steiner D F, Carroll R J, Paigen B J, Leiter E H. Nat Genet. 1995;10:135–142. doi: 10.1038/ng0695-135. [DOI] [PubMed] [Google Scholar]
- 19.Rouillé Y, Westermark G, Martin S K, Steiner D F. Proc Natl Acad Sci USA. 1994;91:3242–3246. doi: 10.1073/pnas.91.8.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rouillé Y, Martin S K, Steiner D F. J Biol Chem. 1995;270:26488–26496. doi: 10.1074/jbc.270.44.26488. [DOI] [PubMed] [Google Scholar]
- 21.Rothenberg M E, Eilertson C D, Klein K, Mackin R B, Noe B D. Mol Endocrinol. 1996;10:331–341. doi: 10.1210/mend.10.4.8721979. [DOI] [PubMed] [Google Scholar]
- 22.Dhanvantari S, Seidah N G, Brubaker P L. Mol Endocrinol. 1996;10:342–355. doi: 10.1210/mend.10.4.8721980. [DOI] [PubMed] [Google Scholar]
- 23.Unger R, Orci L. In: Endocrinology. 3rd Ed. DeGroot L, editor. Philadelphia: Saunders; 1995. , Chapter 78, pp. 1337–1353. [Google Scholar]
- 24.Weir G C, Mojsov S, Hendrick G K, Habener J F. Diabetes. 1989;38:328–342. doi: 10.2337/diab.38.3.338. [DOI] [PubMed] [Google Scholar]
- 25.Ørskov C. Diabetologia. 1992;35:701–711. [PubMed] [Google Scholar]
- 26.Marcinkiewicz M, Day R, Seidah N G, Chrétien M. Proc Natl Acad Sci USA. 1993;90:4922–4926. doi: 10.1073/pnas.90.11.4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou A, Mains R E. J Biol Chem. 1994;269:17440–17447. [PubMed] [Google Scholar]
- 28.Birch N P, Hakes D J, Dixon J E, Mezey E. Neuropeptides. 1994;27:307–322. doi: 10.1016/0143-4179(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 29.Breslin M B, Lindberg I, Benjannet S, Mathis J P, Lazure C, Seidah N G. J Biol Chem. 1993;268:27084–27093. [PubMed] [Google Scholar]
- 30.Nillni E A, Luo L-G, Jackson I M D, McMillan P. Endocrinology. 1996;137:5651–5661. doi: 10.1210/endo.137.12.8940396. [DOI] [PubMed] [Google Scholar]
- 31.Joyner A L. Gene Targeting: A Practical Approach. Oxford: IRL; 1993. [Google Scholar]
- 32.Tager H S, Rubenstein A H, Steiner D F. Methods Enzymol. 1995;37:326–345. doi: 10.1016/s0076-6879(75)37030-4. [DOI] [PubMed] [Google Scholar]
- 33.Irminger J-C, Meyer K, Halban P. Biochem J. 1996;320:11–15. doi: 10.1042/bj3200011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brakch N, Galanopoulou A S, Patel Y C, Boileau G, Seidah N G. FEBS Lett. 1995;362:143–146. doi: 10.1016/0014-5793(95)00229-3. [DOI] [PubMed] [Google Scholar]
- 35.Brand C L, Jørgensen P N, Svendsen I, Holst J J. Diabetes. 1996;45:1076–1083. doi: 10.2337/diab.45.8.1076. [DOI] [PubMed] [Google Scholar]
- 36.Logothetopoulos J. Handbook of Physiology. Washington, DC: Am. Physiol. Soc.; 1972. , Section 7, Chapter 3, pp. 67–76. [Google Scholar]
- 37.Swenne I. Diabetologia. 1992;35:193–201. doi: 10.1007/BF00400917. [DOI] [PubMed] [Google Scholar]
- 38.Ohagi S, LaMendola J, LeBeau M M, Espinosa R, III, Takeda J, Smeekens S P, Chan S J, Steiner D F. Proc Natl Acad Sci USA. 1992;89:4977–4981. doi: 10.1073/pnas.89.11.4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou A, Bloomquist B T, Mains R E. J Biol Chem. 1993;268:1763–1769. [PubMed] [Google Scholar]
- 40.Zhou A, Mains R E. J Biol Chem. 1994;269:17440–17447. [PubMed] [Google Scholar]
- 41.Braks J A M, Martens G J M. Cell. 1994;78:263–273. doi: 10.1016/0092-8674(94)90296-8. [DOI] [PubMed] [Google Scholar]
- 42.Zhu X, Lindberg I. J Cell Biol. 1995;129:1641–1650. doi: 10.1083/jcb.129.6.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holst J J, Bersani M, Johnsen A H, Kofod H, Hartmann B, Ørskov C. J Biol Chem. 1994;269:18827–18833. [PubMed] [Google Scholar]
- 44.Bersani M, Thim L, Baldissera F G A, Holst J J. J Biol Chem. 1989;264:10633–10636. [PubMed] [Google Scholar]
- 45.Johanning K, Mathis J P, Lindberg I. J Neurochem. 1996;66:898–907. doi: 10.1046/j.1471-4159.1996.66030898.x. [DOI] [PubMed] [Google Scholar]
- 46.König M, Zimmer A M, Steiner H, Holmes P V, Crawley J M, Brownstein M J, Zimmer A. Nature (London) 1996;383:535–538. doi: 10.1038/383535a0. [DOI] [PubMed] [Google Scholar]
- 47.Weibel E R. Int Rev Cytol. 1969;26:235–302. doi: 10.1016/s0074-7696(08)61637-x. [DOI] [PubMed] [Google Scholar]
- 48.Baetens D, Stefan Y, Ravazzola M, Malaisse-Lagae F, Coleman D L, Orci L. Diabetes. 1978;27:1–7. doi: 10.2337/diab.27.1.1. [DOI] [PubMed] [Google Scholar]
- 49.Tanaka S, Kurabuchi S, Mochida H, Kato T, Takahashi S, Watanabe T, Nakayama K. Arch Histol Cytol. 1996;3:261–271. doi: 10.1679/aohc.59.261. [DOI] [PubMed] [Google Scholar]