Abstract
The pathogenesis of malarial anemia is multifactorial, and the mechanisms responsible for its high mortality are poorly understood. Studies indicate that host mediators produced during malaria infection may suppress erythroid progenitor development (Miller, K.L., J.C. Schooley, K.L. Smith, B. Kullgren, L.J. Mahlmann, and P.H. Silverman. 1989. Exp. Hematol. 17:379–385; Yap, G.S., and M.M. Stevenson. 1991. Ann. NY Acad. Sci. 628:279–281). We describe an intrinsic role for macrophage migration inhibitory factor (MIF) in the development of the anemic complications and bone marrow suppression that are associated with malaria infection. At concentrations found in the circulation of malaria-infected patients, MIF suppressed erythropoietin-dependent erythroid colony formation. MIF synergized with tumor necrosis factor and γ interferon, which are known antagonists of hematopoiesis, even when these cytokines were present in subinhibitory concentrations. MIF inhibited erythroid differentiation and hemoglobin production, and it antagonized the pattern of mitogen-activated protein kinase phosphorylation that normally occurs during erythroid progenitor differentiation. Infection of MIF knockout mice with Plasmodium chabaudi resulted in less severe anemia, improved erythroid progenitor development, and increased survival compared with wild-type controls. We also found that human mononuclear cells carrying highly expressed MIF alleles produced more MIF when stimulated with the malarial product hemozoin compared with cells carrying low expression MIF alleles. These data suggest that polymorphisms at the MIF locus may influence the levels of MIF produced in the innate response to malaria infection and the likelihood of anemic complications.
Malaria is a systemic disease caused by infection with parasitic protozoa of the genus Plasmodium (1). Death results principally from the complications of infection: cerebral disease leading to intractable coma and a severe and refractory anemia producing hypoxemia and cardiac decompensation. These complications of Plasmodium infection have been estimated to account for at least 1–2 million deaths yearly, mostly in African children under the age of five (1, 2).
The anemia of malaria infection is the result of pathologic processes that act both to accelerate red cell destruction and to inhibit new red cell production (3–5). Once infected by malarial parasites, red cells undergo lysis as a result of the process of schizogony, wherein the cell ruptures to release newly formed merozoites. Immune-mediated lysis, phagocytosis, and sequestration also occur, and these contribute to the increased clearance of nonparasitized as well as parasitized cells (6, 7). Importantly, recent studies have led to the conclusion that enhanced red cell clearance alone does not adequately explain the development of malarial anemia, especially in those patients who develop a severe, life-threatening disease (8, 9). Severe anemia can occur in patients despite low parasitemia or as a result of chronic subclinical infection, and it can persist for weeks after the patient has been cured of infection and relocated to a nonmalarial region (8, 10).
Detailed hematological studies in patients with severe malarial anemia emphasize that bone marrow abnormalities such as ineffective erythropoiesis, dyserythropoiesis, and lower erythroblast proliferative rates contribute importantly to the development of severe refractory anemia (9, 11–13). Malaria-infected patients frequently show a suboptimal reticulocyte count for the degree of anemia even in the face of an appropriately high level of circulating erythropoietin, which is the hormone critical for bone marrow erythropoiesis (14–16). These findings have been supported by experimental studies in mice (17–20). Collectively, these observations have served to focus attention on the pathogenesis of the bone marrow suppression that occurs during malaria infection and on the mechanisms that may contribute to the resistance of erythroid progenitor cells to the action of circulating erythropoietin (21).
Several investigators have proposed that a dysregulation in host immunologic pathways is responsible for the suppression of erythropoiesis during malaria infection (22, 23). Potential mechanisms include an excessive or a sustained innate immune response (24) and a polarization of the adaptive T cell response toward the production of mediators that might suppress normal pathways of erythropoietic development (15, 25, 26). Experimental studies in mice support the concept that malaria infection induces in the host the production of a potent circulating inhibitor of erythropoiesis (19, 27, 28). This erythropoiesis inhibitor has been partially characterized with respect to its biologic and biophysical properties (27, 28). Cytokines such as TNFα, IL-1β, and IFNγ that are produced systemically during malaria infection have been considered as candidates for this inhibitory mediator, but experimental studies have ruled out an important role for these cytokines in mediating erythroid suppression (20). A recent and unexpected set of observations from malaria vaccine trials also has focused attention on the immunopathogenesis of malarial anemia (29). Vaccination and challenge infection in Aotus monkeys produces severe anemia in a subset of animals that achieves initial immunity. The precise explanation for this vaccine-related anemia is unknown, but hematologic investigations in these hosts have supported a role for impaired erythropoiesis.
In this study, we have investigated whether the immunoregulatory cytokine macrophage migration inhibitory factor (MIF) plays a role in the pathogenesis of malaria anemia. MIF has been proposed to contribute to the pathogenesis of malaria based on its abundant expression in an experimental mouse model and on the biophysical features it shares with the previously characterized circulating inhibitor of erythropoiesis (30). Using a combination of in vitro studies of erythroid progenitors and in vivo studies in MIF-KO mice, we show that MIF has an intrinsic role in the pathogenesis of the bone marrow suppression that occurs during malaria infection.
RESULTS
Effect of MIF and proinflammatory cytokines on erythropoiesis in vitro
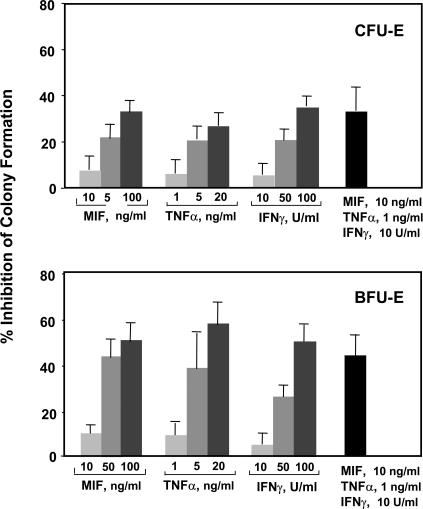
Several inflammatory cytokines interfere with hematopoiesis, and there is evidence that MIF may inhibit progenitor development in vitro (30). We examined the effect of MIF on mouse erythroid precursor development using defined erythropoietin-dependent colony assays, including both the early burst-forming unit erythroid (BFU-E) and the late CFU erythroid (CFU-E). We also studied the two inflammatory cytokines TNFα and IFNγ, which are produced as a consequence of macrophage and T cell activation and are implicated in severe malarial anemia (4). The appearance of BFU-E and CFU-E were quantified after culture with these cytokines, which were applied either alone or in combination. As shown in Fig. 1, the addition of 10–100 ng/ml MIF, 1–20 ng/ml TNFα, or 10–100 U/ml IFNγ dose dependently inhibited BFU-E and CFU-E colony formation. The early stage BFU-E showed greater inhibition than the late stage CFU-E colonies at each of the doses tested. Notably, the addition of subinhibitory concentrations of MIF together with TNFα and IFNγ resulted in a profound and synergistic inhibitory action. Synergism in effector function is not unusual for cytokine action, and the ability of MIF to lower the response threshold to other cytokines has been noted in prior studies of other cell systems (31–33).
Figure 1.
Dose-dependent impact of MIF, TNFα, and IFNγ on colony formation in mouse bone marrow progenitor cultures in vitro. Bone marrow cells were harvested and plated in a methylcellulose-based medium, and colony numbers were scored after the addition of mouse cytokines (see Materials and methods). Individual assays were performed in duplicate, and the data shown is a compilation of three to six independently performed experiments. Percent inhibition of colony formation is calculated with reference to a cytokine-minus control. All values shown are the mean ± SD (error bars) and are significant when compared with wells with no cytokine addition (P < 0.05). CFU-E, CFU erythroid; BFU-E, burst-forming unit erythroid.
MIF suppresses erythroid differentiation in progenitor cell lines
We next sought to identify the potential pathways responsible for the MIF-mediated impairment of erythroid differentiation. Erythroid progenitors such as the Friend mouse erythroleukemia (MEL; reference 34) or the human K562 (35) cell line undergo cytodifferentiation in vitro, leading to the initiation of hemoglobin synthesis and to a more mature erythroid phenotype. We examined these model cell systems because they replicate certain features of erythroid differentiation and they are more amenable to biochemical analysis than methylcellulose cultures of primary progenitor cells.
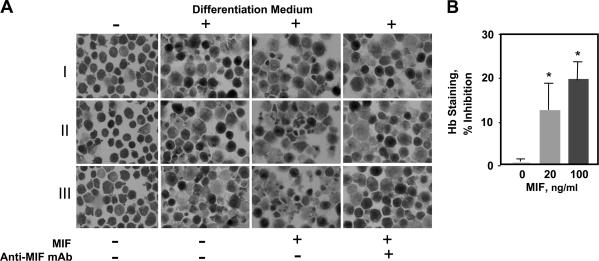
Cultured MEL cells were induced to undergo cytodifferentiation in the presence of recombinant MIF and were analyzed for intracellular hemoglobin content by benzidine staining. At a test concentration of 200 ng/ml, which is an amount of MIF that may circulate during severe systemic infection (36, 37), MIF decreased the cytodifferentiation response of MEL cells (Fig. 2 A). The specificity of this effect was verified by the application of a neutralizing anti-MIF mAb. Quantification of cellular hemoglobin by a sensitive chemical analysis also showed that lower amounts of MIF (20 and 100 ng/ml) reduced hemoglobin content by 12 and 20%, respectively (Fig. 2 B).
Figure 2.
MIF inhibits cytodifferentiation and hemoglobin synthesis of MEL cells. (A) Photomicrographic images of benzidine-stained cells from three experiments (I, II, and III) cultured with or without differentiation medium for 96 h together with 200 ng/ml recombinant mouse MIF or MIF plus 100 μg/ml anti-MIF mAb. (B) Intracellular hemoglobin quantification of lysed MEL cells (4 × 105 cells per experiment) as described in Materials and methods. Each value represents the mean ± SD (error bars) of five different experiments. *, P < 0.01 versus control.
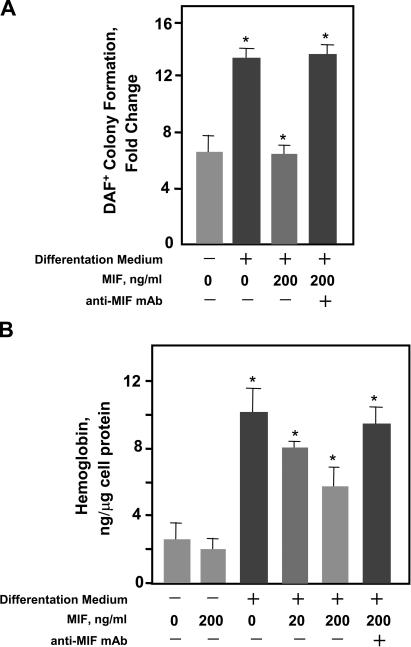
We then tested the effect of MIF stimulation on the human K562 progenitor cell line by coculturing these cells under differentiation conditions in the presence of MIF. As shown in Fig. 3 A, MIF inhibited the terminal erythropoietic differentiation of these cells as measured by a sensitive diaminofluorene (DAF) stain (38). The impact of MIF on hemoglobin production in these cells also was verified by biochemical quantification of intracellular hemoglobin content (Fig. 3 B). Neither MIF nor anti-MIF was found to exert cytotoxic effects on erythroid progenitors as assessed by trypan blue exclusion (unpublished data).
Figure 3.
MIF inhibits cytodifferentiation and hemoglobin production in human (K562) erythroid progenitors. (A) Terminal erythropoietic differentiation was assayed as described in Materials and methods with diaminofluorene (DAF) after culture in differentiation medium together with 200 ng/ml MIF for 96 h as described in Materials and methods. The neutralizing anti-MIF mAb was added at 100 μg/ml. DAF-positive cells were enumerated and expressed as fold change over total input cells. (B) Cellular hemoglobin content of cultured K562 progenitor cells. An isotypic control (IgG1) added in the same concentration had no impact on MIF's inhibitory action, nor did anti-MIF influence differentiation in the absence of MIF (not depicted). Each value represents the mean ± SD (error bars) of at least three different experiments. *, P < 0.01 versus corresponding controls.
MIF modulates MAP kinase activation
Having uncovered a prominent inhibitory effect of MIF on the differentiation of K562 cells, MEL cells, and primary erythroid progenitors, we next examined the signaling mechanisms influenced by MIF stimulation. Erythropoiesis requires the coordinate activation of several growth factor–dependent signal transduction pathways (39, 40), and some of these pathways may be faithfully represented in model progenitor cell systems. For example, both primary erythroid and K562 progenitor cells exhibit differentiation-dependent modulation in the different subfamilies of the mitogen-activated protein (MAP) kinases (extracellular signal-related kinase [ERK]–1/2, c-Jun NH2-terminal kinase [JNK]–1/2, and p38; reference 35).
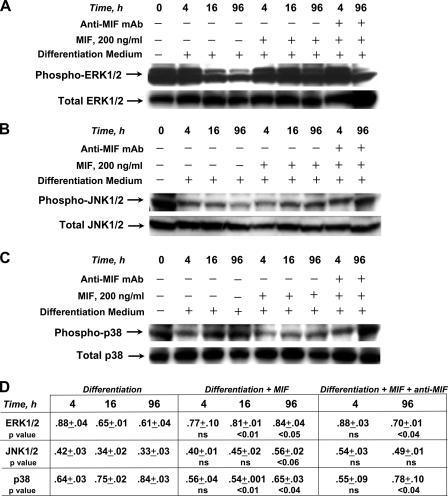
MIF promotes proinflammatory functions in monocytes/macrophages and fibroblasts by activating the ERK-1/2 family of MAP kinases (41). A distinguishing feature of MIF action is that it induces a sustained rather than a transient pattern of ERK-1/2 activation (41), which is noteworthy because the kinetics of ERK-1/2 activation influence the differentiation pathway of different progenitor cell types (42). We cultured K562 progenitors in differentiation medium together with MIF or MIF plus a neutralizing anti-MIF mAb and analyzed the cell lysates using phospho-specific antibodies directed against different MAP kinases. In agreement with published results (35), we observed that the erythroid differentiation of K562 progenitors is associated with a time-dependent inhibition of ERK-1/2 and JNK-1/2 phosphorylation (beginning at 16 and 96 h, respectively) and a complementary activation of the p38 phosphorylation (beginning at 16 h; Fig. 4, A–C). MIF addition markedly affected these differentiation-associated MAP kinase responses. In the case of the ERK-1/2 subfamily, phosphorylation was high at 16 h after MIF stimulation and, in a pattern that is consistent with other primary cell types (41), was sustained for a period of at least 96 h (16 h, P < 0.01; 96 h, P < 0.05; Fig. 4 A). MIF addition also appeared to up-regulate JNK-1/2 phosphorylation when compared with differentiation medium alone; however, this effect was not as pronounced as for ERK-1/2 and did not reach statistical significance (96 h, P < 0.06; Fig. 4 B). Nevertheless, the differentiation-associated changes in p38 MAP kinase phosphorylation were reduced by MIF (16 h, P < 0.01; 96 h, P < 0.04; Fig. 4 C). The phosphorylation patterns induced by MIF were normalized by anti-MIF mAb (96 h: ERK-1/2, P < 0.04; p38, P < 0.04; for anti-MIF mAb treatment vs. nontreatment), and the time course for these effects was consistent with the increase in hemoglobin synthesis observed after anti-MIF treatment (Figs. 3 and 4). These data support the role of MIF in inhibiting erythroid differentiation by modulating MAP kinase activation.
Figure 4.
Western blot analysis of the phosphorylation of MAP kinase proteins ERK-1/2, JNK-1/2, and p38 in response to cytodifferentiation and MIF treatment of K562 erythroid progenitors. For each experiment, 30 μg of cell lysates were subjected to SDS-gel electrophoresis and electroblotting followed by incubation with specific antiphospho–ERK-1/2 or total ERK (A), antiphospho–JNK-1/2 or total JNK antibodies (B), and antiphospho-p38 or total p38 antibodies (C). 100 μg/ml of neutralizing anti-MIF mAb showed no influence on MAP kinase activation in the absence of exogenously added MIF (not depicted). One representative blot is shown from at least three independently performed cell culture experiments for each kinase analysis. (D) Densitometric values were calculated as the ratio of phosphorylated to total MAP kinase as described in Materials and methods (n = 3 blots from independent experiments; mean ± SEM). Significance was calculated for each treatment condition (MIF or MIF + anti-MIF) versus nontreatment for each time point (4, 16, and 96 h).
MIF mediates anemia in experimental malaria infection
After intraperitoneal injection with a modest inoculum of Plasmodium chabaudi–parasitized red blood cells (106 per mouse), the BALB/c mouse strain develops an acute parasitemia that peaks on approximately day 8 of infection (43). More than 50% of mice will succumb to infection by 3 wk, and anemia notably contributes to death because the administration of a blood transfusion late in infection can rescue up to 90% of the infected mice (44).
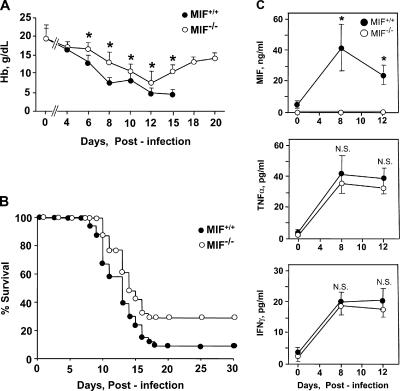
To determine the role of MIF in the anemic complications of acute malaria infection in vivo, we backcrossed a recently developed MIF-KO strain (45) into the BALB/c genetic background for experimental infection with P. chabaudi. Before study, we first assessed the hematopoietic competence of the MIF-KO strain by bone marrow histochemistry and enumeration of the different hematopoietic lineages. There were no differences between wild-type controls and MIF-KO mice with respect to the number of mature peripheral blood cells or in the numbers of CFU-E and BFU-E in bone marrow (unpublished data). Infection of wild-type or MIF-KO mice with P. chabaudi AS resulted in a prominent parasitemia that peaked on postinfection day 8 at 47 ± 15%. Peripheral blood was sampled every 2 d, and there was no appreciable difference in the mean level of parasitemia in the wild-type versus the MIF-deficient mice over the 4-wk course of the study (unpublished data). Despite the similar levels of parasitemia, however, the severity of anemia that developed in the two different experimental groups was quite different, especially as the infection progressed (Fig. 5 A). Hemoglobin levels progressively declined in the wild-type mice, with the lowest levels recorded on postinfection day 15, after which time >90% of the animals in this group perished. In comparison, the MIF-KO mice experienced a less severe anemia than the wild-type mice, and this difference remained statistically significant after the first week. Notably, the onset of death for mice in the MIF-KO group was delayed by 2 d when compared with wild-type mice (P = 0.013; Fig. 5 B). Moreover, although almost 30% of the MIF-KO mice survived to postinfection day 30, only 9% of wild-type mice survived this long (P < 0.04).
Figure 5.
Malaria-infected MIF-KO mice (MIF−/−) suffer from less severe anemia and show increased survival when compared with genetically matched wild-type controls (MIF+/+). (A) Time course for the development of anemia as assessed by peripheral blood sampling every other day. The data shown are the means ± SD (error bars) of 10 mice per group from one of two experiments that yielded similar results. For differences in mean hemoglobin concentrations between the MIF+/+ and MIF−/− mice, P values are as follows: *, P < 0.01 for days 6, 8, and 15; *, P < 0.05 for days 10 and 12. Because of low numbers of survivors, the wild-type mice were not further studied after day 15. (B) Kaplan-Meyer survival curves for MIF+/+ and MIF−/− mice after infection with P. chabaudi AS. The data shown are for all mice studied (MIF+/+, n = 30; MIF−/−, n = 31). The median survival was 13 d for MIF+/+ mice and 15 d for MIF−/− mice. P = 0.0113 (two-tailed Mann-Whitney test); for overall survival, P < 0.04 (χ2 test). (C) Plasma MIF, TNFα, and IFNγ concentrations measured by ELISA in P. chabaudi–infected wild-type (MIF+/+) and MIF-KO (MIF−/−) mice. Cytokines were measured in triplicate. (The MIF-KO mice do not produce any immunoreactive MIF product). For TNFα and IFNγ, there were statistically significant increases on postinfection days 8 and 12 compared with uninfected controls in both strains (*, P < 0.001).
To better assess the role of the host inflammatory response in the development of anemia and lethality from P. chabaudi infection, we measured the production of MIF and the cytokines TNFα and IFNγ, which serve as useful indicators of systemic macrophage and T cell activation, respectively. Plasma MIF concentrations reached 40 ± 20 ng/ml in wild-type mice at day 8 (Fig. 5 C). However, serum TNFα and IFNγ levels showed no differences when measured in the MIF-KO and wild-type strain during the period critical for anemia and lethality. Collectively, these findings support an important role for MIF, independent of the contribution of TNFα or IFNγ, in the development of the malaria infection–related complications of anemia and death.
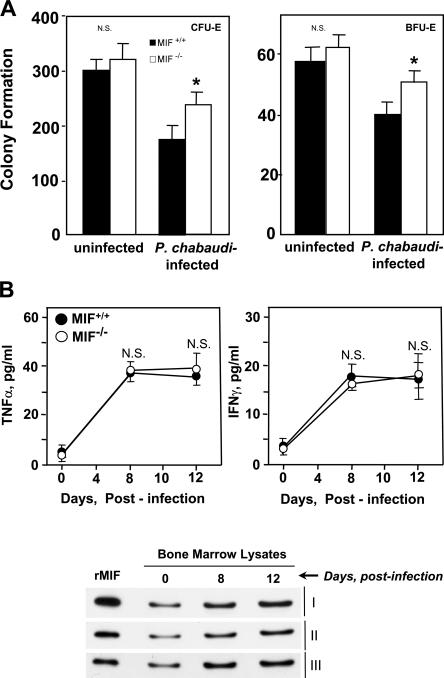
To further evaluate MIF's role in the development of anemia and erythroid suppression in this model of malaria infection, we next examined hematopoietic parameters in these mice. Malaria infection produced a reticulocytosis in both the wild-type and MIF-KO strains, but the corrected reticulocyte count was much greater in the setting of MIF deficiency (MIF+/+, 8 ± 5% vs. MIF−/−, 23 ± 10%; day 10 after infection; P < 0.01). We also measured CFU-E and BFU-E in bone marrow, and colony formation was significantly greater in the P. chabaudi–infected MIF-KO mice than in the corresponding P. chabaudi–infected wild-type mice (P < 0.01; Fig. 6 A). Notably, the levels of bone marrow production of the cytokines TNFα and IFNγ were indistinguishable in the infected MIF-KO and wild-type strains, and the measured values closely mirrored the circulating levels of these mediators (Figs. 5 C and 6 B). The wild-type mice nevertheless showed a prominent induction of MIF protein in the bone marrow that increased over time during the period critical for anemia development. These data collectively support the role of MIF in erythroid suppression and that MIF deficiency is associated with better compensation of erythropoiesis during experimental malarial infection.
Figure 6.
Malaria-infected MIF-KO mice show enhanced bone marrow erythroid progenitor maturation. (A) CFU-E and BFU-E formation in cultured bone marrow cells harvested from P. chabaudi–infected or uninfected mice on postinfection day 13. Colonies were scored as described in Materials and methods, and the data shown are the mean ± SD (error bars) of three mice per group. *, P < 0.01 for MIF−/− versus MIF+/+. (B) TNFα, IFNγ, and MIF production in bone marrow lysates obtained from P. chabaudi–infected wild-type (MIF+/+) and MIF-KO (MIF−/−) mice. TNFα and IFNγ were measured by ELISA, and the data shown are the mean ± SD for five mice per group. The time-dependent increase in bone marrow MIF content was assessed by Western blotting of bone marrow samples (240 ng of total protein per lane) as described in Materials and methods. The blots show the bone marrow analysis of three mice that were studied (I, II, and III). The standard lane (first) shows the immunoblotting intensity of 90 ng of recombinant mouse MIF protein.
MIF circulates in high concentrations in malaria patients
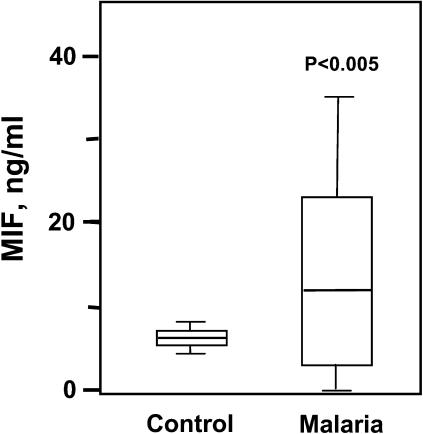
Recent investigations in human malaria have provided evidence for MIF production in the intervillous blood of pregnant women with placental infection (46, 47). To determine whether clinical malaria infection is associated with an increase in MIF in the systemic circulation, we used a specific sandwich ELISA to quantify MIF in patients residing in a Plasmodium falciparum–endemic region of Zambia. MIF was measured in 20 children who were presented sequentially for clinical evaluation and diagnosed with malaria by peripheral blood smear. As shown in Fig. 7, there was a significant increase in the plasma concentration of MIF in malaria-infected patients when compared with uninfected controls obtained from the same geographic region. The median plasma concentrations of MIF in the malarial patients was 11.8 ng/ml (0.9–49.5 ng/ml), which is comparable with the level reported previously in patients with bacterial sepsis (median of 12.2 ng/ml; reference 36). These data support the conclusion that Plasmodium infection is a potent stimulus for the systemic expression of MIF in human subjects.
Figure 7.
Plasma MIF concentrations are elevated in malaria-infected individuals. MIF was quantified by sandwich ELISA in 20 sequentially diagnosed subjects with malaria presented to the Macha Mission Hospital in Zambia. Control plasmas (n = 20) were obtained contemporaneously from uninfected hospital controls. The bottom, middle, and top lines of the box mark the 25th, 50th, and 75th percentiles, respectively. The vertical line shows the range of values comprised between the 5th and 95th percentiles. The mean plasma MIF concentrations for the control and malaria groups were 5.4 and 13.6 ng/ml, respectively (P < 0.005 by a two-tailed Student's t test). Error bars represent SD.
A functional promoter polymorphism in human MIF influences the MIF response to the malarial product hemozoin
The evidence that MIF plays a role in malarial anemia prompted us to examine whether genetic polymorphisms in the human MIF gene influence the human host response to malaria infection. Functional promoter polymorphisms in human MIF have been linked recently both to the incidence or the severity of several inflammatory diseases in different populations (48–51). These genetic polymorphisms include a tetranucleotide sequence (CATT) that is repeated between five and eight times in the MIF promoter (position −794). An increase in CATT repeat number produces a corresponding increase in MIF promoter activity (48). Individuals with the 5-CATT (low repeat) MIF allele may be considered low MIF expressors, and those bearing non–5-CATT repeats (6-CATT, 7-CATT, or 8-CATT) may be high MIF expressors.
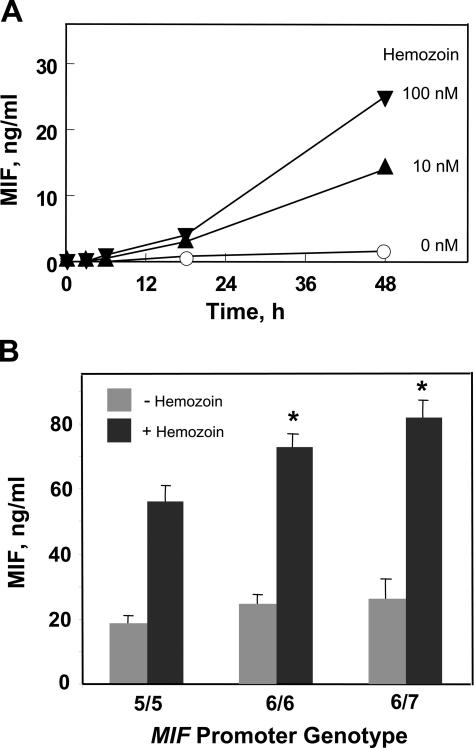
Plasmodium-infected red cells stimulate MIF secretion by cultured macrophages (30). To study the MIF response of human cells, we elected to use hemozoin as a stimulant. Hemozoin is a metabolite of Plasmodium hemoglobin degradation that accumulates within the reticuloendothelial system of the infected host (52). Hemozoin induces cytokine release from monocytes/macrophages (53, 54), and it constitutes a Plasmodium-specific, pathogen-associated molecular pattern for innate immunity by interaction with the innate receptor TLR9 (55). We confirmed that hemozoin, which was prepared synthetically from heme chloride (53) and, thus, was free of other Plasmodium-produced components, induces MIF release from macrophages in a standardized culture system (Fig. 8 A). We then added hemozoin to human monocyte cultures of predetermined MIF genotype and found that cells bearing the low expression 5-CATT (homozygous) allele secreted lower levels of MIF than cells encoding the high expression 6-CATT (homozygous) or 6-CATT/7-CATT (heterozygous) alleles (Fig. 8 B). These experimental data, which were obtained in vitro, suggest that the host MIF genotype may influence MIF production in response to the malarial product hemozoin.
Figure 8.
Hemozoin-induced MIF release from mononuclear cells is regulated by low and high expression MIF alleles. (A) Hemozoin induces MIF release by cultured mouse macrophages. 2 × 106/ml thioglycollate-elicited peritoneal macrophages were cultured with synthetically prepared hemozoin for the indicated times, and the conditioned medium was harvested for MIF ELISA. Values shown are the mean of triplicate determinations. (B) Hemozoin-stimulated human mononuclear cells encoding the high expression MIF alleles 6-CATT/6-CATT (6/6) or 6-CATT/7-CATT (6/7) release more MIF protein than cells encoding the low expression MIF allele 5-CATT/5-CATT (5/5). Cells were stimulated with 42 nM hemozoin for 48 h before sampling supernatants for MIF content. Values shown are mean ± SD (error bars) of duplicate analyses. *, P < 0.002 for 6/6 versus 5/5; *, P < 0.001 for 6/7 versus 5/5; *, P < 0.02 for 6/7 versus 6/6.
DISCUSSION
Clinical observations have led to the concept that the nature of the host response critically influences the pathologic manifestations of malaria infection. Young children and the immunologically naive suffer disproportionately from lethal malaria, and it is hypothesized that death results from an exaggerated immunologic response such that the mechanisms initially recruited to fight infection become dysregulated and produce life-threatening tissue damage (56, 57). Investigators proposed many years ago that the specific pathogenic manifestations of malaria, such as severe anemia and cerebral malaria, result from proinflammatory mediator release by host cells interacting with malaria parasites or their products (22). Others reported evidence of a role for activated leukocytes in the erythropoietic changes observed during malaria (18), and some studies (19, 27) identified a host-derived circulating mediator that acts to suppress erythropoiesis. Individual cytokines such as TNFα, IL-1, and IFNγ have been examined for their effects on hematopoiesis, but their effects have been judged to be minor or indirect; for instance, by inducing other mediators or by affecting the distribution and availability of heme iron (20, 28, 58).
MIF has emerged to be an important regulator of innate immunity (59). Its proximate actions are to promote downstream cytokine and Toll-like receptor expression (32, 60, 61) and to sustain cellular responses in the face of activation-induced apoptosis (62). MIF release from macrophages in response to Plasmodium-infected red cells and its prominent expression in the plasma, spleen, and bone marrow during experimental malaria have suggested that MIF may mediate erythropoietic suppression (30). Indeed, MIF shares each of the properties that have been reported for the malaria-induced erythropoiesis inhibitor. MIF levels closely correlate with peak parasitemia, and its tissue and bone marrow production mimic the expression pattern of the malaria-induced erythropoiesis inhibitor (19, 20, 28). MIF's biophysical properties also match those that have been reported for the circulating inhibitor (31).
Hematopoietic colony assays confirm that MIF, in concentrations that circulate in patients with malaria infection, is a direct and potent inhibitor of erythroid progenitor development. Our finding of synergistic inhibition by MIF together with low, subinhibitory combinations of TNFα or IFNγ is not without precedent because similar synergism has been observed in studies of MIF's impact on proinflammatory monocyte/macrophage activation (31, 32). In the progenitor cell systems that were examined, MIF's action was to inhibit cytodifferentiation and hemoglobin production.
These data add to our understanding of the inflammatory pathogenesis of malarial anemia, but it should be emphasized that they do not exclude a role for yet additional mediators in influencing erythroid development. Differential TH1 and TH2 immunologic responses may influence the pathologic course of malaria infection, and IL-10 and IL-12 deficiencies also may contribute to anemia (15, 24, 26, 63). Whether these effects can be attributed to the direct action of these cytokines on erythroid progenitors or to regulatory effects on other mediators, including MIF, remains to be examined. There also is evidence from other disease models that proinflammatory cytokines may inhibit erythropoiesis by repressing the synthesis of erythropoietin (64). Clinical studies of malarial anemia, however, suggest that erythropoietin levels are appropriately elevated for the degree of anemia (14–16), although this finding is not uniform (65, 66). High circulating erythropoietin levels point to an acquired resistance by progenitor cells to erythropoietin stimulation, and this effect has been recently verified in a study of P. chabaudi–infected mice (21). Signal transduction through MAP kinases has been implicated in the functional effects of erythropoietin (35, 40), and our data indicate that signals delivered by MIF antagonize the normal pattern of MAP kinase phosphorylation that is associated with the erythroid differentiation in a progenitor line. The potential involvement of other signaling pathways that are relevant for erythroid differentiation remain to be examined, but an MIF-induced dysregulation in signaling may contribute to the suppression of erythropoiesis that accompanies other inflammatory disease states as well (58).
The idea that MIF has an intrinsic role in the development of malarial anemia was verified by examining mice genetically deficient in MIF. MIF-KO mice infected with P. chabaudi showed less anemia and decreased mortality when compared with their wild-type infected counterparts. Although it may be argued that the impact of MIF deficiency on either mortality or anemia in this experimental mouse model is not dramatic, it is consistent with MIF's influence on the component of anemia that is attributable to erythroid suppression. Indeed, enumeration of bone marrow cultures revealed increases in CFU-E and BFU-E of the malaria-infected MIF-KO mice when compared with the wild-type controls (P < 0.01).
Longitudinal studies in malaria patients to examine the relationship between MIF production and hematologic indices will be important to perform. The levels of plasma MIF that we detected in children with malaria are similar to those reported previously in patients with bacterial sepsis, although patients with septic shock show even higher circulating MIF levels (36). Several case control studies have linked high expression human MIF alleles (6-CATT, 7-CATT, and 8-CATT) or an associated single-nucleotide polymorphism with different inflammatory diseases (48–51). It is noteworthy that there is considerable population stratification in the distribution of MIF alleles, suggesting that this locus may have been subject to differential selective pressure in different regions or ethnic groups (67). We found that malarial hemozoin stimulates MIF protein production from primary human mononuclear cells in amounts that reflect the low (5-CATT) versus the higher (6-CATT and 7-CATT) expression genotype of the cells. Our data indicate that the MIF locus may regulate the monocyte/macrophage response to this proinflammatory malarial product. Hemozoin is poorly degraded in vivo, and, once formed in host tissues, it may act as a persistent inflammatory focus to sustain the production of MIF and other cytokines. Hemozoin deposition within the bone marrow (30) also may explain why reticulocytosis and bone marrow recovery are often delayed despite the cure of infection in human malaria (8, 10). Our studies point to the deleterious effect of MIF on the clinical expression of malarial anemia; however, it is important to consider that in the appropriate context, MIF also may exert important functions in the immunologic response to Plasmodium infection.
Whether the human MIF gene influences the clinical expression of malaria and the development of complications such as severe anemia is of interest, and this question can be addressed by examining the association between MIF alleles and disease manifestations in a malaria-endemic population. Such information may facilitate the identification of individuals who are at high risk for severe malarial anemia or other complications so that steps can be taken to prevent or better treat their disease.
MATERIALS AND METHODS
Cytokines, antibodies, and reagents.
Recombinant mouse or human MIF were prepared, and the proteins were purified essentially free of endotoxin (<1 pg endotoxin/μg protein) as described previously (31). The neutralizing anti-MIF mAb (IgG1 isotype) and an isotypic control antibody (5D4-11; American Type Culture Collection) were purified by Mono-Q fast protein liquid chromatography. 2.7 × 105 U/μg recombinant mouse TNFα and 8.4 × 103 U/μg IFNγ were obtained from R&D Systems. Hemozoin was prepared synthetically from hemin chloride (Sigma-Aldrich) under endotoxin-free conditions (53).
Cytokine quantification.
MIF was measured by sandwich ELISA (36) using human or mouse MIF-specific antibodies developed in our laboratory. The MIF content of bone marrow was determined by Western blotting and detection with rabbit polyclonal antibody. TNFα and IFNγ levels in sera and in supernatants of bone marrow lysates were measured by the Quantikine M TNFα or IFNγ Immunoassay kit (R&D Systems).
Mice and mouse cell cultures.
The MIF−/− (MIF-KO) mice (45) were bred onto the BALB/c genetic background and studied between 8–10 wk of age. The mice were at generation N6. All mouse studies were performed in accordance with protocols approved by our Institutional Animal Care and Use Committees.
Bone marrow precursors were harvested from the femora and tibia of mice in 3 ml of Iscove's modified Dulbecco's medium containing 2% FBS. The viability of marrow cells was determined to be >97% by trypan blue exclusion staining. Bone marrow lysates were collected by flushing two femurs and two tibias per mouse with 1 ml of lysis buffer (150 mM NaCl, 50 mM Tris, pH 7.5, 1% NP-40, 0.5% deoxycholic acid, 0.1% SDS, and 2 mM EDTA) using a 25 G 5/8-gauge needle. The bone marrow plug was homogenized, the cellular debris was pelleted, and the lysate supernatant was concentrated using a Centricon 10 membrane (Amico). Mouse macrophages were prepared from the adherent cultures of thioglycollate-elicited peritoneal macrophages as described previously (60).
BFU-E and CFU-E progenitor cell assays.
The progenitor cell (colony) assays were performed according to the standardized methods described previously (30) and protocols from StemCell Technologies Inc. In brief, washed mouse bone marrow cells were plated in sterile 35-mm dishes containing a methylcellulose-based medium and growth factors. The total number of bone marrow cells plated in duplicate culture dishes were 2 × 105 for CFU-E and 5 × 105 for BFU-E. MethoCult M3334 was used for the CFU-E and BFU-E colony assays. The media for CFU-E and BFU-E was volume adjusted by adding one part Isocove's modified Dulbecco's medium to nine parts MethoCult M3334 to give final concentrations of the following components: 15% FBS, 1% BSA, 200 mg/ml transferrin, 10 mg/ml insulin, 1% methylcellulose, 10−4 M 2-mercaptoethanol, 2 mM l-glutamine, and 3 units/ml erythropoietin. Each assay of different bone marrow progenitor cells was performed independently three to six times.
Erythroid progenitor cell lines.
The Friend MEL cell line was cultured in Dulbecco's modified Eagle's medium supplemented with 10% FBS. Differentiation was induced with dimethyl sulfoxide differentiation medium for 4 d as described previously (34). Hemoglobin synthesis was assessed by cytocentrifugation onto glass slides followed by staining with benzidine (Worthington) and a hemoglobin kit (Sigma-Aldrich).
The human K562 progenitor cell line (CCL-243; American Type Culture Collection) was cultured in RPMI/10% FBS supplemented with penicillin and streptomycin. For differentiation, sodium butyrate (Sigma-Aldrich) was added to the medium (35). Terminal erythropoietic differentiation was measured as described previously (38), and positive cells were enumerated after staining with DAF (Sigma-Aldrich). To quantify hemoglobin synthesis, the cells were centrifuged and washed in PBS, and the cell pellet was resuspended in lysis buffer (100 mM potassium phosphate, pH 7.8, and 0.2% Triton X-100) before disruption by aspiration through a 21-gauge needle. After microcentrifugation, the supernatant was collected, and the hemoglobin concentration was determined using a hemoglobin kit (Sigma-Aldrich). Cell viability was assessed by trypan blue dye exclusion and found to be equivalent (85–90% viability) between all samples studied.
Western blot analysis.
Immunoblotting with antibodies directed against phospho–ERK-1/2, total ERK-1/2, phospho–JNK-1/2, total JNK-1/2, phospho-p38, and total p38 was performed according to the manufacturer's instructions (New England Biolabs, Inc.). The secondary antibody was an anti–rabbit IgG conjugated to horseradish peroxidase, and detection was by chemiluminescence according to the manufacturer's instructions (GE Healthcare). For quantitation of signal intensity of phospho-specific kinase results, Western blots were scanned using a scanner (PowerLook IIII; UMAX), and the images were analyzed using UN-SCAN-IT gel Automated Digitizing system software version 5.1 (Silk Scientific). The ratio between phosphorylated and total MAP kinase was determined and expressed as a fold change for each lane (mean ± SEM for three blots from independent cell cultures). The P values were calculated for each of the time course comparisons (4, 16, and 96 h) of three different experiments (two-tailed Student's t test).
Mouse malaria studies.
To maintain uniformity of infections, P. chabaudi AS was maintained by serial passage in A/J mice, and no more than 10 passages were allowed before a fresh inoculum was prepared from frozen stocks. For simple passages, infection was initiated with a dose of 106–107 parasitized erythrocytes. For clinical infections, mice were inoculated intraperitoneally with 106 parasitized red blood cells. The course of experimental infection in mice was monitored every other day by examining DiffQuik (Baxter Scientific Products)-stained thin smears from blood. Parasitemias were determined by counting a minimum of 200 erythrocytes per blood sample. Hemoglobin was determined by using Drabkin's procedure (Sigma-Aldrich) on 1 μl of tail vein blood prepared on every second day, after infection, for 3 wk. Mice were observed daily for at least 30 d, and mortality was recorded. At predetermined times after infection, five animals per group were killed by CO2, and the blood was collected by cardiac puncture.
Human specimens and cell culture.
Blood samples from 20 malaria patients and 20 uninfected, healthy controls were obtained from the Macha Mission Hospital in Choma, Zambia, where P. falciparum is endemic. The blood samples were obtained in accordance with research protocols approved by the institutional review boards of the University of Zambia and Yale University. The subjects had acute onset malaria and were diagnosed for clinical symptoms (fever, splenomegaly, headache, and malaise) and a positive, thick blood film for parasites. The subjects (12 male and 8 female) were between 5–52 mo of age (mean = 25.5 mo) and had a mean hemoglobin of 5.9 g/deciliter. Co-infection by organisms likely to contribute to a systemic inflammatory response was excluded by clinical evaluation and laboratory testing. Numerical values obtained by MIF-specific sandwich ELISA were confirmed semiquantitatively by Western blotting.
Human mononuclear cells were prepared from the adherent leukocyte fraction of volunteers and genotyped for the (−794) CATT tetranucleotide repeat and for a (−173) G/C single-nucleotide polymorphism (48). Two homozygous and one heterozygous MIF promoter genotypes were studied: 5-CATT/5-CATT, 6-CATT/6-CATT, and 6-CATT/7-CATTC. The mononuclear cells were cultured in 24-well plates (104 cells/well) and stimulated with 42 nM hemozoin for 48 h. Cells from three individuals were studied within each genotyped group, and the results were repeated twice.
Statistics.
Data are presented as means ± SD. The significance level for all statistical tests was set at α = 0.05. One-way analysis of variance was performed to determine whether there were differences in the effects of MIF, anti-MIF, TNFα, and IFNγ at various doses with respect to colony formation of bone marrow progenitor cells. Upon finding differences, Bonferroni-adjusted pairwise (post-hoc) comparisons were performed to determine which mediator–dose combination differed from one another. Comparisons between cytokine levels, colony formation, and cytokine production between the MIF+/+ and MIF−/− mice and between normal and malaria-infected mice were performed using the two-tailed Student's t test. For mouse survival, the median survival time was compared by the Mann-Whitney test, and the overall survival was compared using the χ2 tests (68).
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants (1RO1-AI051306, AR49610, and 2RO1-AI042310 to R. Bucala and grants 1R01-AI44587-05 and UH1-HL03679-07), the Office of Research on Minority Health, a Howard University General Clinical Research Center grant (MO1-RR10284 to V.R. Gordeuk), and the NIH Medical Scientist Training Program to Yale University (J. Griffith). R. Mitchell and R. Bucala are co-inventors on patents pertaining to the inhibition of MIF for the treatment of inflammatory disease. C.N. Metz is a co-inventor on several patents related to MIF.
The authors have no conflicting financial interests.
Abbreviations used: BFU-E, burst-forming unit erythroid; CFU-E, CFU erythroid; DAF, diaminofluorene; ERK, extracellular signal-related kinase; JNK, c-Jun NH2-terminal kinase; MAP, mitogen-activated protein; MEL, mouse erythroleukemia; MIF, macrophage migration inhibitory factor.
M.A. McDevitt, J. Xie, and G. Shanmugasundaram contributed equally to this paper.
J. Xie's present address is Department of Medicine, The University of Chicago Pritzker School of Medicine, Chicago, IL 60637.
References
- 1.Miller, L., D. Baruch, K. Marsh, and O. Doumbo. 2002. The pathogenic basis of malaria. Nature. 415:673–679. [DOI] [PubMed] [Google Scholar]
- 2.Breman, J.G. 2001. The ears of the hippopotamus: manifestations, determinants, and the estimate of the malaria burden. Am. J. Trop. Med. Hyg. 64:S1–11. [DOI] [PubMed] [Google Scholar]
- 3.Nagel, R. 2002. Malarial anemia. Hemoglobin. 26:329–343. [DOI] [PubMed] [Google Scholar]
- 4.McDevitt, M., J. Xie, V. Gordeuk, and R. Bucala. 2004. The anemia of malaria infection: role of inflammatory cytokines. Curr. Hematol. Rep. 3:97–106. [PubMed] [Google Scholar]
- 5.Chang, K.-H., and M.M. Stevenson. 2004. Malarial anaemia: mechanisms and implications of insufficient erythropoiesis during blood-stage malaria. Int. J. Parasitol. 34:1501–1516. [DOI] [PubMed] [Google Scholar]
- 6.Jakeman, G.N., A. Saul, W.L. Hogarth, and W.E. Collins. 1999. Anaemia of acute malaria infections in non-immune patients primarily results from destruction of uninfected erythrocytes. Parasitology. 119:127–133. [DOI] [PubMed] [Google Scholar]
- 7.Waitumbi, J., M. Opollo, R. Muga, A. Misore, and J. Stoute. 2000. Red cell surface changes and erythrophagocytosis in children with severe Plasmodium falciparum anemia. Blood. 95:1481–1486. [PubMed] [Google Scholar]
- 8.Weatherall, D., and P. Abdalla. 1982. The anaemia of P. falciparum malaria. Br. Med. Bull. 38:147–151. [DOI] [PubMed] [Google Scholar]
- 9.Wickramasinghe, S.N., and S.H. Abdalla. 2000. Blood and bone marrow changes in malaria. Baillieres. Best Pract. Res. Clin. Haematol. 13:277–299. [DOI] [PubMed] [Google Scholar]
- 10.Camacho, L., V. Gordeuk, P. Wilairatana, P. Pootrakul, G. Brittenham, and S. Looareesuwan. 1998. The course of anaemia after the treatment of acute, falciparum malaria. Ann. Trop. Med. Parasitol. 92:525–537. [DOI] [PubMed] [Google Scholar]
- 11.Srichaikul, T., M. Wasanasomsithi, V. Poshyachinda, N. Panikbutr, and T. Rabieb. 1969. Ferrokinetic studies and erythropoiesis in malaria. Arch. Intern. Med. 124:623–628. [PubMed] [Google Scholar]
- 12.Abdalla, S.H. 1990. Hematopoiesis in human malaria. Blood Cells. 16:401–416. [PubMed] [Google Scholar]
- 13.Das, B., N. Nanda, P. Rath, R. Satapathy, and D. Das. 1999. Anaemia in acute Plasmodium falciparum malaria in children from the Orissa state, India. Ann. Trop. Med. Parasitol. 93:109–118. [DOI] [PubMed] [Google Scholar]
- 14.Burchard, G., P. Radloff, J. Philipps, M. Nkeyi, J. Knobloch, and P. Kremsner. 1995. Increased erythropoietin production in children with severe malarial anemia. Am. J. Trop. Med. Hyg. 53:547–551. [DOI] [PubMed] [Google Scholar]
- 15.Nussenblatt, V., G. Mukasa, A. Metzger, G. Ndeezi, E. Garrett, and R.D. Semba. 2001. Anemia and interleukin-10, tumor necrosis factor alpha, and erythropoietin levels among children with acute, uncomplicated Plasmodium falciparum malaria. Clin. Diagn. Lab. Immunol. 8:1164–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurtzhals, J.A., O. Rodrigues, M. Addae, J.O. Commey, F.K. Nkrumah, and L. Hviid. 1997. Reversible suppression of bone marrow response to erythropietin in Plasmodium falciparum malaria. Br. J. Haematol. 97:169–174. [DOI] [PubMed] [Google Scholar]
- 17.Rencricca, N.J., J.P. Stout, and R.M. Coleman. 1974. Erythropoietin production in virulent malaria. Infect. Immun. 10:831–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maggio-Price, L., D. Brookoff, and L. Weiss. 1985. Changes in hematopoietic stem cells in bone marrow of mice with Plasmodium berghei malaria. Blood. 66:1080–1085. [PubMed] [Google Scholar]
- 19.Silverman, P.H., J.C. Schooley, and L.J. Mahlmann. 1987. Murine malaria decreases hemtopoietic stem cells. Blood. 69:408–413. [PubMed] [Google Scholar]
- 20.Yap, G.S., and M.M. Stevenson. 1994. Inhibition of in vitro erythropoiesis by soluble mediators during Plasmodium chabaudi AS malaria: lack of a major role for interleukin-1, tumor necrosis factor-α, and γ-interferon. Infect. Immun. 62:357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang, K.-H., M. Tam, and M.M. Stevenson. 2004. Inappropriately low reticulocytisis in severe malarial anemia correlates with suppression in the development of late erythroid precursorse. Blood. 103:3727–3735. [DOI] [PubMed] [Google Scholar]
- 22.Clark, I., J.-L. Virelizier, E. Carswell, and P. Wood. 1981. Possible importance of macrophage-derived mediators in acute malaria. Infect. Immun. 32:1058–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunt, N., and G.E. Grau. 2003. Cytokines: accelerators and brakes in the pathogenesis of cerebral malaria. Trends Immunol. 24:491–499. [DOI] [PubMed] [Google Scholar]
- 24.Biemba, G., V. Gordeuk, P. Thuma, G.F. Mabeza, and G. Weiss. 1998. Prolonged macrophage activation and persistent anemia in children with complicated malaria. Trop. Med. Int. Health. 3:60–65. [DOI] [PubMed] [Google Scholar]
- 25.Mohan, K., and M.M. Stevenson. 1998. Dyserythropoiesis and severe anaemia associated with malaria correlate with deficient interleukin-12 production. Br. J. Haematol. 103:942–949. [DOI] [PubMed] [Google Scholar]
- 26.Perkins, D.J., J.B. Weinberg, and P.G. Kremsner. 2000. Reduced interleukin-12 and transforming growth factor-β1 in severe childhood malaria: relationship of cytokine balance with disease severity. J. Infect. Dis. 182:988–992. [DOI] [PubMed] [Google Scholar]
- 27.Miller, K.L., J.C. Schooley, K.L. Smith, B. Kullgren, L.J. Mahlmann, and P.H. Silverman. 1989. Inhibition of erythropoiesis by a soluble factor in murine malaria. Exp. Hematol. 17:379–385. [PubMed] [Google Scholar]
- 28.Yap, G.S., and M.M. Stevenson. 1991. Production of soluble inhibitor of erythropoiesis during Plasmodium chabaudi as infection in resistant and susceptible mice. Ann. NY Acad. Sci. 628:279–281. [DOI] [PubMed] [Google Scholar]
- 29.Jones, T.R., D.F. Stroncek, A.S. Gozalo, N. Obaldia, E.M. Andersen, C. Lucas, D.L. Narum, A.J. Magill, B.K.L. Sim, and S.L. Hoffman. 2002. Anemia in parasite- and recombinant protein-immunized Aotus monkeys infected with Plasmodium falciparum. Am. J. Trop. Med. Hyg. 66:672–679. [DOI] [PubMed] [Google Scholar]
- 30.Martiney, J.A., B. Sherry, C.N. Metz, M. Espinoza, A.S. Ferrer, T. Calandra, H.E. Broxmeyer, and R. Bucala. 2000. Macrophage migration inhibitory factor release by macrophages after ingestion of Plasmodium chabaudi-infected erythrocytes: possible role in the pathogenesis of malarial anemia. Infect. Immun. 68:2259–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernhagen, J., R.A. Mitchell, T. Calandra, W. Voelter, A. Cerami, and R. Bucala. 1994. Purification, bioactivity, and secondary structure analysis of mouse and human macrophage migration inhibitory factor (MIF). Biochemistry. 33:14144–14155. [DOI] [PubMed] [Google Scholar]
- 32.Calandra, T., J. Bernhagen, C.N. Metz, L.A. Spiegel, M. Bacher, T. Donnelly, A. Cerami, and R. Bucala. 1995. MIF as a glucocorticoid-induced modulator of cytokine production. Nature. 377:68–71. [DOI] [PubMed] [Google Scholar]
- 33.Donnelly, S.C., C. Haslett, P.T. Reid, I.S. Grant, W.A. Wallace, C.N. Metz, L.J. Bruce, and R. Bucala. 1997. Regulatory role for macrophage migration inhibitory factor in acute respiratory distress syndrome. Nat. Med. 3:320–323. [DOI] [PubMed] [Google Scholar]
- 34.Terada, M., J. Fried, U. Nudel, R.A. Rifkind, and P.A. Marks. 1977. Transient inhibition of initiation of S-phase associated with dimethylsulfoxide induction of murine erythroleukemia cells to erythroid differentiation. Proc. Natl. Acad. Sci. USA. 74:248–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Witt, O., K. Sand, and A. Pekrun. 2000. Butyrate-induced erythroid differentiation of human K562 leukemia cells involves inhibition of ERK and activation of p38 MAP kinase pathways. Blood. 95:2391–2396. [PubMed] [Google Scholar]
- 36.Calandra, T., B. Echtenacher, D. Le Roy, J. Pugin, C.N. Metz, L. Hültner, D.M.D. Heumann, R. Bucala, and M.P. Glauser. 2000. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat. Med. 6:164–169. [DOI] [PubMed] [Google Scholar]
- 37.Beishuizen, A., L.G. Thijs, C. Haanen, et al. 2001. Macrophage migration inhibitory factor and hypothalamic-pituitary-adrenal function during critical illness. J. Clin. Endocrinol. Metab. 86:2811–2816. [DOI] [PubMed] [Google Scholar]
- 38.McGuckin, C.P., N. Forraz, and W.M. Liu. 2003. Diaminofluorene stain detects erythroid differentiation in immature haemopoietic cells treated with EPO, IL-3, SCF, TGF beta(1), MIP-1 alpha and IFN gamma. Eur. J. Haematol. 70:106–114. [DOI] [PubMed] [Google Scholar]
- 39.Arcasoy, M.O., and X. Jiang. 2005. Co-operative signalling mechanisms required for erythroid precursor expansion in response to erythropoietin and stem cell factor. Br. J. Haematol. 130:121–129. [DOI] [PubMed] [Google Scholar]
- 40.Klingmuller, U. 1997. The role of tyrosine phosphorylation in proliferation and erythropoietin-induced erythroid differentiation - signals emanating from the erythropietin receptor. Eur. J. Biochem. 249:637–647. [DOI] [PubMed] [Google Scholar]
- 41.Mitchell, R.A., C.N. Metz, T. Peng, and R. Bucala. 1999. Sustained mitogen-activated protein kinase (MAPK) and cytoplasmic phospholipase A2 activation by macrophage migration inhibitory factor (MIF). Regulatory role in cell proliferation and glucocorticoid action. J. Biol. Chem. 274:18100–18106. [DOI] [PubMed] [Google Scholar]
- 42.Howe, A.K., and R.L. Juliano. 1998. Distinct mechanisms mediate the initial and sustained phases of integrin-mediated activation of the Raf/MEK/mitogen-activated protein kinase cascade. J. Biol. Chem. 273:27268–27274. [DOI] [PubMed] [Google Scholar]
- 43.Stevenson, M.M., and E.M. Riley. 2004. Innate immunity to malaria. Nat. Rev. Immunol. 4:169–180. [DOI] [PubMed] [Google Scholar]
- 44.Yap, G.S., and M.M. Stevenson. 1994. Blood transfusion alters the course and outcome of Plasmodium chabaudi AS infection in mice. Infect. Immun. 62:3761–3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bozza, M., A.R. Satoskar, G. Lin, B. Lu, A.A. Humbles, C. Gerard, and J.R. David. 1999. Targeted disruption of migration inhibitory factor gene reveals its critical role in sepsis. J. Exp. Med. 189:341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chaisavaneeyakorn, S., J.M. Moore, C. Othoro, J. Otieno, S. Chaiyaroj, Y. Shi, B. Nahlen, A. Lal, and V. Udhayakumar. 2002. Immunity to placental malaria. IV. Placental malaria is associated with up-regulation of macrophage migration inhibitory factor in intervillous blood. J. Infect. Dis. 186:1371–1375. [DOI] [PubMed] [Google Scholar]
- 47.Chaiyaroj, S.C., A.S.M. Rutta, K. Muenthaisong, P. Watkins, M.N. Ubol, and S. Looareesuwan. 2004. Reduced levels of transforming growth factor-beta 1, interleukin-12 and increased migration inhibitory factor are associated with severe malaria. Acta. Trop. 89:319–327. [DOI] [PubMed] [Google Scholar]
- 48.Baugh, J.A., S. Chitnis, S.C. Donnelly, J. Monteiro, X. Lin, B.J. Plant, F. Wolfe, P.K. Gregersen, and R. Bucala. 2002. A functional promoter polymorphism in the macrophage migration inhibitory factor (MIF) gene associated with disease severity in rheumatoid arthritis. Genes Immun. 3:170–176. [DOI] [PubMed] [Google Scholar]
- 49.Donn, R., Z. Aloufi, F. De Benedetti, C. Meazza, E. Zeggini, M. Lunt, A. Stevens, E. Shelley, R. Lamb, W. Ollier, et al. 2002. Mutation screening of the macrophage migration inhibitory factor gene: positive association of a functional polymorphism of macrophage migration inhibitory factor with juvenile idiopathic arthritis. Arthritis Rheum. 46:2402–2409. [DOI] [PubMed] [Google Scholar]
- 50.Hizawa, N., E. Yamaguchi, D. Takahashi, J. Nishihira, and M. Nishimura. 2004. Functional polymorphisms in the promoter region of macrophage migration inhibitory factor and atopy. Am. J. Respir. Crit. Care Med. 169:1014–1018. [DOI] [PubMed] [Google Scholar]
- 51.Mizue, Y., S. Ghani, L. Leng, C. McDonald, P. Kong, J. Baugh, S.J. Lane, J. Craft, J. Nishihira, S.C. Donnelly, et al. 2005. Role for macrophage migration inhibitory factor (MIF) in asthma. Proc. Natl. Acad. Sci. USA. 102:14410–14415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Slater, A.F. 1992. Malaria pigment. Exp. Parasitol. 74:362–365. [DOI] [PubMed] [Google Scholar]
- 53.Sherry, B.A., G. Alava, K.J. Tracey, J. Martiney, A. Cerami, and A.F. Slater. 1995. Malaria-specific metabolite hemozoin mediates the release of several potent endogenous pyrogens (TNF, MIP-1α, and MIP-1β) in vitro, and altered thermoregulation in vivo. J. Inflamm. 45:85–96. [PubMed] [Google Scholar]
- 54.Jaramillo, M., I. Plante, N. Ouellet, K. Vandal, P.A. Tessier, and M. Olivier. 2004. Hemozoin-inducible proinflammatory events in vivo: potential role in malaria infection. J. Immunol. 172:3101–3110. [DOI] [PubMed] [Google Scholar]
- 55.Coban, C., K.J. Ishii, T. Kawai, H. Hemmi, S. Sato, S. Uematsu, M. Yamamoto, O. Takeuchi, S. Itagaki, N. Kumar, et al. 2005. Toll-like receptor 9 mediates innate immune activation by the malaria pigment hemozoin. J. Exp. Med. 201:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Newton, C., T.E. Tayler, and R.O. Whitten. 1998. Pathophysiology of fatal falciparum malaria in African children. Am. J. Trop. Med. Hyg. 58:673–683. [DOI] [PubMed] [Google Scholar]
- 57.Day, N.P., T.T. Hien, T. Schollaardt, P.P. Loc, L.V. Chuong, T.T. Chau, N.T. Mai, N.H. Phu, D.X. Sinh, N.J. White, and M. Ho. 1999. The prognostic and pathophysiologic role of pro- and anti-inflammatory cytokiens in severe malaria. J. Infect. Dis. 180:1288–1297. [DOI] [PubMed] [Google Scholar]
- 58.Means, R.T., and S.B. Krantz. 1992. Progress in understanding the pathogenesis of anemia of chronic disease. Blood. 80:1639–1646. [PubMed] [Google Scholar]
- 59.Calandra, T., and T. Roger. 2003. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat. Rev. Immunol. 3:791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Calandra, T., J. Bernhagen, R.A. Mitchell, and R. Bucala. 1994. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J. Exp. Med. 179:1895–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roger, T., J. David, M.P. Glauser, and T. Calandra. 2001. MIF regulates innate immune responses through modulation of Toll-like receptor 4. Nature. 414:920–924. [DOI] [PubMed] [Google Scholar]
- 62.Mitchell, R.A., H. Liao, J. Chesney, G. Fingerle-Rowson, J. Baugh, J. David, and R. Bucala. 2002. Macrophage migration inhibitory factor (MIF) sustains macrophage proinflammatory function by inhibiting p53: regulatory role in the innate immune response. Proc. Natl. Acad. Sci. USA. 99:345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mohan, K., and M.M. Stevenson. 1998. Interleukin-12 corrects severe anemia during blood-stage Plasmodium chabaudi AS in susceptible A/J mice. Exp. Hematol. 26:45–52. [PubMed] [Google Scholar]
- 64.Jelkmann, W., H. Pagel, M. Wolff, and J. Fandrey. 1992. Monokines inhibiting erythropoietin production in human hepatoma cultures and in isolated perfused rat kidneys. Life Sci. 50:301–308. [DOI] [PubMed] [Google Scholar]
- 65.Burgmann, H., S. Looareesuwan, S. Kapiotis, C. Viravan, S. Vanijanonta, U. Hollenstein, E. Wiesinger, E. Presterl, S. Winkler, and W. Graninger. 1996. Serum levels of erythropoietin in acute Plasmodium falciparum malaria. Am. J. Trop. Med. Hyg. 54:280–283. [DOI] [PubMed] [Google Scholar]
- 66.el Hassan, A.M., A.M. Saeed, J. Fandrey, and W. Jelkmann. 1997. Decreased erythropoietin response in Plasmodium falciparum malaria-associated anaemia. Eur. J. Haematol. 59:299–304. [DOI] [PubMed] [Google Scholar]
- 67.Zhong, X., L. Leng, R. Chen, C. McDonald, A. Beitin, R. Jenison, A. Lee, P. Gregersen, P. Thuma, D.C. Ward, and R. Bucala. 2005. Rapid detection of tetranucleotide repeat and single promoter polymorphisms in the macrophage migration inhibitory factor (Mif) gene by thin-film biosensor chips. Nucleic Acids Res. 33:e121–e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zar, J.H. 1984. Biostatistical Analysis. Prentice-Hall, Englewood Cliffs, NJ. 718 pp.