Abstract
Mouse and human natural killer T (NKT) cells recognize a restricted set of glycosphingolipids presented by CD1d molecules, including self iGb3 and microbial α-glycuronosylceramides. The importance of the canonical Vα14-Jα18 TCR α chain for antigen recognition by NKT cells is well recognized, but the mechanisms underlying the Vβ8, Vβ7, and Vβ2 bias in mouse have not been explored. To study the influences of thymic selection and the constraints of pairing with Vα14-Jα18, we have created a population of mature T cells expressing Vα14-Jα18 TCR α chain in CD1d-deficient mice and studied its recognition properties in vitro and in vivo. Transgenic cells expressed a diverse Vβ repertoire but their recognition of endogenous ligands and synthetic iGb3 was restricted to the same biased Vβ repertoire as expressed in natural NKT cells. In contrast, α-GalCer, a synthetic homologue of microbial α-glycuronosylceramides, was recognized by a broader set of Vβ chains, including the biased NKT set but also Vβ6, Vβ9, Vβ10, and Vβ14. These surprising findings demonstrate that, whereas Vβ8, Vβ7, and Vβ2 represent the optimal solution for recognition of endogenous ligand, many Vβ chains that are potentially useful for the recognition of foreign lipids fail to be selected in the NKT cell repertoire.
Mouse Vα14-Jα18 and human Vα24-Jα18 NKT cells are innate-like, CD1d-restricted, autoreactive lymphocytes that regulate several infectious, autoimmune, and cancerous conditions (1). Their TCR structure combines a canonical TCR α chain with TCR β chains that belong to a restricted set of Vβ families including Vβ8, Vβ7, Vβ2 in mice and Vβ11 in humans (2). Unlike the invariant TCR α chain, these TCR β chains exhibit a wide diversity of CDR3 junctions (3–5), nearly all of which allow for the recognition of the self-glycosphingolipid iGb3 (6), the microbial cell wall α-glycuronosylceramides (7, 8), and their mimic α-GalCer. In addition, a fraction of NKT cells was reported to recognize mycobacterial phosphatidylinositolmannosides (9).
The mechanisms leading to the striking Vβ bias during development and the consequences on microbial ligand recognition have remained unexplained. For example, it is unclear whether the Vβ bias reflects pairing preferences with the invariant TCR α chain or the predominant influence of positive selection or negative selection during thymic development. There are reports of pairing defects of the invariant TCR α chain with a Vβ11 chain in vitro (10) and with Vβ chains lacking the solvent-exposed Cβ FG loop in vitro and in vivo (11), suggesting that many Vα14-Jα18/Vβ dimers are unstable. In addition, different contributions of individual Vβ8, Vβ7, and Vβ2 families to the affinity of recognition of α-GalCer have been shown (12), suggesting that the Vβ bias may also reflect thymic selection by lipid ligands. Indeed, recent studies suggested Vβ-specific differences in avidity for CD1d/endogenous ligand, namely a higher avidity of Vβ7 chains (13, 14). However, because these studies did not examine the recognition properties of the nonbiased Vβ repertoire before selection by CD1d/ligands, the forces shaping the Vβ bias during thymic development have not been explored.
Here, we have created a population of mature Vα14-Jα18 T cells expressing a diverse, nonbiased Vβ repertoire by expressing a Vα14-Jα18 TCR α transgene in CD1d-deficient (CD1d −/−) mice. Our studies demonstrate that the invariant TCR α chain could pair indiscriminately with the entire Vβ repertoire, ruling out pairing biases as a substantial factor. Second, we found that only Vβ7, Vβ8, and Vβ2 allowed for recognition of endogenous ligand and iGb3, with a Vβ7>Vβ8>Vβ2 hierarchy of responses paralleling the corresponding Vβ enrichment by thymic selection in vivo, demonstrating a predominant role of positive selection over negative selection in shaping the Vβ repertoire. In contrast, many Vβ families that are not represented in the natural NKT cell repertoire were nevertheless capable of recognition of foreign and microbial lipids, revealing a surprisingly inefficient selection of potentially useful TCRs.
RESULTS
Vα14-Jα18 transgenic (Tg) cells in CD1d −/− mice
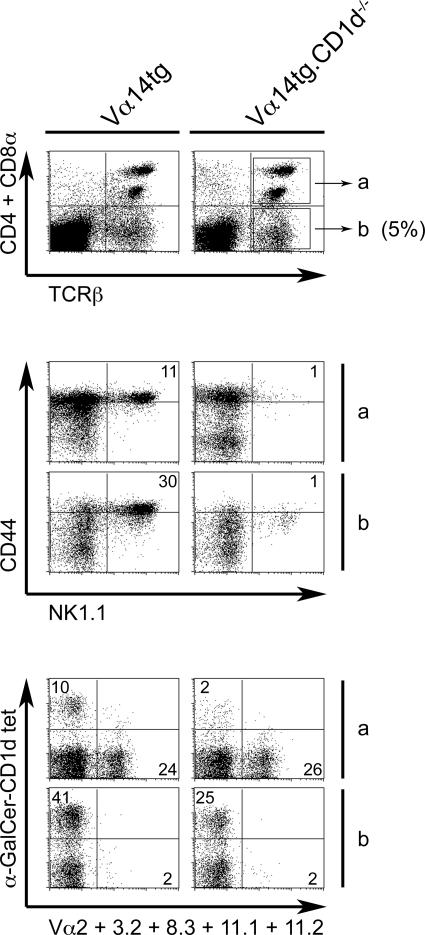
Vα14-Jα18 TCR α chain Tg mice have an increased population of canonical CD44highNK1.1+ NKT cells with the expected Vβ8, Vβ7, Vβ2 bias and CD4 or double negative (DN) phenotype. We previously reported that some Tg lines also harbored in their thymus and spleen a peculiar subset of mature T cells with a CD44low/intNK1.1− DN phenotype and a conspicuous absence of Vβ bias (15). By crossing the Vα14-Jα18 transgene onto a CD1d −/− background, we found that the canonical NK1.1+ NKT cells had disappeared, as expected, whereas the CD44low/intNK1.1− DN T cells persisted (Fig. 1, top and middle). Most of the CD44low/intNK1.1− DN T cells lacked endogenous Vα chains because only 2% were stained by a combination of antibodies against Vα2, Vα3.2, Vα8, and Vα11, which otherwise accounted for 26% of the TCR α chains expressed by other T cells (Fig. 1, bottom). Thus, it is likely that the DN T cells express the invariant Vα14-Jα18 TCR α chain encoded by the transgene, whereas other T cells express endogenous Vαs. Consistent with the expression of Vα14-Jα18, up to 25% of the DN T cells were brightly stained by the α-GalCer-CD1d tetramers (Fig. 1, bottom). Such a peculiar DN lineage maturing in the absence of thymic ligand has been described previously in many TCR Tg systems and is believed to be induced by the early expression of the Tg TCR α chain at the DN3 stage (as we observed in this Tg line; unpublished data) competing with the pre-TCR α chain for signaling (16–20). Importantly, these Tg cells were reported to respond to their ligand in a dosage-dependent manner despite the absence of thymic selection (21).
Figure 1.
Mature DN T cells in Vα14tg.CD1d−/− mice. (top) The spleen of Vα14tg mice harbors a sizeable population of CD4−CD8α−CD3ɛ+ DN T cells (boxed in gate b) that is independent of CD1d expression. (middle) Gated DN T cells have a uniform CD44low/intNK1.1− phenotype in Vα14tg.CD1d −/− mice. (bottom) The DN T cells of Vα14tg.CD1d −/− mice do not express endogenous TCR α chains; 25% of them are α-GalCer-CD1d tetramer+. Note that, in contrast, the CD4 and CD8 T cells (boxed in gate a) in these mice express endogenous Vαs but are α-GalCer-CD1d tetramer-negative. Data are representative of four independent experiments with two mice per group.
Nonbiased Vβ repertoire of Vα14-Jα18 Tg DN cells in CD1d −/− mice
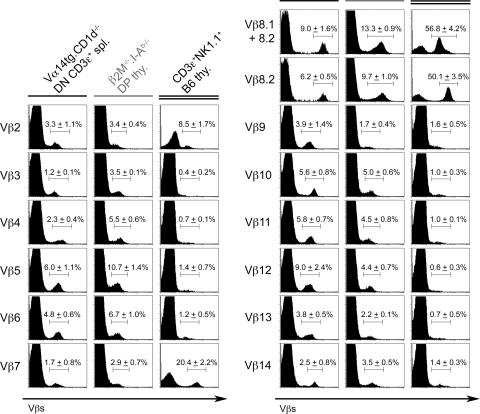
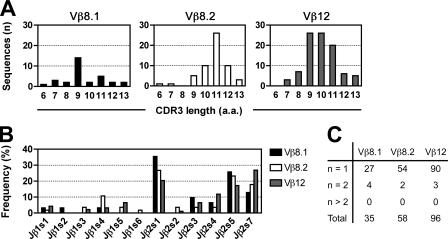
We next studied the Vβ repertoire associated with the invariant TCR α chain in the Vα14-Jα18 Tg DN T cells of CD1d −/− mice. For comparison with another nonselected Vβ repertoire, we used CD4+CD8+ double positive (DP) thymocytes from β2m −/−.I-A b−/− “MHC-null” mice that express high levels of surface TCR readily stained by anti-Vβ antibodies (22). For comparison with the CD1d-restricted repertoire, we used CD3+NK1.1+ thymocytes from WT B6 mice thymuses, which contain >95% canonical tetramer+ NKT cells (23). Fig. 2 shows that, in dramatic contrast with the biased Vβ8, Vβ7, Vβ2 repertoire of natural NKT cells in WT mice, the CD1d −/− Tg DN cells expressed a very diverse repertoire. This repertoire appeared relatively close to the nonselected DP thymocytes in MHC-null mice, although significant differences were found for some Vβs suggesting that, in the absence of selection by CD1d, Vα14-Jα18 imparts only modest pairing bias compared with the average Vα chain. To further evaluate the diversity of the Vβ repertoire associated with Vα14-Jα18, we cloned and sequenced multiple CDR3 segments generated by RT-PCR from sorted, CD1d −/− Tg DN cells. We chose three TCR β chains, Vβ8.2, Vβ8.1, and Vβ12, as representatives of Vβs highly, poorly, or nonenriched in natural NKT lineage cells, respectively. Fig. 3 shows a broad distribution of CDR3 lengths and a broad usage of Jβ segments for each of these TCR β chains, with a similar overrepresentation of the Jβ2 over the Jβ1 cluster as previously reported for conventional T cells (24, 25). We found 77–94% of unique sequences in each set of TCR β chains analyzed, similar to previous findings reported for other T cell populations (26, 27). Together, these results establish the broad diversity of the Vβ repertoire expressed by the CD1d −/− Tg DN cells. Thus, pairing of Vα14-Jα18 with TCR β chains is largely promiscuous and unlikely to be a significant factor in the Vβ repertoire bias of Vα14 NKT cells. Furthermore, comparison of the nonselected Vβ repertoire of CD1d −/− Tg DN cells with the natural Vβ repertoire of mature NKT thymocytes allowed a precise calculation of the degree of enrichment associated with thymic selection for each Vβ family. Thus, Vβ2 was enriched 2.6-fold, Vβ8.2 was enriched 8-fold, and Vβ7 was enriched 12-fold. A similar percentage of Vβ7 and Vβ8.1+8.2 as in the CD1d −/− Tg DN cells was also found in DP thymocytes obtained from another CD1d −/− Vα14-Jα18 TCR Tg strain where the transgene was driven by a CD4 promoter to avoid premature expression at the DN3 stage (unpublished data). Thus, this striking Vβ7>Vβ8.2>Vβ2 hierarchy contrasts with previous estimates derived merely from the relative frequency of Vβs expressed by NKT cells, where Vβ8.2>Vβ7 because it was six times more frequent than Vβ7. It also differs significantly from estimates derived by comparing the NKT lineage repertoire with mainstream DP thymocytes where Vβ7 “enrichment” was similar to that of Vβ8.2 (14).
Figure 2.
Vβ repertoire of Vα14tg.CD1d−/− DN T cells. The Vβ repertoires of splenic CD3ɛ+ DN T cells in Vα14tg.CD1d −/− mice, DP thymocytes in MHC-null mice, and CD3ɛ+NK1.1+ thymic NKT cells in C57BL/6 mice are compared. Histograms show staining by a panel of anti-Vβ mAbs with percentages as indicated. Data are means ± SD of three independent experiments with at least two mice per group.
Figure 3.
Vα14tg.CD1d−/− DN T cells have diverse CDR3 sequences. Vβ8.1- (n = 35), Vβ8.2- (n = 58), and Vβ12-CDR3 (n = 96) sequences amplified by RT-PCR from sorted Vα14tg.CD1d −/− DN T cells were cloned and sequenced. (A) Bar graphs of CDR3 lengths (a.a., amino acid). (B) Frequencies of Jβ usage expressed as percentage of total sequences. Note that a similar bias in Jβ2 cluster usage has been reported for conventional T cells. (C) Number of unique and duplicate sequences among Vβ8.1, Vβ8.2, and Vβ12.
Vβ-specific binding of α-GalCer-CD1d tetramers
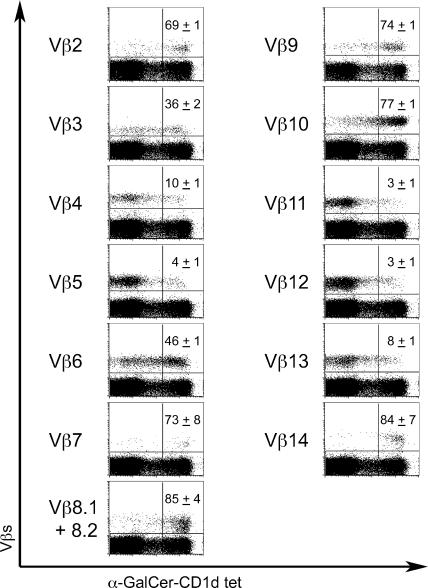
α-GalCer-CD1d tetramers stain nearly all mouse NKT cells expressing Vα14-Jα18 combined with Vβ8, Vβ7, Vβ2 (23, 28). In the nonselected Vα14-Jα18 Tg DN T cells, 69–85% of cells expressing Vβ8, Vβ7, and Vβ2 were tetramer+ (Fig. 4), indicating that these Vβs are usually, though not always, compatible with α-GalCer recognition independently of the CDR3 sequence. Surprisingly, however, 36–84% of cells expressing additional Vβ families that are not significantly represented in the natural NKT cell repertoire (Fig. 2), including Vβ3, 6, 9, 10, and 14, were also stained by the tetramers. Other Vβs, such as Vβ4, 5, 11, 12, and 13 were mostly negative, although they consistently included 3–10% positive cells. Thus, these results reveal many new Vβ families that allow for α-GalCer recognition but had escaped prior recognition because of their absence from the natural NKT cell repertoire. As expected, an extended Vβ repertoire was also found for the microbial lipid α-glucuronylceramide of which α-GalCer is a mimic (unpublished data).
Figure 4.
Vβ-specific binding of α-GalCer-CD1d tetramers to Vα14tg.CD1d−/− DN T cells. Freshly isolated splenocytes from Vα14tg.CD1d −/− mice were stained with α-GalCer-CD1d tetramer, CD3ɛ, CD4 and CD8α, and a panel of anti-Vβ mAbs. Dot plots are gated on CD3ɛ+CD4−CD8α− cells. Numbers in the top right quadrants indicate the percentages of tetramer+ cells among the Vβ positive cells. Data are mean ± SD from three individual mice analyzed in three independent experiments.
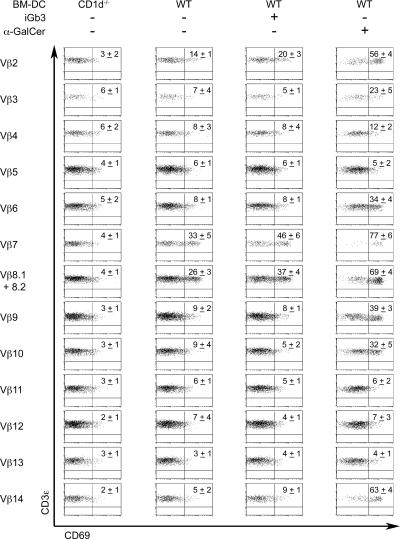
Vβ-specific induction of CD69 by CD1d/lipid ligands
Because iGb3-CD1d tetramers could not stain NKT cells (6), we devised T cell stimulation assays to compare the Vβ repertoires specific for iGb3 and α-GalCer. Thymocytes have very poor endocytic properties and are therefore defective in the presentation of exogenous lipid ligands requiring endosomal access for processing or loading onto CD1 molecules (reference 29 and unpublished data). Therefore, we used DCs pulsed with antigen to stimulate DN T cells obtained from the spleen of Vα14tg.CD1d −/− mice. After 4–6 h, DN T cells were harvested and stained for CD69. Fig. 5 shows that a large fraction of Vβ2, 7, and 8 cells up-regulated CD69 in response to DCs pulsed with either iGb3 (20–46%) or α-GalCer (56–77%) when compared with unpulsed DCs from CD1d −/− mice (3–4%). Consistent with the tetramer staining experiments, many additional Vβs were stimulated by α- GalCer, including Vβ3, 6, 9, 10, 14 (23–63%), whereas others (Vβ4, 5, 11, 12, 13) gave very weak responses (4–12%). Interestingly, when compared with CD1d −/− DCs, unpulsed WT DCs weakly but consistently stimulated Vβ2, 7, and 8 cells (14–33%), suggesting that natural ligand expression was sufficient to induce CD69 on a subset of cells expressing the Vβ families naturally associated with NKT cells. Thus, in contrast with α-GalCer, both the natural ligands expressed by DCs and exogenously added synthetic iGb3 elicited the very same Vβ repertoire as expressed by natural NKT cells. Importantly, neither synthetic iGb3 nor naturally expressed ligands stimulated other Vβs, suggesting that the Vβ bias of natural NKT cells must reflect positive selection of these Vβs rather than negative selection of other Vβs.
Figure 5.
Vβ-specific expression of CD69 by antigen-stimulated Vα14tg.CD1d−/− DN T cells. Enriched DN T cells from Vα14tg.CD1d −/− spleens were cocultured with CD1d −/− DCs or WT DCs pulsed with 100 ng/ml iGb3, 1 ng/ml α-GalCer, or no antigen. T cells were stimulated for 4 h with α-GalCer or for 6 h with iGb3. Percentages of CD69-positive DN T cells gated according to Vβ usage are indicated in top right quadrants as mean ± SD from four independent experiments.
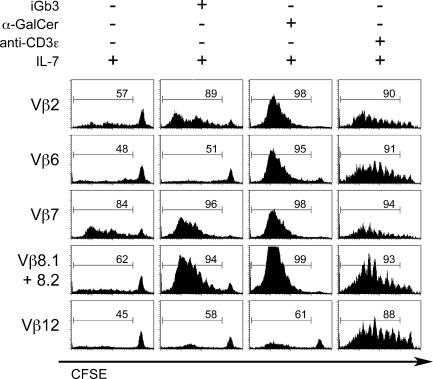
Vβ-specific induction of cell division by CD1d/lipid ligands
Although CD69 can be induced by partial TCR signaling, T cell proliferation requires stronger “agonist” signals (30). Indeed, iGb3 was reported to be an agonist ligand for mouse and human NKT cells and a prime candidate for mediating the positive selection and the expansion of NKT cell precursors in the thymus (6). Therefore, to model the expansion of selective Vβs during NKT development, we used CFSE-labeled DN T cells to compare the mitogenic properties of endogenous and exogenous NKT ligands in a cell culture system. IL-7 was added to prevent the death of unstimulated cells over the 4-d culture period with antigen-pulsed DCs. When DN T cells were incubated with CD1d −/− DCs alone, a background CFSE dilution of ∼50%, likely the result of IL-7, was observed for all Vβs in different experiments. Remarkably, CD1d-expressing WT DCs induced a significant and selective expansion of Vβ7 cells (84%) in the absence of added ligands, above the background CFSE dilution observed in Vβ6 and Vβ12 cells (45–48%) (Fig. 6). Vβ2 (57%) and Vβ8 (62%) were slightly higher than Vβ6 and Vβ12. These data suggest that naturally expressed ligands can act as true albeit weak agonists capable of inducing cell proliferation, with the highest affinity for Vβ7, similar to the Vβ7>Vβ8>Vβ2 hierarchy observed in the CD69 induction assay (compare with Fig. 5). When a low dose of anti-CD3ɛ antibody was used to induce polyclonal activation, all Vβs underwent similar expansion, demonstrating that the selective Vβ response to naturally expressed NKT ligands was specific to these antigens. DCs pulsed with synthetic iGb3 induced the selective expansion of Vβ2, 7, and 8 cells (89–96%) well above the levels induced by naturally expressed ligands but with a similar Vβ7>Vβ8>Vβ2 hierarchy. In contrast, consistent with the experiments using tetramer staining (Fig. 4) and short-term induction of CD69 (Fig. 5), α-GalCer induced a strong expansion not only of Vβ2, 7, and 8 cells (98–99%) but also Vβ6 cells (95%), whereas Vβ12 cells remained close to background, as expected from their lack of CD69 induction.
Figure 6.
Vβ-specific proliferation of antigen-stimulated Vα14tg.CD1d−/− DN T cells. CFSE-labeled Vα14tg.CD1d −/− DN T cells were cocultured with antigen-pulsed DCs in the presence of 10 ng/ml IL-7 for 4 d and CFSE dilution profiles were gated according to Vβ expression. DCs were pulsed with no antigen, iGb3 at 50 ng/ml or α-GalCer at 10 ng/ml. Control stimulation was with a low dose of 50 ng/ml of anti-CD3ɛ directly added to the culture wells. Data are representative of three independent experiments.
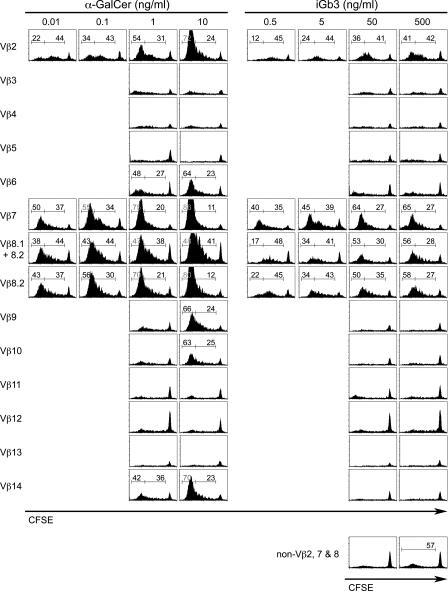
Vβ-specific hierarchy in responses to glycolipid ligands
To extend the aforementioned findings to the larger Vβ repertoire and to more rigorously quantitate the apparent differences in individual Vβ responses, DCs were pulsed with a range of concentrations of α-GalCer and iGb3 before coculture with CFSE-labeled DN T cells. Fig. 7 shows the CFSE dilution profiles according to Vβ expression, with statistics displayed whenever dilution was >50% background level. Frequencies of CFSE-negative (>7 divisions) and CFSE-low cells were displayed separately to provide a quantitation of the rate of proliferation in addition to the frequency of cells having proliferated. Furthermore, absolute cell recoveries according to Vβ expression were also recorded (Table I). α-GalCer expanded a broad range of Vβ, including Vβ2, 6, 7, 8.2, 9, 10, and 14. These results closely resembled the patterns found with tetramer staining (Fig. 4) and CD69 induction (Fig. 5). However, one notable exception was Vβ3, which stained with tetramers (36%) and induced CD69 (23%), yet failed to induce detectable expansion. Closer examination of Figs. 4 and 5 indicated that the fluorescence intensity of staining with tetramers or CD69 was clearly lower for Vβ3 than for other responder Vβs, suggesting lower affinity as an explanation for the lack of proliferation. Vβ7 and Vβ8.2 cells had comparable responses over a range of α-GalCer concentrations, as judged by the percentage of cells that diluted CFSE to low or negative levels and by the cellular expansion measured in absolute cell numbers (Table I). Slight differences between Vβ7 and Vβ8.1+8.2 cells were the result of the heterogeneity of Vβ8 because Vβ8.1 was much less responsive than Vβ8.2 to α-GalCer (Fig. 6 and Table I). In a previous report (12), staining of Vβ8.2 NKT cells with a dimeric CD1d-αGalCer ligand (CD1d-IgG1) was somewhat brighter than that of Vβ7. This apparent discrepancy with our results may reflect differences between the pre- and postselection NKT cell repertoire or, alternatively, indicate that the affinity estimated by ligand staining does not always correlate well with various forms of functional stimulation. In contrast with Vβ8.2 and Vβ7, Vβ2 cells were ∼10 times less responsive to α-GalCer than either Vβ7 or Vβ8 cells, as judged by the 10 times greater amount of α-GalCer required for Vβ2 cells to achieve similar CFSE dilutions as Vβ8.2 and Vβ7 cells. The other responder Vβ chains, including Vβ6, 9, 10, and 14, required concentrations of α-GalCer in the ng/ml range for cell expansion, somewhat higher than Vβ2, though still in the low range compared with peptide antigens of conventional MHC-restricted T cells.
Figure 7.
Vβ-specific hierarchy of responses to graded concentrations of antigens. CFSE-labeled Vα14tg.CD1d −/− DN T cells were stimulated as in Fig. 6 using a range of antigen concentrations. Numbers in each histogram indicate the percentage of cells within the corresponding brackets of CFSE-negative (left) and CFSE-low (right) cells. Histograms with no percentages have <50% CFSE-diluted cells. Data are representative of four independent experiments.
Table I.
Vβ-specific expansion of Vα14tg.CD1d −/− DN T cells stimulated by lipid antigens
| α-GalCer (ng/ml) | iGb3 (ng/ml) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0.01 | 0.1 | 1 | 10 | 0.5 | 5 | 50 | 500 | |
| Vβ2 | 0.6 | 0.9 | 4.2 | 9.2 | 0.5 | 0.7 | 3.7 | 4.3 |
| Vβ6 | ND | ND | 1.7 | 2.7 | ND | ND | <0.5 | <0.5 |
| Vβ7 | 2.1 | 4.0 | 15.6 | 27.3 | 1.4 | 3.0 | 10.2 | 14.6 |
| Vβ8.1 + 8.2 | 1.2 | 1.9 | 8.7 | 17.3 | 0.6 | 1.2 | 5.1 | 6.8 |
| Vβ8.2 | 1.5 | 2.6 | 12.0 | 23.1 | 0.7 | 1.3 | 6.0 | 7.2 |
| Vβ9 | ND | ND | <0.5 | 4.7 | ND | ND | <0.5 | <0.5 |
| Vβ10 | ND | ND | <0.5 | 4.1 | ND | ND | <0.5 | <0.5 |
| Vβ14 | ND | ND | 3.2 | 9.4 | ND | ND | <0.5 | <0.5 |
| non-Vβ2, 7, & 8 | ND | ND | 0.6 | 0.7 | ||||
The fold increase in the absolute number of cells expressing indicated Vβs at the end of a 4-d culture period is shown for a range of concentrations of α-GalCer and iGb3. Data are representative of four independent experiments. ND, not determined.
Stimulation by iGb3 demonstrated remarkably distinct features (Fig. 7). In general, iGb3 exerted its stimulatory effects at concentrations 30–50 times greater than those needed for α-GalCer, but it is unclear at present whether this difference in potency reflects a lower affinity (weak agonist) or different requirement for uptake and presentation by DCs. In contrast with α-GalCer and as noted in the CD69 induction assay in Fig. 5, iGb3 nearly exclusively stimulated Vβ2, Vβ7, and Vβ8, thus faithfully mimicking the natural NKT cell bias. This highly selective effect was further illustrated by the near background CFSE dilution (57%) found for all the non-Vβ2, 7, 8 cells examined together at the highest concentration of iGb3 (Fig. 7, bottom right). Furthermore, iGb3 was 10 times more stimulatory for Vβ7 than for Vβ8.2 cells, as judged by the proportion of cells reaching the CFSE-negative stage and by the cell expansion measured in absolute numbers (Table I). For example, 40% of Vβ7 cells had reached the CFSE-negative stage at 0.5 ng/ml of iGb3 compared with only 34% of Vβ8.2 at 5 ng/ml (Fig. 7), and the level of expansion of Vβ7 at 0.5 ng/ml was not reached until 5 ng/ml for Vβ8.2 (Table I). Thus, the data establish a clear hierarchy of Vβ stimulation by iGb3, with Vβ7>Vβ8.2>Vβ2, fully consistent with their respective 12-, 8-, and 2.6-fold enrichments during thymic selection (Fig. 2). This good correlation between the hierarchy measured in vitro and the selection bias in vivo is consistent with iGb3 being a major thymic ligand for NKT precursors and further suggests that positive selection rather than negative selection plays the primary role in shaping the Vβ repertoire of Vα14 NKT cells.
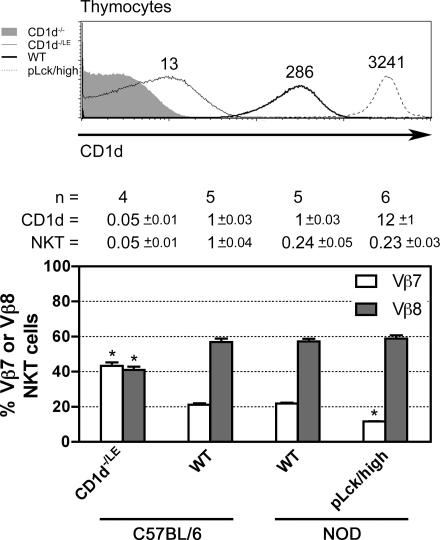
Correlations between Vβ selection and CD1d levels in vivo
Previous reports have shown that changes in Vβ selection occurred in mice expressing lower or higher levels of CD1d, and were hypothesized to result from changes in positive and negative selection according to Vβ affinities for naturally expressed ligands. Thus, in mice expressing very high levels of CD1d (∼50 times greater than WT) under a MHC class I promoter (13), NKT cells were decreased in absolute numbers to 1/3 of WT, suggesting negative selection, with Vβ2 NKT cells being relatively overrepresented compared with Vβ8 and Vβ7 cells. Conversely, Vβ7 were overrepresented in BALB/c.CD1d +/− mice as compared with WT (14). Although the intricacies of positive and negative selection and clonal competition preclude a definitive interpretation of such results when considered alone, the data are fully consistent with the hierarchy of Vβ responsiveness to iGb3 and naturally expressed ligands as determined in the present study. Here, we present two additional CD1d expression models to further test this hierarchy in vivo. In pLck/high mice expressing CD1d under the control of the proximal Lck promoter in a NOD.CD1d −/− background, the level of CD1d was 12 times higher than that of WT mice (31). These Tg mice exhibited a dramatic decrease in the proportion of Vβ7+ NKT cells from 21 to 12%, without significant changes in total cell numbers (Fig. 8). Although this Tg model was studied in the NOD background, which is known to be a low NKT expressor, the results were significant because they could be compared with littermate controls in the very same genetic background. Conversely, in C57BL/6 mice expressing a CD1d null allele and a CD1d low allele (an unintended consequence of a loxP insertion in the 5′UTR of CD1d1 locus) (CD1d −/LE mice), the percentage of Vβ7 cells reached 43% as compared with 22% in WT controls, with a corresponding decrease of Vβ8 cells from 57 to 41% (Fig. 8). Overall, NKT cell numbers were drastically reduced, suggesting that in the presence of a limited amount of ligands, positive selection could only rescue the highest affinity cells. Thus, combining these two new models with the other two previously published, the in vivo thymic selection outcomes are remarkably consistent with the Vβ hierarchy of affinity directly established from our ligand stimulation assays.
Figure 8.
Vβ repertoire changes in mice expressing high or low levels of CD1d. (top) Levels of CD1d expression (mean fluorescence intensity indicated above histograms) by thymocytes of indicated mice. CD1d −/LE are mice with a null (−) allele and a low expression (LE) allele the result of a loxP insertion in the 5′UTR of CD1d1. pLck/high are mice with high level expression of a CD1d transgene driven by the proximal Lck promoter. (bottom) Percentages of Vβ7 and Vβ8 cells among α-GalCer-CD1d tetramer+ cells were determined by FACS analysis of indicated mice. Bars represent mean and SD (white, Vβ7; gray, Vβ8) based on indicated “n” number of mice examined. “CD1d =”: relative CD1d expression level (mean fluorescence intensity) compared with B6 WT mice; “NKT =”: relative numbers of NKT thymocytes compared with B6 WT mice. *, statistical significance (P < 0.05) using unpaired Student's t test.
Vβ-specific induction of CD69 upon intrathymic cell transfer
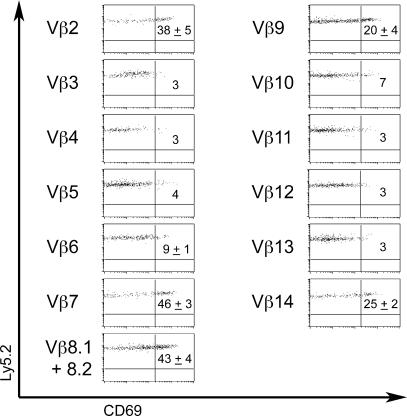
To further study the recognition of naturally expressed NKT ligands, we transferred Vβ nonbiased Vα14-Jα18 Tg DN T cells into the thymus of Ly5.1 congenic recipients. Cells were recovered from the host thymuses after 6 h and subjected to an analysis of Vβ and CD69 expression. In the absence of exogenous antigen, CD69 was induced on a subset of transferred cells with a selective Vβ7 (46%)>Vβ8 (43%)>Vβ2 (38%) pattern (Fig. 9) reminiscent of iGb3 stimulation assays in vitro. Surprisingly, however, two additional Vβs, Vβ9 and Vβ14, exhibited significant induction of CD69, albeit to lower levels than the canonical Vβ7, Vβ8, Vβ2, suggesting weaker signaling. This surprising result was consistently observed in 3/3 independent transfer experiments and may suggest that, in addition to iGb3, other thymic ligands may be able to activate NKT precursors. These putative antigens, however, must be unable to meet the critical signaling requirements for inducing cell expansion and/or survival. Unfortunately, the transferred Vα14-Jα18 Tg DN T cells could not be efficiently tracked over the longer period of time required to evaluate cell proliferation, precluding a more complete assessment of the fate of these Vβ9 and Vβ14 cells.
Figure 9.
Vβ-specific induction of CD69 after intrathymic transfer of Vα14tg.CD1d−/− DN T cells. DN T cells were intrathymically injected into Ly5.1 congenic host. After 6 h, CD69 expression was determined by FACS analysis of Ly5.2+CD4−CD8α−-gated cells according to Vβ expression. Data are means ± SD from three independent experiments.
DISCUSSION
By transgenically expressing the Vα14-Jα18 transgene in a CD1d-deficient background, we have created a DN T cell population with a TCR repertoire made of the canonical invariant α chain of NKT cells combined with a broad, nonbiased Vβ repertoire. Several features suggest that these Tg cells display a faithful representation of the preselection repertoire of TCR β chains associated with Vα14-Jα18 α chain. First, they originate in the thymus in the absence of selection by CD1d/ligand. Second, they express an endogenous TCR β repertoire of breadth and diversity comparable to that of nonselected DP thymocytes. Third, their ontogeny is related to the premature expression of the Tg TCR α chain that binds to the TCR β chains expressed at the DN3 stage. It is believed that, by modifying the signals that normally emanate from the pTα–TCRβ complexes, the TCR α transgene bypasses the subsequent need for TCR-mediated positive selection (16–20, 32). Finally, similar to a subset of mature NKT cells, these Tg cells do not express CD4 or CD8. These Tg DN cells provide, therefore, a unique tool to explore the structure and the functional properties of the preselection repertoire of NKT precursor thymocytes and to contrast them with those of natural mature NKT cells.
Our findings elucidate some long-standing issues regarding the mechanisms underlying NKT cell repertoire selection. Because the nonselected Vβ repertoire associated with Vα14-Jα18 differed only slightly from the one found in the nonselected DP thymocytes of MHC-null mice, which represents an average of all Vαs, we conclude that the Vβ-pairing bias of Vα14-Jα18 α chain must be modest compared with other Vαs. This finding contrasts with isolated or indirect evidence suggesting an inherent instability of many Vα14–Jα18/Vβ complexes (10, 11).
Second, by comparing the frequency of Vβ families in the unselected and postselection repertoire, it was possible for the first time to measure the degree of enrichment for individual families, with Vβ7 (x12) clearly above Vβ8.2 (x8) and Vβ2 (x2.6) at the bottom. The picture would be significantly different if the baseline for comparison was the DP repertoire because Vβ7 and Vβ8.2 would appear to be equally selected (14). Remarkably, these data matched precisely the hierarchy of Vβ responses observed after stimulating the nonselected Vα14-Jα18 Tg T cells with naturally expressed ligands or with synthetic iGb3. Thus, we conclude that the Vβ repertoire associated with Vα14-Jα18 in NKT lineage cells has undergone predominant positive selection and only modest negative selection.
Importantly, the close resemblance between stimulation by iGb3 and stimulation by the naturally expressed NKT ligands provides new and independent support for the notion that iGb3 is the main NKT ligand involved in these thymic processes. A large proportion ranging from 20 to 50% of the Vβ8-, Vβ7-, and Vβ2-expressing cells responded to iGb3 in the CD69 induction assay, whereas nearly all of them responded in the CFSE dilution assay. Although the different nature and kinetics of these assays may underlie these differences, the results converge to indicate that a substantial proportion of the naturally arising Vβ8, Vβ7, and Vβ2 TCRs may be selected during NKT cell development. Surprisingly, Vβ9- and Vβ14-expressing cells consistently exhibited some level of CD69 induction upon intrathymic transfer, despite the absence of response to iGb3 and despite the absence of corresponding cells in the natural NKT cell pool. This observation could indicate that additional unidentified ligands partially activate NKT precursors during thymic development, yet may not promote their selection or expansion, perhaps as a result of lower affinity.
Another important insight gained from this study relates to the much larger repertoire elicited by synthetic α-GalCer, a mimic of microbial glycuronosylceramides, than by endogenous ligands. Thus, many Vβs that are not stimulated by iGb3 and are not found in the natural NKT repertoire, including Vβ3, 6, 9, 10, 14, could nevertheless be stimulated by nanomolar concentrations of α-GalCer. These unexpected findings demonstrate that the NKT cell repertoire against foreign and microbial antigens is severely limited because thymic exposure to natural ligands failed to select potentially useful TCRs. This stands in sharp contrast with positive selection driven by polymorphic MHC ligands, where the opposite outcome is observed, namely a gain in recognition of foreign peptides after positive selection by self-ligands (33). We propose that these differences may be inherent to the processes of positive selection by partial agonists/antagonists versus full agonists.
Finally, our results directly confirm and extend to the entire nonselected repertoire recent reports (14) suggesting that different Vβ families generically encode different affinities for lipid ligands. Vβ7 responded much better than Vβ8.2 to low doses of natural ligand, whereas both responded comparably to α-GalCer and microbial ligands. Because of the absence of CDR3 restriction in these responses, the CDR1 and 2 regions of the β chain may confer the differences in affinity. Future structural studies of these interactions, including crystallography and mutational analysis, will shed light on this intriguing mode of TCR recognition. In the meantime, our quantitative determination of the hierarchy of natural ligand recognition by different Vβ families provides potentially useful knowledge to assess the contribution of affinity to the functional properties of NKT cell subsets and to the selection of NKT cells in mice exhibiting NKT cell defects.
MATERIALS AND METHODS
Mice.
C57BL/6 and NOD mice were purchased from The Jackson Laboratory. C57BL/6.β2M −/−.I-A b−/− (MHC-null) and C57BL/6.Ly5.1 congenic mice were purchased from Taconic Farm. C57BL/6.Vα14tg and C57BL/6.CD1d −/− mice were described previously (15, 34), and were intercrossed to obtain C57BL/6.Vα14tg.CD1d −/− mice. The CD1dLE (LE, low expression) allele was the unintended consequence of the insertion of a loxP site in the 5′UTR of the C57BL/6 CD1d1 gene after homologous recombination in ES cell and excision of a neomycinr gene and a STOP sequence cassette. This insertion resulted in the modification of the genomic sequence upstream of the ATG site, 5′-TAGAACTCTGGCG^CTATG-3′, which included a deletion of the underlined GCG and an insertion at the position marked ^ of the sequence 5′-CATAACTTCGTATAGCATACATTATACGAAGTTATATTAAGGGTTATTGAATATGATCGGAATTCCTCGAGCGGC-3′. The C57BL/6.CD1d −/LE mouse carrying a null allele and a LE allele expressed very reduced levels of CD1d on the surface of thymocytes. The LE allele was screened using PCR (forward primer: 5′-TGTGTAGAACTCTGCATAACTTCG-3′, reverse primer: 5′-GGGCCAGGTGTATTCAGGAGCATCTGCACGG-3′). All mice were housed in a specific pathogen-free facility at the University of Chicago according to the guidelines of the institutional animal care and use committee.
Flow cytometry.
Fluorochrome-conjugated mAbs anti-CD1d, CD3ɛ, CD4, CD8α, CD44, CD69, Ly5.2, NK1.1, TCRβ, TCR Vβ2 through Vβ14, TCR Vα2, Vα3.2, Vα8.3, and Vα11.1+11.2, as well as biotinylated Vβ2 through Vβ14 mAbs, were purchased from BD Biosciences. Streptavidin conjugated to allophycocyanin (APC) or R-PE was purchased from eBioscience. Anti-Vβ8.2 mAb (clone F23.2) was biotinylated in our laboratory. CD1d tetramers loaded with α-GalCer were prepared and used as described previously (23). CFSE labeling of enriched splenocytes was done in vitro according to manufacturer's instructions in CellTrace CFSE Cell Proliferation kit (Invitrogen). Flow cytometry analysis was performed on a four-color FACSCalibur with CELLQuest software (BD Biosciences).
CDR3 sequencing.
DN T cells were sorted as TCRβ+CD4−CD8α− cells from C57BL6.Vα14tg.CD1d −/− splenocytes. RNA extraction, reverse transcription PCR, and plasmid cloning and sequencing methods were as described previously (35). Primers were 5′-CCTCATTCTGGAGTTGGCTACCC-3′ for Vβ8.2+8.1, 5′-GAAGATGGTGGGGCTTTCAAGGATC-3′ for Vβ12, and 5′-CTTGGGTGGAGTCACATTTCTC-3′ for Cβ. CDR3 sequence analyses were performed according to the international ImMunoGeneTics (IMGT) information system website (http://imgt.cines.fr) (36).
Cell stimulation assays.
Single cell suspensions prepared from four to five adult C57BL6.Vα14tg.CD1d −/− spleens were first enriched for DN T cells by depleting B, CD4, CD8, and NK cells with B220, CD4, CD8α, and DX5 mAb-conjugated magnetic microbeads and AutoMACS (Miltenyi Biotec) according to manufacturer's instructions. DX5 is not expressed by the DN T cells. For the CFSE expansion assay, this enriched population (>50% were DN CD3ɛ+ cells) was labeled with CFSE before stimulation with lipid-pulsed BM-DCs. The DCs were prepared from cultures of BM cells in a 1:1 mixture of EHAA and RPMI 1640 (Biofluids) supplemented with 10% heat-inactivated fetal calf serum, glutamine, antibiotics, 5 × 10−5 M of 2-mercaptoethanol and 2 ng/ml of mouse GM-CSF (Biosource). After 6 d of culture, BM-DCs were pulsed with different concentrations of α-GalCer or iGb3 overnight, washed, and plated at 106 per well in six-well plates (Corning Costar). 10 × 106 enriched DN T cells were added per well and cultured in the presence of mouse recombinant IL-7 at 10 ng/ml (Biosource) for 4 d before FACS analysis. For the CD69 induction assay, the lipid-pulsed DCs were allowed to adhere to culture wells for 16–24 h before adding the enriched DN T cells. These cocultures were spun down briefly (1,200 revolutions/min) to favor cell-to-cell contact. After 4–6 h, the DN T cells were harvested and processed for FACS analysis, using anti-CD69, Vβs, CD3ɛ, and CD4 and CD8α antibodies.
Intrathymic cell transfers.
DN T cells enriched from C57BL/6.Vα14tg.CD1d −/− spleens (Ly5.2) by depleting B, CD4, CD8, and NK cells with B220, CD4, CD8α, and DX5 mAb-conjugated magnetic microbeads and AutoMACS were injected intrathymically into C57BL6.Ly5.1 congenic hosts as described previously (37). After 6 h, thymocytes were harvested and depleted of CD4- and CD8α-expressing cells using magnetic microbeads and AutoMACS (Miltenyi Biotec) before FACS analysis. Transferred DN T cells cells were identified using anti-Ly5.2, CD69, Vβs, and CD4 and CD8α antibodies.
Acknowledgments
We thank B. Jabri for expert advice in TCR repertoire analysis, C. Borowski and K. Griewank for helpful discussions, and all members of the Bendelac Lab for help and support.
This work was supported by National Institutes of Health grants to A. Bendelac (no. AI038339) and A. Bendelac, P.B. Savage, and L. Teyton (no. PO1 AI053725).
The authors have no conflicting financial interests.
Abbreviations used: DN, double negative; DP, double positive; NKT, natural killer T; Tg, transgenic.
References
- 1.Godfrey, D.I., H.R. MacDonald, M. Kronenberg, M.J. Smyth, and L. Van Kaer. 2004. NKT cells: what's in a name? Nat. Rev. Immunol. 4:231–237. [DOI] [PubMed] [Google Scholar]
- 2.Bendelac, A., M.N. Rivera, S.H. Park, and J.H. Roark. 1997. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu. Rev. Immunol. 15:535–562. [DOI] [PubMed] [Google Scholar]
- 3.Lantz, O., and A. Bendelac. 1994. An invariant T cell receptor α chain is used by a unique subset of major histocompatibility complex class I– specific CD4+ and CD4−8− T cells in mice and humans. J. Exp. Med. 180:1097–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsuda, J.L., L. Gapin, N. Fazilleau, K. Warren, O.V. Naidenko, and M. Kronenberg. 2001. Natural killer T cells reactive to a single glycolipid exhibit a highly diverse T cell receptor β repertoire and small clone size. Proc. Natl. Acad. Sci. USA. 98:12636–12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ronet, C., M. Mempel, N. Thieblemont, A. Lehuen, P. Kourilsky, and G. Gachelin. 2001. Role of the complementarity-determining region 3 (CDR3) of the TCR-β chains associated with the Vα14 semi-invariant TCR α-chain in the selection of CD4+ NK T Cells. J. Immunol. 166:1755–1762. [DOI] [PubMed] [Google Scholar]
- 6.Zhou, D., J. Mattner, C. Cantu III, N. Schrantz, N. Yin, Y. Gao, Y. Sagiv, K. Hudspeth, Y.P. Wu, T. Yamashita, et al. 2004. Lysosomal glycosphingolipid recognition by NKT cells. Science. 306:1786–1789. [DOI] [PubMed] [Google Scholar]
- 7.Mattner, J., K.L. Debord, N. Ismail, R.D. Goff, C. Cantu III, D. Zhou, P. Saint-Mezard, V. Wang, Y. Gao, N. Yin, et al. 2005. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 434:525–529. [DOI] [PubMed] [Google Scholar]
- 8.Kinjo, Y., D. Wu, G. Kim, G.W. Xing, M.A. Poles, D.D. Ho, M. Tsuji, K. Kawahara, C.H. Wong, and M. Kronenberg. 2005. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 434:520–525. [DOI] [PubMed] [Google Scholar]
- 9.Fischer, K., E. Scotet, M. Niemeyer, H. Koebernick, J. Zerrahn, S. Maillet, R. Hurwitz, M. Kursar, M. Bonneville, S.H. Kaufmann, and U.E. Schaible. 2004. Mycobacterial phosphatidylinositol mannoside is a natural antigen for CD1d-restricted T cells. Proc. Natl. Acad. Sci. USA. 101:10685–10690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gui, M., J. Li, L.J. Wen, R.R. Hardy, and K. Hayakawa. 2001. TCRβ chain influences but does not solely control autoreactivity of Vα14J281T cells. J. Immunol. 167:6239–6246. [DOI] [PubMed] [Google Scholar]
- 11.Degermann, S., G. Sollami, and K. Karjalainen. 1999. Impaired NK1.1 T cell development in mice transgenic for a T cell receptor β chain lacking the large, solvent-exposed cβ FG loop. J. Exp. Med. 190:1357–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schumann, J., R.B. Voyle, B.Y. Wei, and H.R. MacDonald. 2003. Cutting edge: influence of the TCR V beta domain on the avidity of CD1d: α-galactosylceramide binding by invariant Vα14 NKT cells. J. Immunol. 170:5815–5819. [DOI] [PubMed] [Google Scholar]
- 13.Chun, T., M.J. Page, L. Gapin, J.L. Matsuda, H. Xu, H. Nguyen, H.S. Kang, A.K. Stanic, S. Joyce, W.A. Koltun, et al. 2003. CD1d-expressing dendritic cells but not thymic epithelial cells can mediate negative selection of NKT cells. J. Exp. Med. 197:907–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schumann, J., M.P. Mycko, P. Dellabona, G. Casorati, and H.R. Macdonald. 2006. Cutting edge: influence of the TCR Vβ domain on the selection of semi-invariant NKT cells by endogenous ligands. J. Immunol. 176:2064–2068. [DOI] [PubMed] [Google Scholar]
- 15.Bendelac, A., R.D. Hunziker, and O. Lantz. 1996. Increased interleukin 4 and immunoglobulin E production in transgenic mice overexpressing NK1 T cells. J. Exp. Med. 184:1285–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Boehmer, H., J. Kirberg, and B. Rocha. 1991. An unusual lineage of α/β T cells that contains autoreactive cells. J. Exp. Med. 174:1001–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruno, L., H.J. Fehling, and H. von Boehmer. 1996. The αβ T cell receptor can replace the γδ receptor in the development of γδ lineage cells. Immunity. 5:343–352. [DOI] [PubMed] [Google Scholar]
- 18.Terrence, K., C.P. Pavlovich, E.O. Matechak, and B.J. Fowlkes. 2000. Premature expression of T cell receptor (TCR) αβ suppresses TCR γδ gene rearrangement but permits development of γδ lineage T cells. J. Exp. Med. 192:537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borowski, C., X. Li, I. Aifantis, F. Gounari, and H. von Boehmer. 2004. Pre-TCRα and TCRα are not interchangeable partners of TCRβ during T lymphocyte development. J. Exp. Med. 199:607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baldwin, T.A., M.M. Sandau, S.C. Jameson, and K.A. Hogquist. 2005. The timing of TCRα expression critically influences T cell development and selection. J. Exp. Med. 202:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caveno, J., Y. Zhang, B. Motyka, S.J. Teh, and H.S. Teh. 1999. Functional similarity and differences between selection-independent CD4−CD8− αβ T cells and positively selected CD8 T cells expressing the same TCR and the induction of anergy in CD4−CD8− αβ T cells in antigen-expressing mice. J. Immunol. 163:1222–1229. [PubMed] [Google Scholar]
- 22.Zerrahn, J., W. Held, and D.H. Raulet. 1997. The MHC reactivity of the T cell repertoire prior to positive and negative selection. Cell. 88:627–636. [DOI] [PubMed] [Google Scholar]
- 23.Benlagha, K., A. Weiss, A. Beavis, L. Teyton, and A. Bendelac. 2000. In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers. J. Exp. Med. 191:1895–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato, T., H. Sasakawa, M. Kurokawa, K. Masuko-Hongo, C. Hamada, K. Yamamoto, and K. Nishioka. 1999. Comparison of T-cell receptor Jβ gene usage in spleen cells of different mouse strains. Microbiol. Immunol. 43:93–97. [DOI] [PubMed] [Google Scholar]
- 25.Wallace, M.E., M. Bryden, S.C. Cose, R.M. Coles, T.N. Schumacher, A. Brooks, and F.R. Carbone. 2000. Junctional biases in the naive TCR repertoire control the CTL response to an immunodominant determinant of HSV-1. Immunity. 12:547–556. [DOI] [PubMed] [Google Scholar]
- 26.Hsieh, C.S., Y. Liang, A.J. Tyznik, S.G. Self, D. Liggitt, and A.Y. Rudensky. 2004. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 21:267–277. [DOI] [PubMed] [Google Scholar]
- 27.Pannetier, C., M. Cochet, S. Darche, A. Casrouge, M. Zoller, and P. Kourilsky. 1993. The sizes of the CDR3 hypervariable regions of the murine T-cell receptor β chains vary as a function of the recombined germ-line segments. Proc. Natl. Acad. Sci. USA. 90:4319–4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuda, J.L., O.V. Naidenko, L. Gapin, T. Nakayama, M. Taniguchi, C.R. Wang, Y. Koezuka, and M. Kronenberg. 2000. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J. Exp. Med. 192:741–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moody, D.B., V. Briken, T.Y. Cheng, C. Roura-Mir, M.R. Guy, D.H. Geho, M.L. Tykocinski, G.S. Besra, and S.A. Porcelli. 2002. Lipid length controls antigen entry into endosomal and nonendosomal pathways for CD1b presentation. Nat. Immunol. 3:435–442. [DOI] [PubMed] [Google Scholar]
- 30.Rabinowitz, J.D., C. Beeson, C. Wulfing, K. Tate, P.M. Allen, M.M. Davis, and H.M. McConnell. 1996. Altered T cell receptor ligands trigger a subset of early T cell signals. Immunity. 5:125–135. [DOI] [PubMed] [Google Scholar]
- 31.Wei, D.G., H. Lee, S.H. Park, L. Beaun, L. Teyton, A. Lehuen, and A. Bendelac. 2005. Expansion and long-range differentiation of the NKT cell lineage in mice expressing CD1d exclusively on cortical thymocytes. J. Exp. Med. 202:239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eberl, G., H.J. Fehling, H. von Boehmer, and H.R. MacDonald. 1999. Absolute requirement for the pre-T cell receptor α chain during NK1.1+ TCR αβ cell development. Eur. J. Immunol. 29:1966–1971. [DOI] [PubMed] [Google Scholar]
- 33.Bevan, M.J. 1977. In a radiation chimaera, host H-2 antigens determine immune responsiveness of donor cytotoxic cells. Nature. 269:417–418. [DOI] [PubMed] [Google Scholar]
- 34.Chen, Y.H., N.M. Chiu, M. Mandal, N. Wang, and C.R. Wang. 1997. Impaired NK1+ T cell development and early IL-4 production in CD1-deficient mice. Immunity. 6:459–467. [DOI] [PubMed] [Google Scholar]
- 35.Park, S.H., A. Weiss, K. Benlagha, T. Kyin, L. Teyton, and A. Bendelac. 2001. The mouse CD1d-restricted repertoire is dominated by a few autoreactive T cell receptor families. J. Exp. Med. 193:893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lefranc, M.P., V. Giudicelli, Q. Kaas, E. Duprat, J. Jabado-Michaloud, D. Scaviner, C. Ginestoux, O. Clement, D. Chaume, and G. Lefranc. 2005. IMGT, the international ImMunoGeneTics information system. Nucleic Acids Res. 33:D593–D597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benlagha, K., D.G. Wei, J. Veiga, L. Teyton, and A. Bendelac. 2005. Characterization of the early stages of thymic NKT cell development. J. Exp. Med. 202:485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]