Abstract
Dendritic cell (DC) function will likely be important for immunological cancer therapies, but our knowledge of the roles of DCs in tumors is limited, in part because most studies were performed before DC subtypes were defined. We studied the distributions of immature epidermal, immature dermal, mature, and plasmacytoid DCs in 63 primary tumors and 8 lymph node metastases from oral squamous cell carcinoma patients without evidence of distant metastasis. Immature CD207/Langerin+ and CD209/DC-SIGN+ DCs were present in the primary tumor region, but CD209/DC-SIGN+ cells rarely infiltrated the tumor. Mature DCs were rare, and presence of CD123+ plasmacytoid DCs was associated with poorer outcome. Unexpectedly, migration and maturation of DCs was associated with a worse outcome. Overall, the distribution of DC subtypes indicated that ineffective DC response to tumor is a failure of DC function rather than recruitment, suggesting that a strategy of shifting the balance of secreted factors in the tumor milieu may be more effective in restoring antitumor immune function than current methods restoring only one population of DCs to the immunosuppressive tumor region.
Introduction
The complexity of the interactions of the immune system with tumors becomes increasingly apparent with more detailed analysis. Dendritic cells (DCs), as the master regulators of the adaptive immune system, are being pursued as agents for immunotherapy for multiple malignancies [1, 2], but their usefulness may be limited without a better understanding of the roles of DCs during the development and progression of human cancer.
Multiple subsets of DCs play roles in the normal immune response in the oral cavity. Two subsets of myeloid immature dendritic cells, the dermal/interstitial and epidermal, collect antigen from tissues, then migrate to the lymph nodes and mature into antigen-presenting cells to induce an adaptive immune response [2]. Plasmacytoid dendritic cells, although historically known for amplifying antiviral immune responses in the lymph nodes, have recently been found in the primary tumor region of multiple types of human cancer [3-7].
Although many studies have examined the presence of DCs in cancer, they have generally been confined to one or two subtypes of DCs. These limited results are often contradictory, a problem exacerbated by the observation that tumors arising at different primary sites show significant differences in their interactions with the immune system. It is difficult to extrapolate the limited findings from one site to an overall understanding of the roles of different DC populations in cancer [3, 5, 8, 9].
To gain a more comprehensive understanding of the specific roles of DC subtypes in a primary tumor, we investigated the recruitment and infiltration of dendritic cells and other leukocytes in primary squamous cell carcinoma of the oral cavity (OSCC) using a panel of antibodies that recognize a wide array of subsets and maturation stages. More than 300,000 new cases of OSCC are diagnosed each year around the world. Despite advances in treatment, this malignancy is associated with a long-term survival rate of around 50%, which has been slow to improve over the last forty years [10-12]. Since surgical removal of the primary tumor precedes treatment with radiation and/or chemotherapy for the majority of patients, a better understanding of the immune response in the primary tumor may improve treatment decisions.
Materials and Methods
Study Population
Paraffin-embedded slides from 63 patients with newly diagnosed squamous cell carcinoma of the oral cavity were selected from the archives of the Hospital of the University of Pennsylvania upon the basis of pathological lymph node status, absence of distant metastasis, and availability of material. Clinical factors and survival data were obtained from chart review and the Social Security Death Index and subsequently de-identified at the University of Pennsylvania under IRB approval. Tumors were pathologically staged according to AJCC guidelines. No attempt was made to identify sentinel lymph nodes. Four patients with and without lymph node involvement were selected for lymph node examination. Forty-one (65%) patients were alive at the time of statistical analysis; for these patients, the median follow-up for survival was 4.8 years, with a range of 1.8-7.2 years.
Immunohistochemical Staining
For DC-LAMP (clone 104.G4, 1:50, Beckman Coulter, Fullerton, CA) staining, sections were incubated with trypsin for 10 minutes at 37°C. For Langerin (clone 12D6, 1:50, Vision BioSystems Inc., Norwell, MA) and DC-SIGN (clone DCN46, 1:40, Pharmingen, San Diego, CA), sections were incubated in 10 mM sodium citrate buffer (pH 6.0) for 10 minutes at 95°C. Slides were allowed to cool for 20 minutes, then blocked against endogenous peroxidase by incubation in 0.6% H2O2 in PBS at room temperature for 20 minutes. Slides were washed in PBS, incubated at 37°C for 1 hour with diluted antibody, then washed and incubated with biotinylated secondary antibody (Vector Laboratories, Burlingame, CA), washed again, and bound with a streptavidin-peroxidase conjugate (Dako, CA). Bound antibody was visualized using DAB or NovaRed (Vector Laboratories, CA). For CD123 staining (clone 9F5, 1:400, Pharmingen, CA), the streptavidin conjugate was amplified by a 3-minute incubation with tyramide (Perkin-Elmer, Wellesley, MA) and then a second incubation with streptavidin-peroxidase before visualization. Slides were counterstained in hematoxylin (Dako, CA), washed and dehydrated, then permanently mounted with Clarion (Sigma-Aldrich Corp., St. Louis, MO). For CD56 (clone 123C3, 1:10, Caltag, Burlingame, CA), sections were microwaved in citrate buffer (Lab Vision Corp., Fremont, CA) twice for 4 minutes and staining was performed using a similar method with a Dako Autostainer (Dako, CA).
Evaluation of Stains
Slides were scored blind to outcome. For each antibody, slides were scored semiquantitatively into four groups: 0, 1+, 2+, and 3+ based on approximate numbers of stained cells (see Supplementary Figures 1-4). For cell quantitation in lymph nodes, 4-10 lymph nodes were examined per patient. Lymph nodes were obtained from varying levels on the affected side of the neck for seven patients (one N0 patient included lymph nodes from the opposite side of the neck) and examined using a Leica DMRA2 microscope (Leica Microsystems Inc., Bannockburn, IL). Four representative pictures of each lymph node at 10x were captured and analyzed using the Nuance Multispectral Imaging System (Cambridge Research and Instrumentation Inc., Woburn, MA). Nonspecific background staining was subtracted from each image individually. Spectra were defined for the Nuance system by using known positive cells. Images were unmixed using Nuance software, cells were counted using the UTHSCSA ImageTool program (developed at the University of Texas Health Science Center at San Antonio, Texas and freely available from the Internet), and mean cell count was calculated for each lymph node.
Tests of association between categorical variables were performed with Fisher’s exact test. Survival was estimated by the method of Kaplan and Meier and differences in survival among groups were assessed by the log rank test. Differences in mean values of lymph node measurements between groups were evaluated using random effects models [13], which take into account the correlation between repeated measures that arise from multiple lymph node samples taken from the same patient. All reported p-values are two-sided. Significance was defined as p < 0.05. Statistical analyses were performed with SPSS V12.0 (SPSS Inc., Chicago, IL), Stata V8.0 (Stata Corp., College Station, TX), or the chi-square and Fisher’s exact probability calculators at http://faculty.vassar.edu/lowry/VassarStats.html and http://faculty.vassar.edu/lowry/fisher.html.
Results
Dendritic Cell Infiltration of the Primary Tumor
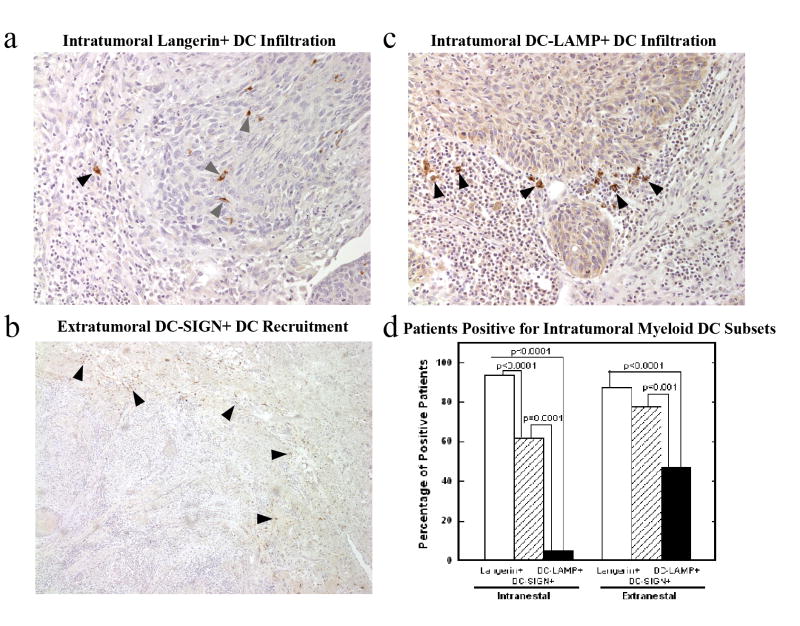
A series of 63 patients with squamous cell carcinoma of the oral cavity (Table 1) was examined for the presence and pattern of leukocyte infiltration of the primary tumor. Both intranestal (infiltration between the malignant cells) and extranestal (located in bands of tissue surrounding groups of malignant cells) invasion of the tumor area was seen (Figure 1). Two subsets of immature DCs (Langerin+ epidermal and DC-SIGN+ dermal/interstitial) were distinguished by specific antibody staining and showed differing patterns of infiltration of the primary tumor (Figure 1a, b). A high number of intranestal immature Langerin+ epidermal DCs was significantly associated with the ability of the tumor to invade angio/lymphatic vessels (p=0.009) but not the ability to invade perineurally (p=0.795, Table 2). Mature DC-LAMP+ DCs were present in significantly fewer patients than the immature DC subsets (p<0.001), and were predominantly extranestal when present (Figure 1c, d). The presence of mature DC-LAMP+ DCs was not significantly associated with any clinical factor including lymph node metastasis (p=0.380, Table 2). Lymph node metastasis was not significantly associated with presence or absence of any dendritic cell population in the primary tumor (data not shown).
Table 1. Characteristics of 63 oral squamous cell carcinoma patients.
| All Patients | n=63 | 100 % |
|---|---|---|
| Age | ||
| <= 60 | 35 | 55.6 |
| > 60 | 28 | 44.4 |
| Gender | ||
| Male | 46 | 73.0 |
| Female | 17 | 27.0 |
| Pathological T Stage* | ||
| 1 | 17 | 27.9 |
| 2 | 17 | 27.9 |
| 3 | 6 | 9.8 |
| 4 | 21 | 34.4 |
| Pathological N Stage | ||
| N0 | 30 | 47.6 |
| N1 | 8 | 12.7 |
| N2b | 19 | 30.2 |
| N2c | 6 | 9.5 |
| Angio/Lymphinvasion | ||
| Negative | 54 | 85.7 |
| Positive | 9 | 14.3 |
| Perineural Invasion | ||
| Negative | 39 | 61.9 |
| Positive | 24 | 38.1 |
| Prior Treatment | ||
| No Prior Treatment | 60 | 95.2 |
| Prior Surgery | 2 | 3.2 |
| Prior Surgery/Chemotherapy/Radiation | 1 | 1.6 |
n=61
Figure 1. Myeloid dendritic cell subsets display different patterns of primary tumor infiltration.
Immature Langerin+ dendritic cells were predominantly present within the tumor area, both intranestal (
 ) and extranestal (▲), 20x (a). Immature DC-SIGN+ dendritic cells were predominantly present outside the tumor area (▲), 5x (b). Mature DC-LAMP+ dendritic cells (▲) 20x (c), were present in fewer patients than the immature DC subsets (d).
) and extranestal (▲), 20x (a). Immature DC-SIGN+ dendritic cells were predominantly present outside the tumor area (▲), 5x (b). Mature DC-LAMP+ dendritic cells (▲) 20x (c), were present in fewer patients than the immature DC subsets (d).
Table 2. Specific clinical factors are associated with the extent of infiltration of dendritic cell subsets.
High intranestal infiltration of Langerin+ immature dendritic cells were correlated with angiolymphinvasion but not perineural invasion by the primary tumor. High levels of DC-LAMP+ mature dendritic cell invasion of the primary tumor were not associated with lymph node metastasis. ( ) indicates the column percentage for each clinical factor.
| Clinical Factor | Present | Dendritic Cell Factor | P-value | |
|---|---|---|---|---|
| Langerin+ Intranestal Infiltration | ||||
| Low(0-1+) | High(2-3+) | |||
| n=34 | n=29 | |||
| Angio/Lymphinvasive | No | 33 (97.1) | 21 (72.4) | |
| Yes | 1 (2.9) | 8 (27.6) | 0.009 | |
| Perineurally Invasive | No | 22 (64.7) | 17 (58.6) | |
| Yes | 12 (35.3) | 12 (41.4) | 0.795 | |
| DC-LAMP+ Extranestal Infiltration | ||||
| Low(0-1+) | High(2-3+) | |||
| n=48 | n=14 | |||
| Node Positive at Time of Surgery | No | 24 (50.0) | 5 (35.7) | |
| Yes | 24 (50.0) | 9 (64.3) | 0.380 | |
Multiple dendritic cell patterns were associated with decreased survival. Although the majority of DC-SIGN+ immature dermal DCs were present outside the tumor margin, their presence intratumorally was significantly associated with decreased survival (p<0.0001, Figure 2c). The presence of CD123+ plasmacytoid dendritic cells (pDCs) was also significantly associated with decreased survival (p<0.0001, Figure 2d). Since pDCs are known to activate NK cells, we examined 25 of 63 tumors for NK cell infiltration (Figure 3a). High numbers of pDCs were not associated with the presence of CD56+ NK cells, which were rare in all cases studied (p=0.712, Figure 3b).
Figure 2. Patient survival is correlated with specific dendritic cell patterns and extents of infiltration of the primary tumor.
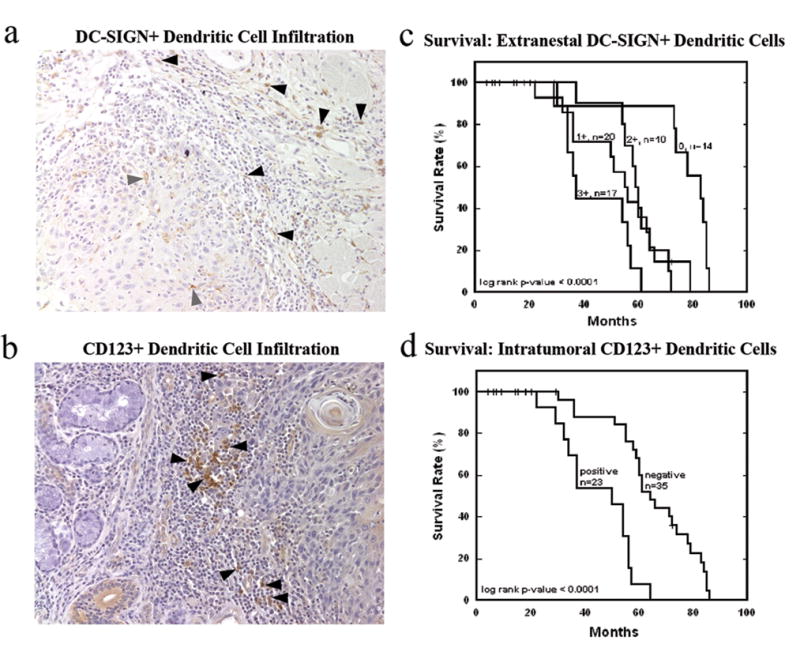
High levels of extranestal (▲) but not intranestal (
 ) DC-SIGN+ immature dendritic cell infiltration, 20x (a, c) and the presence of predominantly extranestal (▲) CD123+ plasmacytoid dendritic cells within the primary tumor, 20x (b, d) were correlated with decreased survival.
) DC-SIGN+ immature dendritic cell infiltration, 20x (a, c) and the presence of predominantly extranestal (▲) CD123+ plasmacytoid dendritic cells within the primary tumor, 20x (b, d) were correlated with decreased survival.
Figure 3. Presence of intratumoral plasmacytoid dendritic cells does not correlate with NK cell recruitment into the primary tumor.
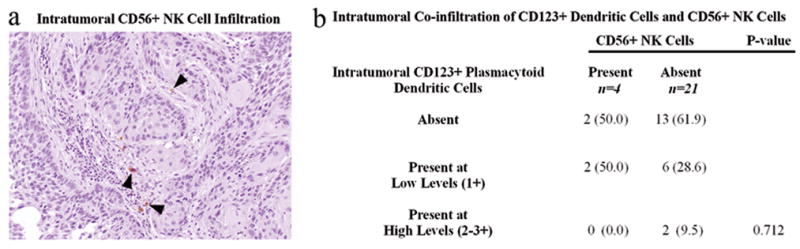
CD56+ NK cells were rare in patients with and without CD123+ plasmacytoid dendritic cell infiltration, 20x (a,b). Patients with high levels of CD123+ plasmacytoid dendritic cell infiltration did not display increased NK cell infiltration (b). ( ) indicates the column percentage.
Leukocyte Response in the Draining Lymph Nodes
The draining lymph nodes of 8 patients (4 N0, 1 N1, 2 N2b, 1 N2c) were quantitatively examined for the presence of immature epidermal Langerin+ DCs, mature DC-LAMP+ DCs, and CD123+ pDCs, with a total of 18 lymph nodes from N+ patients and 43 lymph nodes from N- patients. The extremely high numbers of DC-SIGN+ cells normally present in lymph nodes prevented a similar quantitation process for the immature dermal DCs (data not shown). We compared DC measures in uninvolved lymph nodes between the four metastatic and four nonmetastatic patients, stratified by level (a clinical measure of distance from the tumor), using a random effects model, which takes into account the multiple correlated data points within each patient. For all DC types examined, the numbers of DCs in tumor-burdened lymph nodes were comparable to the numbers in the uninvolved lymph nodes at the same level (Figure 4a-c). The numbers of mature DC-LAMP+ DCs were significantly higher in the uninvolved lymph nodes of metastatic patients as compared with nonmetastatic patients at level 2 (p<0.001) and level 3 (p=0.03) (Figure 4a). The analysis also showed a trend of decreasing numbers of DC-LAMP+ DCs with increased distance from the primary tumor (e.g. level 1 versus level 3) in both metastatic and nonmetastatic patients (Figure 4a). A similar number of Langerin+ immature epidermal DCs were noted in metastatic and nonmetastatic patients at all levels (Figure 4c). Numbers of CD123+ pDCs were lower in the uninvolved lymph nodes of metastatic patients as compared with nonmetastatic patients at all levels, but the differences were not statistically significant (Figure 4b). The large interpatient variation yet low intrapatient variation among multiple data points within the group of nonmetastatic patients (Figure 4d) likely resulted in lower power to detect group differences in the statistical model.
Figure 4. Metastatic patients display a different pattern of lymph node immune response from nonmetastatic patients, even in tumor-negative nodes.
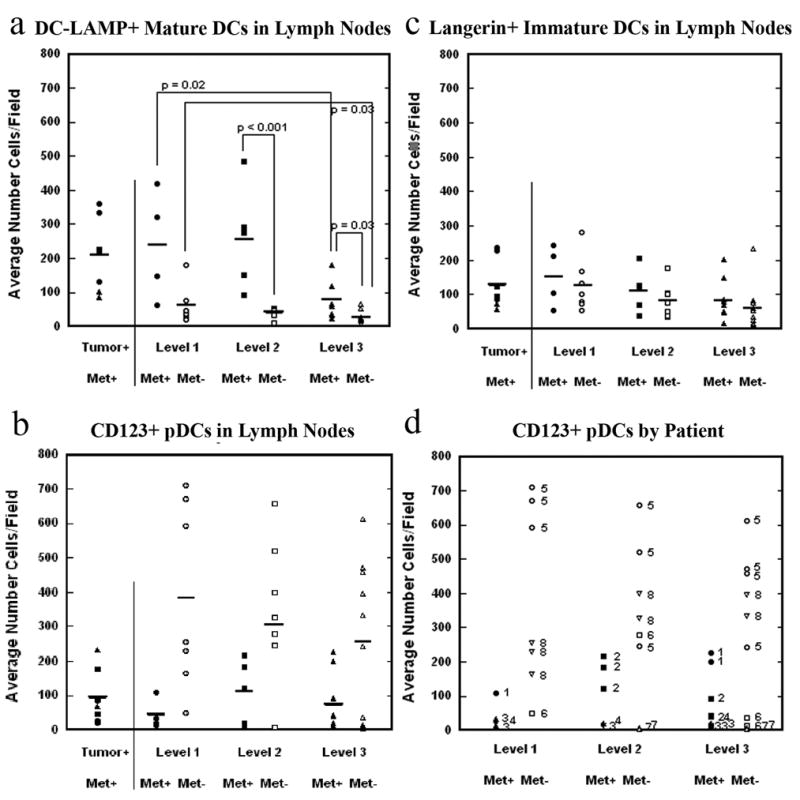
Tumor-burdened lymph nodes and uninvolved lymph nodes, stratified by metastatic status and level, were examined for the presence of DC-LAMP+ (4a), CD123+ (4b) and Langerin+ (4c) dendritic cells. Closed and open symbols represent lymph nodes in metastatic and nonmetastatic patients, respectively. For CD123, mean differences between metastatic and nonmetastatic patients were not significant, due to large interpatient variation yet low intrapatient variation in the nonmetastatic patients (4d). Lymph nodes from individual patients are labeled by patient identification number.
Discussion
Although dendritic cells are believed to be important in stimulating and regulating an antitumor immune response, the specific mechanisms of that control are not yet understood. Previous work in primary tumors, although extensive, has evaluated an incomplete set of DC populations. By examining multiple populations of DCs in a single patient cohort, we show that the dendritic cell response at the primary tumor is impaired at multiple steps.
The first step of DC response to a primary tumor involves the capture of antigens by immature DCs [2]. However, immature DCs from the DC-SIGN+ subset were generally unable to infiltrate the primary tumor area, suggesting a deficiency in antigen capture. Immature DCs from the Langerin+ subset were able to infiltrate the primary tumor, but were impaired in the second steps of maturation and migration. The low numbers of mature DC-LAMP+ DCs compared with the high numbers of Langerin+ DCs inside the primary tumor area indicates that the Langerin+ immature DCs are not maturing in situ, but neither do they appear to be migrating to the lymph nodes, as there is no accumulation of Langerin+ DCs in the lymph nodes anatomically nearest to the primary tumor. The third step of tumor immune response, presentation of antigen by mature DCs and initiation of an adaptive immune response that results in tumor regression, is also not evident in these patients. The presence of mature DC-LAMP+ DCs in the primary tumor area was not correlated with any clinical factor, indicating that any local immune response they may initiate is insufficient to cause tumor regression. There are increased numbers of DC-LAMP+ DCs in the draining lymph nodes, but this potential evidence of a vigorous response is associated with metastasis instead of with tumor regression. Lastly, the presence of plasmacytoid DCs is correlated with negative outcome. Although pDCs are known to recruit and activate NK cells that may possess antitumor abilities [14-17], NK cells were not found in the primary tumor region. Also, the lymph nodes of metastatic patients displayed a pattern of a high ratio of pDCs to DC-LAMP+ DCs, which is reversed in nonmetastatic patients.
Failure of the immune system to counteract the tumor in this patient group is clearly not due to a lack of DCs; Langerin+ DCs are present intratumorally in the vast majority of patients and immature DC-SIGN+ DCs are recruited in large numbers to the tumor region. We must conclude that effective recruitment of these populations is not sufficient for effective antitumor response. High numbers of Langerin+ cells are not correlated with better prognosis, and the DC-SIGN+ DCs which penetrate the primary tumor are ineffective in combating it, as shown by their association with poor survival.
Our work also shows that the interaction of DCs with the primary tumor is more complex than has been previously realized, as different subsets of DCs display different patterns of interaction with the primary tumor and are associated with different pathologies, such as the association between intranestal immature Langerin+ DCs and angiolymphatic vessel invasion and the association between extranestal immature DC-SIGN+ DCs and decreased survival. Our work also reveals similarities of DC phenotypes in OSCC with other types of cancer, including the presence of immature Langerin+ dendritic cell invasion into the primary tumor in breast cancer [8, 9], the exclusion of the majority of DC-SIGN+ immature dendritic cells from the tumor region in melanoma [5], the association of pDCs with poor prognosis in breast cancer [8], and the extranestal presence of smaller numbers of mature DC-LAMP+ cells in multiple human carcinoma models [3, 5, 8, 9, 18, 19].
The comprehensive nature of the failure of the dendritic cell response in these patients indicates that the current immunotherapy paradigm of supplementing a single population of DCs is unlikely to dramatically affect the overall immune response to the tumor. The recent disappointments in DC immunotherapy clinical trials should promote further research into the mechanisms of DC interactions with tumor [20]. Our work shows an ineffective dendritic cell response to tumor. This result suggests that the dysfunction of the immune response is not due to a lack of DC recruitment, but to the inability of the recruited DCs to induce an effective immune response.
Although there are multiple methods by which HNSCC may suppress the immune system (reviewed in [21]), the widespread nature of the dendritic cell suppression may most simply be explained by a change in the cytokine balance within the tumor that shifts the immune system from an effective to an ineffective response. It may be possible to restore the effective tumor response through treatment with agents that promote an antitumor response, such as IL-12 or anti-IL-10. Although it may not be possible to rescue the DCs residing within the primary tumor before treatment, the active recruitment of these cells to the tumor area may provide a new population that can respond effectively if the milieu of the tumor area can be changed and the overall balance of power shifted from the tumor to the immune system.
Supplementary Material
Semiquantitative evaluation of Langerin+ immature dendritic cells.
Semiquantitative evaluation of DC-SIGN+ immature dendritic cells.
Semiquantitative evaluation of CD123+ plasmacytoid dendritic cells.
Semiquantitative evaluation of DC-LAMP+ mature dendritic cells.
Acknowledgments
The authors thank Wendy Snyder, Clinical Research Coordinator of the Department of Otorhinolaryngology at the Hospital of the University of Pennsylvania, for her assistance in collecting patient data.
Work was supported by the University of Pennsylvania Cancer Center Support Grant CA-0165202, U54 CA1050081, and NIH R01 CA468301.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rossi M, Young JW. Human dendritic cells: potent antigen-presenting cells at the crossroads of innate and adaptive immunity. J Immunol. 2005;175:1373–1381. doi: 10.4049/jimmunol.175.3.1373. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nature Reviews. 2005;5:296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 3.Tsuge K, Takeda H, Kawada S, Maeda K, Yamakawa M. Characterization of dendritic cells in differentiated thyroid cancer. J Pathol. 2005;205:565–76. doi: 10.1002/path.1731. [DOI] [PubMed] [Google Scholar]
- 4.Bontkes H, Ruizendaal J, Kramer D, Meijer C, Hooijberg E. Plasmacytoid dendritic cells are present in cervical carcinoma and become activated by human papillomavirus type 16 virus-like particles. Gynecol Oncol. 2005;96:897–901. doi: 10.1016/j.ygyno.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 5.Vermi W, Bonecchi R, Facchetti F, Bianchi D, Sozzani S, Festa S, Berenzi A, Cella M, Colonna M. Recruitment of immature plasmacytoid dendritic cells (plasmacytoid monocytes) and myeloid dendritic cells in primary cutaneous melanomas. J Pathol. 2003;200:255–68. doi: 10.1002/path.1344. [DOI] [PubMed] [Google Scholar]
- 6.Hartmann E, Wollenberg B, Rothenfusser S, Wagner M, Wellisch D, Mack B, Giese T, Gires O, Endres S, Hartmann G. Identification and functional analysis of tumor-infiltrating plasmacytoid dendritic cells in head and neck cancer. Cancer Res. 2003;63:6478–87. [PubMed] [Google Scholar]
- 7.Salio M, Cella M, Vermi W, Facchetti F, Palmowski M, Smith CL, Shepherd D, Colonna M, Cerundolo V. Plasmacytoid dendritic cells prime IFN-gamma-secreting melanoma-specific CD8 lymphocytes and are found in primary melanoma lesions. Eur J Immunol. 2003;33:1052–62. doi: 10.1002/eji.200323676. [DOI] [PubMed] [Google Scholar]
- 8.Treilleux I, Blay J-Y, Bendriss-Vermare N, Ray-Coquard I, Bachelot T, Guastalla J-P, Bremond A, Goddard S, Pin J-J, Barthelemy-Dubois C, Lebecque S. Dendritic cell infiltration and prognosis of early stage breast cancer. Clin Cancer Res. 2004;10:7466–474. doi: 10.1158/1078-0432.CCR-04-0684. [DOI] [PubMed] [Google Scholar]
- 9.Bell D, Chomarat P, Broyles D, Netto G, Harb GM, Lebecque S, Valladeau J, Davoust J, Palucka KA, Banchereau J. In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas. J Exp Med. 1999;190:1417–26. doi: 10.1084/jem.190.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenlee R, Murray T, Bolden S, Wingo P. Cancer statistics, 2000. CA Cancer J Clin. 2000;50:7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- 11.Mork J. Forty years of monitoring head and neck cancer in Norway--no good news. Anticancer Res. 1998;18:3705–8. [PubMed] [Google Scholar]
- 12.Edwards B, Brown M, Wingo P, Howe H, Ward E, Ries L, Schrag D, Jamison P, Jemal A, Wu X, Friedman C, Harlan L, Warren J, Anderson R, Pickle L. Annual report to the nation on the status of cancer, 1975-2002, featuring population-based trends in cancer treatment. J National Cancer Institute. 2005;97:1407–27. doi: 10.1093/jnci/dji289. [DOI] [PubMed] [Google Scholar]
- 13.Diggle P, Liang K, Zeger S. Analysis of Longitudinal Data. Oxford University Press; New York: 1994. [Google Scholar]
- 14.Krug A, French AR, Barchet W, Fischer JAA, Dzionek A, Pingel JT, Orihuela MM, Akira S, Yokoyama WM, Colonna M. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 2004;21:107–119. doi: 10.1016/j.immuni.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Romagnani C, Della Chiesa M, Kohler S, Moewes B, Radbruch A, Moretta L, Moretta A, Thiel A. Activation of human NK cells by plasmacytoid dendritic cells and its modulation by CD4+ T helper cells and CD4+CD25hi T regulatory cells. Eur J Immunol. 2005;35:2452–8. doi: 10.1002/eji.200526069. [DOI] [PubMed] [Google Scholar]
- 16.Megjugorac N, Young H, Amrute S, Olshalsky S, Fitzgerald-Bocarsly P. Virally stimulated plasmacytoid dendritic cells produce chemokines and induce migration of T and NK cells. J Leukoc Biol. 2004;75:504–14. doi: 10.1189/jlb.0603291. [DOI] [PubMed] [Google Scholar]
- 17.Wallace M, Smyth MJ. The role of natural killer cells in tumor control--effectors and regulators of adaptive immunity. Springer Semin Immunopathol. 2005;27:49–64. doi: 10.1007/s00281-004-0195-x. [DOI] [PubMed] [Google Scholar]
- 18.Iwamoto M, Shinohara H, Miyamoto A, Okuzawa M, Mabuchi H, Nohara T, Gon G, Toyoda M, Tanigawa N. Prognostic value of tumor-infiltrating dendritic cells expressing CD83 in human breast carcinomas. Int J Cancer. 2003;104:92–7. doi: 10.1002/ijc.10915. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki A, Masuda A, Nagata H, Kameoka S, Kikawada Y, Yamakawa M, Kasajima T. Mature dendritic cells make clusters with T cells in the invasive margin of colorectal carcinoma. J Pathol. 2002;196:37–43. doi: 10.1002/path.1018. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberg S, Yang J, Restifo N. Cancer immunotherapy: moving beyond current vaccines. Nature Medicine. 2004;10:909–15. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young MRI. Protective mechanisms of head and neck squamous cell carcinomas from immune assault. Head & Neck. 2006;28:462–70. doi: 10.1002/hed.20331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Semiquantitative evaluation of Langerin+ immature dendritic cells.
Semiquantitative evaluation of DC-SIGN+ immature dendritic cells.
Semiquantitative evaluation of CD123+ plasmacytoid dendritic cells.
Semiquantitative evaluation of DC-LAMP+ mature dendritic cells.