Abstract
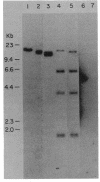
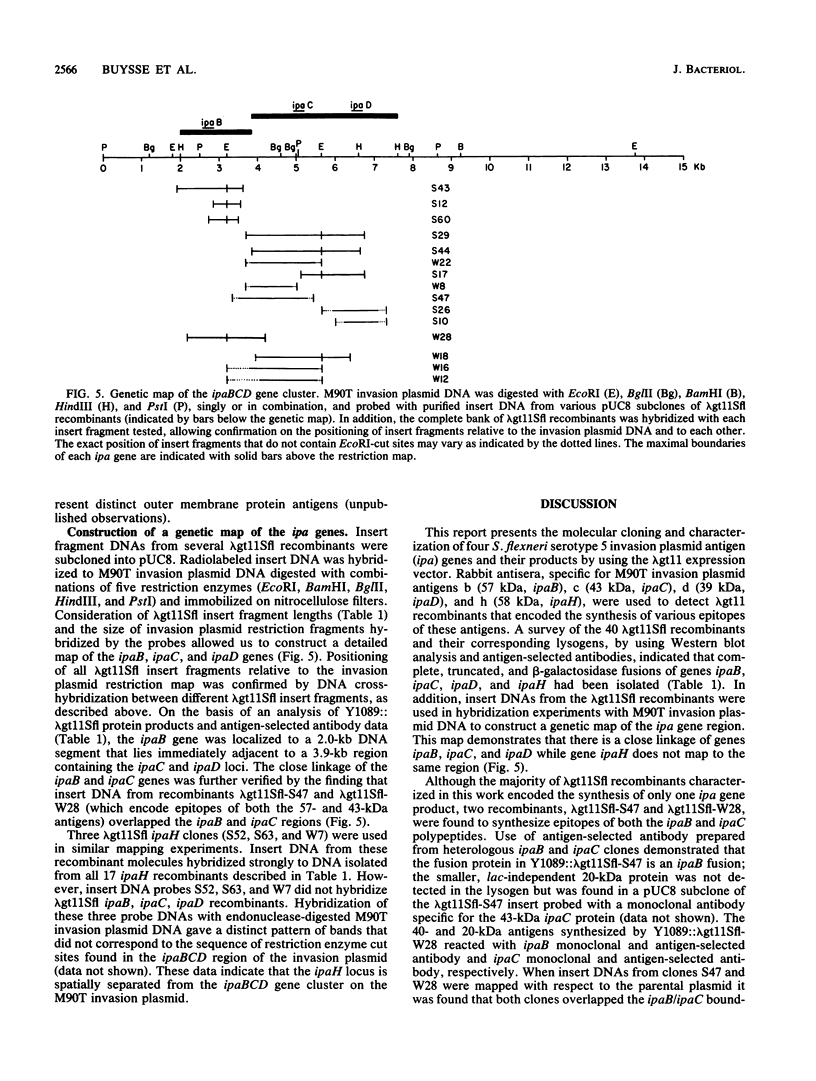
Tn5-tagged invasion plasmid DNA (pWR110) from Shigella flexneri serotype 5 (strain M90T) was cloned into the expression vector lambda gt11. Recombinant phage (lambda gt11Sfl) expressing pWR110-encoded polypeptide antigens were identified by using rabbit antisera directed against S. flexneri M90T invasion plasmid antigens. Antigens encoded by lambda gt11Sfl recombinant phage were characterized by reacting affinity-purified antibodies, eluted from nitrocellulose-bound plaques of lambda gt11Sfl recombinants, with virulent, wild-type S. flexneri M90T polypeptides in Western blot analyses. lambda gt11Sfl clones directing the synthesis of complete, truncated, and beta-galactosidase fusion versions of three previously identified outer membrane polypeptides (57-, 43-, and 39-kilodalton [kDa] antigens) were isolated. A fourth polypeptide, similar in size to the 57-kDa antigen (ca. 58 kDa) but unrelated as determined by DNA homology and serological measurements, was also identified. Southern blot analysis of S. flexneri M90T invasion plasmid DNA hybridized with lambda gt11Sfl insert DNA probes was used to construct a map of invasion plasmid antigen genes (ipa) corresponding to the 57-kDa (ipaB), 43-kDa (ipaC), and 39-kDa (ipaD) polypeptides. Genes ipaB, ipaC and ipaD mapped to contiguous 4.6-kilobase (kb) and 1.0-kb HindIII fragments contained within a larger (23-kb) BamHI fragment. The ipaH gene, which encodes the synthesis of the 58-kDa polypeptide, did not map in or near the ipaBCD gene cluster, suggesting a distinct location of ipaH on the invasion plasmid.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chirala S. S. The nucleotide sequence of the lac operon and phage junction in lambda gt11. Nucleic Acids Res. 1986 Jul 25;14(14):5935–5935. doi: 10.1093/nar/14.14.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskaleros P. A., Payne S. M. Cloning the gene for Congo red binding in Shigella flexneri. Infect Immun. 1985 Apr;48(1):165–168. doi: 10.1128/iai.48.1.165-168.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. L., Formal S. B. Protein synthesis in HeLa or Henle 407 cells infected with Shigella dysenteriae 1, Shigella flexneri 2a, or Salmonella typhimurium W118. Infect Immun. 1981 Apr;32(1):137–144. doi: 10.1128/iai.32.1.137-144.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. L., Oaks E. V., Formal S. B. Identification and antigenic characterization of virulence-associated, plasmid-coded proteins of Shigella spp. and enteroinvasive Escherichia coli. Infect Immun. 1985 Dec;50(3):620–629. doi: 10.1128/iai.50.3.620-629.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. L., Sansonetti P. J., Schad P. A., Austin S., Formal S. B. Characterization of virulence plasmids and plasmid-associated outer membrane proteins in Shigella flexneri, Shigella sonnei, and Escherichia coli. Infect Immun. 1983 Apr;40(1):340–350. doi: 10.1128/iai.40.1.340-350.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isberg R. R., Falkow S. A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by Escherichia coli K-12. Nature. 1985 Sep 19;317(6034):262–264. doi: 10.1038/317262a0. [DOI] [PubMed] [Google Scholar]
- Kopecko D. J., Baron L. S., Buysse J. Genetic determinants of virulence in Shigella and dysenteric strains of Escherichia coli: their involvement in the pathogenesis of dysentery. Curr Top Microbiol Immunol. 1985;118:71–95. doi: 10.1007/978-3-642-70586-1_5. [DOI] [PubMed] [Google Scholar]
- Kopecko D. J., Holcombe J., Formal S. B. Molecular characterization of plasmids from virulent and spontaneously occurring avirulent colonial variants of Shigella flexneri. Infect Immun. 1979 May;24(2):580–582. doi: 10.1128/iai.24.2.580-582.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon J. A., Geller R. H., Haynes J. D., Chulay J. D., Weber J. L. Epitope map and processing scheme for the 195,000-dalton surface glycoprotein of Plasmodium falciparum merozoites deduced from cloned overlapping segments of the gene. Proc Natl Acad Sci U S A. 1986 May;83(9):2989–2993. doi: 10.1073/pnas.83.9.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurelli A. T., Baudry B., d'Hauteville H., Hale T. L., Sansonetti P. J. Cloning of plasmid DNA sequences involved in invasion of HeLa cells by Shigella flexneri. Infect Immun. 1985 Jul;49(1):164–171. doi: 10.1128/iai.49.1.164-171.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurelli A. T., Blackmon B., Curtiss R., 3rd Temperature-dependent expression of virulence genes in Shigella species. Infect Immun. 1984 Jan;43(1):195–201. doi: 10.1128/iai.43.1.195-201.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Nyman K., Nakamura K., Ohtsubo H., Ohtsubo E. Distribution of the insertion sequence IS1 in gram-negative bacteria. Nature. 1981 Feb 12;289(5798):609–612. doi: 10.1038/289609a0. [DOI] [PubMed] [Google Scholar]
- Oaks E. V., Hale T. L., Formal S. B. Serum immune response to Shigella protein antigens in rhesus monkeys and humans infected with Shigella spp. Infect Immun. 1986 Jul;53(1):57–63. doi: 10.1128/iai.53.1.57-63.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsubo H., Nyman K., Doroszkiewicz W., Ohtsubo E. Multiple copies of iso-insertion sequences of IS1 in Shigella dysenteriae chromosome. Nature. 1981 Aug 13;292(5824):640–643. doi: 10.1038/292640a0. [DOI] [PubMed] [Google Scholar]
- Ohtsubo H., Ohtsubo E. Nucleotide sequence of an insertion element, IS1. Proc Natl Acad Sci U S A. 1978 Feb;75(2):615–619. doi: 10.1073/pnas.75.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson B. C., Hashimoto H., Rownd R. H. Cointegrate formation between homologous plasmids in Escherichia coli. J Bacteriol. 1982 Sep;151(3):1086–1094. doi: 10.1128/jb.151.3.1086-1094.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SERENY B. Experimental shigella keratoconjunctivitis; a preliminary report. Acta Microbiol Acad Sci Hung. 1955;2(3):293–296. [PubMed] [Google Scholar]
- Sansonetti P. J., Hale T. L., Dammin G. J., Kapfer C., Collins H. H., Jr, Formal S. B. Alterations in the pathogenicity of Escherichia coli K-12 after transfer of plasmid and chromosomal genes from Shigella flexneri. Infect Immun. 1983 Mar;39(3):1392–1402. doi: 10.1128/iai.39.3.1392-1402.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., Kopecko D. J., Formal S. B. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect Immun. 1982 Mar;35(3):852–860. doi: 10.1128/iai.35.3.852-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., Kopecko D. J., Formal S. B. Shigella sonnei plasmids: evidence that a large plasmid is necessary for virulence. Infect Immun. 1981 Oct;34(1):75–83. doi: 10.1128/iai.34.1.75-83.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasakawa C., Kamata K., Sakai T., Murayama S. Y., Makino S., Yoshikawa M. Molecular alteration of the 140-megadalton plasmid associated with loss of virulence and Congo red binding activity in Shigella flexneri. Infect Immun. 1986 Feb;51(2):470–475. doi: 10.1128/iai.51.2.470-475.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasakawa C., Makino S., Kamata K., Yoshikawa M. Isolation, characterization, and mapping of Tn5 insertions into the 140-megadalton invasion plasmid defective in the mouse Sereny test in Shigella flexneri 2a. Infect Immun. 1986 Oct;54(1):32–36. doi: 10.1128/iai.54.1.32-36.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M., Elledge S., Davis R. W. Rapid mapping of antigenic coding regions and constructing insertion mutations in yeast genes by mini-Tn10 "transplason" mutagenesis. Proc Natl Acad Sci U S A. 1986 Feb;83(3):730–734. doi: 10.1073/pnas.83.3.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H., Nakamura A. Identification of Shigella sonnei form I plasmid genes necessary for cell invasion and their conservation among Shigella species and enteroinvasive Escherichia coli. Infect Immun. 1986 Aug;53(2):352–358. doi: 10.1128/iai.53.2.352-358.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yother J., Chamness T. W., Goguen J. D. Temperature-controlled plasmid regulon associated with low calcium response in Yersinia pestis. J Bacteriol. 1986 Feb;165(2):443–447. doi: 10.1128/jb.165.2.443-447.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]