Abstract
INTRODUCTION
In order to tackle increasing waiting lists the UK Government's ‘two-week rule’ was introduced for a number of cancers, including melanoma, in 2000. Whilst there is evidence that secondary prevention (i.e. early diagnosis) improves patient outcome, particularly in melanoma where early surgical excision is the only intervention to improve survival, there is as yet no evidence base for a 2-week limit. Any survival benefit from this Government target will not be demonstrable until long-term follow-up is available, realistically 10-year mortality figures in 2010.
PATIENTS AND METHODS
To investigate an evidence base for the two-week rule in melanoma, we performed a retrospective study on patients with suspected skin cancers referred to a rapid access Pigmented Lesion Clinic (PLC) over a 4-year period with long-term survival data, and compared them to a historical control group.
RESULTS
A total of 4399 patients attended the PLC from January 1993 to December 1996 and all were seen within 2 weeks. Ninety-six melanomas were diagnosed during this period with 96% treated within 2 weeks of GP referral, the majority (74%) excised on the day of PLC attendance. Melanoma patients (n = 78) diagnosed in the 2 years prior to the inception of the PLC waited 3–34 days for consultation and 4–74 days for treatment. Melanoma patients diagnosed in the PLC had significantly thinner tumours (Mann Whitney test, P < 0.001) and improved overall survival (χ2 18.1924; P < 0.001) compared with melanoma patients diagnosed before the inception of the clinic.
CONCLUSIONS
This is, to our knowledge, the first example that consultation within a 2-week time-frame of GP referral impacts patient survival and the first evidence base behind Government guidelines for this particular cancer.
Keywords: Two-week rule, Melanoma, Pigmented lesion clinic
The relatively poor mortality rates for certain cancers in the UK in comparison to other European countries has been attributed, in part, to cancer patients in this country having more advanced disease by the time they receive treatment.1,2 In 1999, the Government White Paper entitled The new NHS – Modern, Dependable was drawn up in an effort to counter increasing NHS waiting lists and minimise delays between presentation, diagnosis and treatment. The document pledged that every patient with a suspected cancer would be able to see a specialist within 2 weeks of referral by their GP. It was introduced for carcinomas of the breast in April 1999 and extended to a range of other cancers, including melanoma in October 2000. Risk factors and referral guidelines for suspected cancers were drawn up by Working Parties appointed by the Department of Health for each individual cancer type and widely distributed to GPs and hospital trusts. Concern was expressed at the time over the potentially large increase in total referral ratios from primary to secondary care, as was seen following introduction of the ‘two-week rule’ for breast cancer, and the overall capacities of hospitals to respond, with a potential prolongation of waiting times for cancers mistakenly referred as ‘non-urgent’. There has also been subsequent attention drawn to the fact these guidelines do not impact delays from first consultation with a hospital specialist to time of definitive treatment.
The incidence of melanoma continues to rise at an alarming rate amongst white populations3,4 thought to be due to increased sun exposure due, in part, to the proliferation of package holidays and cheaper travel to sunnier destinations.4 Melanoma has a good prognosis if excised quickly but survival rates fall sharply once it has spread to the regional lymph nodes.5 The only consistently effective strategy that has been shown to improve survival is early detection, i.e. secondary prevention.5 An early detection melanoma clinic was established with this in mind in the Department of Plastic Surgery at Mount Vernon Hospital in 1993. This aimed to minimise any delays inherent in the referral of patients to a specialist consultation, which has been shown to influence disease outcome.6
The majority of decisions in modern medicine are, or at least should be, evidence based. However, there has been, to our knowledge, no evidence indicating that the ‘two-week rule’ for cancer influences survival. In colorectal cancer, there was no evidence to suggest that a delay to diagnosis measurable in weeks made a significance difference to either pathological stage at operation or patient outcome,7 though delays of 3–6 months in breast cancer have been correlated with reduced survival.8 It will not be known whether the ‘two-week rule’ implemented in 2000 has had an impact on survival in various cancers until long-term fol-low-up (ideally 10-year mortality figures) becomes available. Previously, we have published the results of an audit indicating that implementation of a PLC has a positive impact in terms of Breslow thickness of melanomas diagnosed and disease recurrences rates.9,10 In order to apply an evidence base to the Government's ‘two-week rule’ in melanoma, we looked at the waiting times and outcome of patients attending a rapid-access Pigmented Lesion Clinic at Mount Vernon Hospital, over a 4-year period with long-term follow-up and compared it to patients with suspected skin cancers referred prior to the inception of the clinic.
Patients and Methods
A weekly PLC was started in January 1993 at Mount Vernon Hospital. Consultants in plastic surgery as well as 2–3 plastic surgery skin cancer research fellows staffed the clinic. It was run such that GPs with a reasonable suspicion of the presence of a melanoma or non-melanoma skin cancer might refer patients. It operated on a ‘walk-in’ basis, with no appointments necessary so that patients could turn up to the clinic with their GP's letter of referral. After consultation, patients with suspected skin cancers were offered immediate excision under local anaesthesia in an operating theatre that ran in parallel with the clinic. Lesions that were not clinically skin cancers but merited excision, were given appropriate priority on the hospital waiting list. Patients with benign lesions were reassured and discharged. All patients seen had a standard proforma completed, which allowed immediate feedback to the patient's GP regarding the outcome of the consultation. If surgical excision was performed, patients were seen in the Plastic Dressings Clinic 1–2 weeks later for suture removal and histology results, a copy of which was forwarded to the GP within 7 working days.
An audit of all melanoma patients attending the regional melanoma centre, serving both a rural and urban population of 1.6 million, based at Mount Vernon Hospital, from January 1991 to December 1996 was performed. Before the establishment of the PLC, referral of such lesions was made by letter from the GP to the out-patient department of plastic surgery. All melanomas diagnosed by the pathology services at Mount Vernon Hospital over the study period were sought on the hospital pathology department computer and from hand-written records and relevant case notes retrieved. A proportion of patient records were incomplete. Further details were initially obtained via the Mount Vernon Hospital Cancer Registry. Letters were sent to GPs involved in the patients' care, including a questionnaire about the missing details from the patients' notes and a stamped, self-addressed envelope, to ensure a maximum response rate. After 5 weeks, those GPs who had not replied were contacted via the telephone.
A database was created using Microsoft Access. Patients' demographic details were recorded as well as time from GP referral to PLC consultation, and for melanoma patients, time from PLC visit to treatment. A historical (1991/1992) population of melanoma patients (n = 78) referred to plastic surgery out-patients prior to the inception of the PLC were used for comparison. Patient follow-up was between 6–11 years. Standard pathological parameters on each melanoma diagnosed were recorded such as: Breslow depth, Clark's level, site of primary melanoma, subtype of melanoma, information on metastases (both local and regional) and disease-free intervals.
Kaplan-Meier univariate survival curves were plotted from the database and analysed using the statistical package, JMP©. Log Rank tests were performed on the data, which was then analysed using Chi-squared tests to obtain P-values. The level of statistical significance accepted was P < 0.05. Graphs were constructed using Origin© and SPSS©.
Results
The waiting times from GP referral to specialist consultation, and (where relevant) treatment, of a rapid access PLC were compared to that of melanoma patients previously referred to plastic surgery out-patients. Tumour thickness and survival of patients with melanomas diagnosed via the two routes were compared. A total of 4399 patients attended the PLC over the study period during with an average attendance of 28.2 patients per clinic. This compared with 756 patients with skin lesions referred to out-patients via traditional routes in 1991/1992, of whom 88 were tertiary referrals. Of patients in the study, 96% were still with the same GP practice up to 10 years after initial treatment at the hospital, suggesting a stable population on which the study was performed, within an identical catchment area. Twenty-one case notes were incomplete and these patients' details were completed after letters had been sent, or telephone calls made, to the relevant GPs.
For all patients referred to the rapid access clinic (n = 4399), analysis of delay between GP referral and PLC visit revealed a range of 0–14 days (i.e. some patients had first seen their GP on the morning of the clinic). Of the 96 melanomas diagnosed in the PLC, 96% had excision biopsy within 2 weeks of their GP referral, with the majority (76%) having their surgery on the day of PLC attendance. For melanoma patients not seen in the PLC, the waiting time from GP referral to consultation ranged from 3–37 days and from GP referral to treatment 4–74 days (mean, 22.4 days).
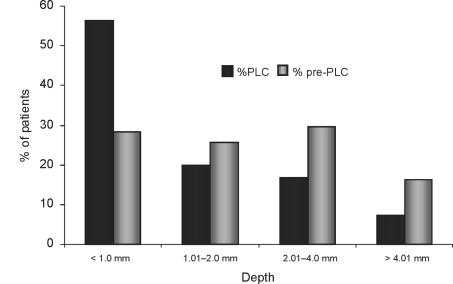
A total of 96 melanomas were diagnosed in the PLC over a 4-year period accounting for 2.1% of all referrals whilst 78 melanomas were diagnosed over a 2-year period from 1991/1992 via plastic surgery out-patients. The clinicopathological variables of the two patient groups are shown in Table 1. Melanomas diagnosed in the PLC within the 2week frame were significantly thinner than those diagnosed in the control group (Mann-Whitney test, P < 0.001), with mean tumour thicknesses of 1.68 mm and 2.39 mm, respectively. Figure 1 shows the difference in the Breslow thickness of melanomas diagnosed and excised within the PLC time-frame when compared with control group. There was no significant variation in tumour thickness between different years of the PLC. The mean thickness of nodular melanomas presenting was significantly higher than for any of the other subtypes (P < 0.0001). In addition to melanoma diagnosis, 748 non-melanoma skin cancers were treated via the PLC making a 19.2% rate of malignancy seen at the clinic.
Table 1.
Details of patients and melanomas diagnosed
| Variable | 1993–6 | 1991–2 |
|---|---|---|
| Number of patients in cohort | 96 | 78 |
| Mean age at diagnosis (years) (range, years) | 54.47 (21–96) | 58.26 (18–93) |
| M:F | 1.5:1 | 1.5:1 |
| Site of melanoma (%) | ||
| Head & neck | 17 (18%) | 13 (17%) |
| Trunk | 29 (31%) | 17 (22%) |
| Upper limb | 21 (23 %) | 11 (14%) |
| Lower limb | 29 (31%) | 37 (47%) |
| Tumour subtypes represented (%) | ||
| Superficial spreading | 64 (67%) | 45 (58%) |
| Lentigo maligna melanoma | 9 (9%) | 4 (5%) |
| Nodular melanoma | 22 (23%) | 28 (36%) |
| Acral lentiginous | 1 (1%) | 1 (1%) |
| Mean tumour thickness (mm) (range, years) | 1.68 (0.1–18) | 2.39 (0.1–10) |
| Median tumour thickness (mm) | 0.90 | 1.80 |
| Waiting time | ||
| GP referral-consultation (days) | 0–14 | 3–34 |
| GP referral-treatment (days) | 0–15 | 4–74 |
| 5-year survival | 79 (82%) | 48 (62%) |
Figure 1.
Results demonstrating differences in Breslow depth between patients seen within 2 weeks in the PLC and the control ‘pre-PLC’ group.
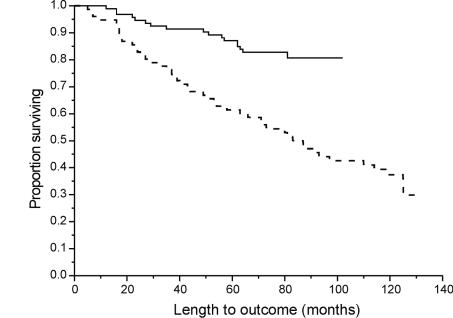
Of the 96 melanoma patients diagnosed via the PLC, 74 were alive at the end-point of the study (77%) with a 5-year survival rate of 82%. This compared with 62% of the control group alive after 5 years. Kaplan-Meier survival curves demonstrated a significantly improved survival for patients seen within 2 weeks (PLC group) compared with the control group (Log rank χ2 18.1924; P < 0.001; Fig. 2). Breslow thickness (P = 0.0005) and ulceration (P = 0.02) both strongly correlated with patient outcome, with Breslow the single most powerful predictor of overall survival, irrespective of other clinicopathological variables. Neither age nor gender had a significance impact on survival.
Figure 2.
Survival curves comparing PLC (1993–1996) patients with pre-PLC (1991–1992) patients. Log rank χ2 18.1924, P < 0.001. PLC patients, solid line; pre-PLC patients, dashed line.
Discussion
The Government's ‘two-week rule’ for cancer referral has raised a number of key questions and concerns for both GPs and hospital specialists alike. Hospitals are under pressure to see the growing number of suspected cancer referrals from primary care within the 2-week time-frame, with resultant delays for routine referrals,11 which invariably contain a number of cancers unsuspected by the GP. Of note, in a retrospective study of colorectal cancer patients seen by a single surgeon, 30% would not have met the current referral criteria.12 Moreover, it has been pointed out that a specialist consultation within 2 weeks is of little benefit if there is no a concomitant reduction in delay to definitive treatment.13 Up to now, whilst in several cancers it has been shown that delays in treatment result in reduced survival,8,14 there has been no evidence base behind selection of 2 weeks as the limit for seeing suspected cancer cases, nor indeed that delays measurable in weeks rather than months affect prognosis.7
In order to apply an evidence base to the Government's ‘two-week rule’, we performed a retrospective study on patients attending a rapid access Pigmented Lesion Clinic for suspected skin cancers. A 4-year period from 1993–1996 was chosen, in order to have long-term follow-up and survival data available and compared to a historical control of melanoma patients, referred to out-patients before the inception of the clinic. The PLC was run on a ‘no-appointment’ basis with no limit on the number of patients seen in a clinic. No formal time-limit was imposed by which patients had to attend, though patients generally attended on the first available Friday following their GP consultation and all had attended within 2 weeks. The waiting time between GP referral and specialist consultation prior to the PLC was up to 5 weeks and up to 11 weeks between referral and definitive treatment. A significant difference in melanoma thickness and improved patient outcome were observed in the patients seen within 2 weeks compared with the historical control. There was no significant difference in tumour thickness or survival between years of the PLC, suggesting that this improvement followed the institution of the rapid access clinic and was not part of a general trend for melanoma patients in the region.
The single most important variable in melanoma prognosis remains maximum tumour thickness,5,15 a finding reflected in this study. Efforts focused on the early detection of melanoma are based on the assumption that early melanomas will be thinner and so have less metastatic potential and thus a better prognosis.9,10,16
Melanoma would appear to be a cancer most likely to benefit from the ‘two-week rule’ in its current form as the diagnosis is largely clinical and there are no lengthy waiting lists for investigative procedures (such as colonoscopies, ultrasonography or CT scans) to delay treatment. The definitive diagnostic test, namely an excision biopsy, is also the first stage of treatment, and these, along with the subsequent wide local excision, are generally fairly quick, straight-forward procedures that can be normally performed under local anaesthesia. Thus, in melanoma, delays between specialist consultation and definitive treatment are usually small when compared to, for example, a patient with a colorectal carcinoma awaiting a laparotomy. In this study, 96% of melanomas diagnosed in the PLC were excised within 2 weeks of GP referral with the majority (76%) removed immediately (i.e. on the day of PLC attendance).
The study also highlights some drawbacks of the ‘two-week rule’. Between January 1993 and December 1996, 4399 lesions were referred to the PLC as suspected skin cancers. Even setting aside the dramatic increase in melanoma incidence in the past decade, under the current Government guidelines this would have equated to over 28 extra patients every week that would have had to be seen within 2 weeks, a huge additional workload on plastic surgery out-patient clinics. This is made all the more problematic when considering that the significant seasonal variation in referral patterns, with the greatest number (range, 50–80) of patients referred in the summer months.9 Of course, it is possible that the ease of referral and rapidity with which patients were seen in the PLC contributed to the increase in numbers referred. Between 1991 and 1992, there were 756 skin lesions referred to plastic surgery out-patients compared with 4399 seen in a 4-year period of running the PLC, representing an approximate 3-fold increase in the average annual number of patients referred. There has been evidence from dermatology clinics that a reduction in waiting times has led to an increase in demand.17 However, all PLC patients required a GP letter referring them as having a suspected skin cancer, and thus one must assume that these patients would have been referred to urgent out-patients as suspected skin cancers had the PLC not existed. It was noted during the study that the PLC received a number of new GP referrals which may have previously gone to other hospitals or specialities (e.g. dermatology). The clinical diagnosis of melanoma can be difficult18,19 as unlike many cancers it occurs across the age range and the recommended guidelines (shown in Table 2), based on signs and symptoms established in the literature, result in a large number of benign lesions being referred. In this series, melanomas made up 2.1% of diagnoses made via the PLC (and non-melanoma skin cancers 19.2%), which is similar to other published series on PLCs.16,20–24
Table 2.
Referral guidelines for urgent referral of suspected melanoma
Pigmented lesions on any part of the body which one or more of the following features
|
The increase in workload coming through a rapid access, no-appointment clinic obviously has implications for a department's working practice. The PLC in this study was staffed by one plastic surgery consultant and three surgical research fellows, the latter being in full-time research and thus not part of the day-to-day clinical rota. We acknowledge that not all skin cancer units have this luxury and to implement a model such as this may, for some units, present certain practical difficulties. However, with or without a rapid access PLC, it is inevitable that numbers of suspected skin cancers or ‘suspicious’ lesions referred from primary care will continue to increase through a combination of rising melanoma incidence, heightened public awareness of skin cancer and the recommended low threshold for GPs to refer patients. The ‘two-week rule’ means hospital departments will have to make some provision in their out-patient services to deal with this rising case-load.
This model of a PLC is a means by which a hospital can meet this increased workload and see all patients that meet referral criteria within the 2-week time-frame. Additionally, the ‘no-appointment’ nature of the clinic enables those cases where the GP has a relatively low index of suspicion also to be seen expeditiously. There is good evidence that cancer specialists are best qualified to assess the urgency of these suspected cancers rather than primary care physicians in melanoma25,26 and in other cancers.8 Ibbotson et al.26 found that those patients whose GPs suspected melanoma were seen within a median of 13 days and the melanomas had an average thickness of 0.9 mm, compared to those in which the GPs did not suspect melanoma, the median time to be seen was 69 days and the mean thickness was 3 mm. The ‘two-week rule’ will evidently not impact the time between patient concern to the time they contact their GP; however, MacKie et al.27 found a significant reduction in patient delay (i.e. time from patient first noticing a suspicious skin lesion to seeing their GP) over a 15-year period from 1986 to 2001, but not a concomitant reduction in time from GP referral to specialist consultation and diagnostic biopsy.
Despite the existence of the PLC, some GPs referred suspected cases to normal out-patients, accounting for the fact 96 melanomas were diagnosed in the PLC over a 4-year period compared with 78 in the preceding 2 years. Of the 96 melanoma patients that were diagnosed, the female to male ratio was 1.5:1, which compares with other data on sex distribution of melanoma.28–30 The site distribution of melanomas was predominantly the legs in women and the trunk in men. This pattern followed that established in the literature and corresponds to the sites most often exposed to the sun in each sex.31–35 The variation in subtype of melanoma was similar to that described elsewhere, with a predominance of superficial spreading melanomas.31,36 Of note, the mean Breslow thickness of nodular melanomas diagnosed in the PLC was significantly greater than both superficial spreading and lentigo maligna melanoma subtypes (P < 0.0001). The explanation probably lies in differences in tumour biology between melanomas. Ideally, a melanoma is detected clinically (and thus excised) in the radial growth phase where there is minimal metastatic potential. Nodular melanomas, however, are notable histologically by a lack of lateral epidermal or junctional spread, i.e. they appear to lack a radial growth phase, and thus are invariably in the vertical growth phase by the time they become evident to the patient or GP. Acral lentiginous melanoma also has a tendency to present late, though relatively few of this subtype were seen in the study.
We have previously demonstrated a significant reduction in average thickness of melanomas diagnosed following the introduction in the PLC at Mount Vernon Hospital.9 A similar effect was seen in Scotland following a publicity campaign raising awareness on melanoma and subsequent PLC referral,16,28 though not following PLC introduction in some other regions.22,24,37,38 The Cancer Research Campaign funded a health education programme and rapid access clinics for the early detection of cutaneous malignant melanoma in seven health authorities in England and Scotland from 1987–1989. This did not demonstrate an improvement in mortality though only 85% of patients were seen within 4 weeks of referral.39
Conclusions
Increasing melanoma incidence, Government referral guidelines and a public increasingly aware of the dangers of sun exposure mean the number of patients referred to the hospital specialists with suspected skin cancers will continue to rise. This study demonstrates that the ‘two-week rule’ has an evidence base in melanoma in terms of reduced tumour thickness and improved survival, though naturally early specialist consultation must be accompanied by prompt surgical treatment. A rapid access, no-appointment Pigmented Lesion Clinic allows hospital specialists to see a large number of referred patients within the 2-week target, offer prompt surgical treatment to those patients that warrant it and provide GPs with a diagnostic service for all lesions that concern them.
Acknowledgments
The authors wish to thank The Restoration of Appearance and Function Trust (RAFT) and The Royal College of Surgeons of England for generously supporting this work. The authors have no commercial interest in the outcome of this study.
References
- 1.Survival of cancer patients in Europe: The EOROCARE-2 study. Lyon: IARC Scientific Publications; 1999. pp. 1–572. [PubMed] [Google Scholar]
- 2.Jones R, Rubin G, Hungin P. Is the two week rule for cancer referrals working? BMJ. 2001;323:24–8. doi: 10.1136/bmj.322.7302.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Severi G, Giles GG, Robertson C, Boyle P, Autier P. Mortality from cutaneous melanoma: evidence for contrasting trends between populations. Br J Cancer. 2000;82:1887–9. doi: 10.1054/bjoc.1999.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacKie RM, Bray CA, Hole DJ, Morris A, Nicolson M, Evans A, et al. Incidence of and survival from malignant melanoma in Scotland: an epidemiological study. Lancet. 2002;360:587–91. doi: 10.1016/S0140-6736(02)09779-9. [DOI] [PubMed] [Google Scholar]
- 5.Balch CM, Soong S-J, Gershenwald JE, Thompson JF, Reintgen DS, Cascinelli N, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19:3622–34. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 6.Temoshok LR, DiClemente J, Sweet DM, Blois MS, Sagebiel RW. Factors related to patient delay in seeking medical attention for cutaneous malignant melanoma. Cancer. 1984;54:3048–53. doi: 10.1002/1097-0142(19841215)54:12<3048::aid-cncr2820541239>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 7.Cancer Guidelines Sub-Group of the COG. Guidance on commissioning cancer services: improving outcomes in colorectal cancer: the research evidence. Leeds: NHS Executive; p. 1997. [Google Scholar]
- 8.Rosjhan Lall C, Leinster S, Mithell S, Holcombe C. Current patterns of referral in breast disease. Breast. 2000;9:334–7. doi: 10.1054/brst.1999.0151. [DOI] [PubMed] [Google Scholar]
- 9.Grover R, Ross DA, McKelvie M, Morgan BD. Improving the early detection of malignant melanoma. Ann R Coll Surg Engl. 1996;78:176–9. [PMC free article] [PubMed] [Google Scholar]
- 10.Pacifico MD, Grover R, Sanders R. Use of an early detection strategy to improve disease control in melanoma patients. Br J Plast Surg. 2004;57:105–11. doi: 10.1016/j.bjps.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 11.Moreea S, Green J, MacFie J, Mitchell CJ. Impact of the two week waiting time standard on the gastroenterology service of a district general hospital. Gut. 2001;48(Suppl):A3. [Google Scholar]
- 12.Soo FY, Winterton R, Plusa SM. Impact of the ‘2 week rule’ on the treatment of colorectal cancer. Gut. 2001;48(Suppl):A53. [Google Scholar]
- 13.Oliver MD. All stages of care pathway need speeding up. BMJ. 2001;323:864. [PubMed] [Google Scholar]
- 14.Richards MA, Westcombe AM, Love SB, Littlejohns P, Ramirez AJ. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet. 1999;353:1119–26. doi: 10.1016/s0140-6736(99)02143-1. [DOI] [PubMed] [Google Scholar]
- 15.Breslow A. Thickness, cross-sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg. 1970;172:902–8. doi: 10.1097/00000658-197011000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herd RM, Cooper EJ, Hunter JA, McLaren K, Chetty U, Watson AC, et al. Cutaneous malignant melanoma. Publicity, screening clinics and survival – the Edinburgh experience 1982–90. Br J Dermatol. 1995;132:563–70. doi: 10.1111/j.1365-2133.1995.tb08712.x. [DOI] [PubMed] [Google Scholar]
- 17.Smethurst DP, Williams HC. Are hospital waiting lists self-regulating? Nature. 2001;410:652–3. doi: 10.1038/35070647. [DOI] [PubMed] [Google Scholar]
- 18.MacKenzie-Wood AR, Milton GW, De Launey JW. Melanoma: accuracy of clinical diagnosis. Aust J Dermatol. 1998;39:31–3. doi: 10.1111/j.1440-0960.1998.tb01238.x. [DOI] [PubMed] [Google Scholar]
- 19.Morton CA, MacKie RM. Clinical accuracy of the diagnosis of cutaneous malignant melanoma. Br J Dermatol. 1998;138:283–7. doi: 10.1046/j.1365-2133.1998.02075.x. [DOI] [PubMed] [Google Scholar]
- 20.Carli P, De Giorgi V, Nardini P, Mannone F, Palli D, Giannotti B. Melanoma detection rate and concordance between self-skin examination and clinical evaluation in patients attending a pigmented lesion clinic in Italy. Br J Dermatol. 2002;146:261–6. doi: 10.1046/j.1365-2133.2002.04580.x. [DOI] [PubMed] [Google Scholar]
- 21.Graham-Brown RA, Osborne JE, London SP, Fletcher A, Shaw D, Williams B, et al. The initial effects on workload and outcome of a public education campaign on early diagnosis and treatment of malignant melanoma in Leicestershire. Br J Dermatol. 1990;122:53–9. doi: 10.1111/j.1365-2133.1990.tb08239.x. [DOI] [PubMed] [Google Scholar]
- 22.Bataille V, Sasieni P, Curley RK, Cook MG, Marsden RA. Melanoma yield, number of biopsies and missed melanomas in a British teaching hospital pigmented lesion clinic: a 9-year retrospective study. Br J Dermatol. 1999;140:243–8. doi: 10.1046/j.1365-2133.1999.02656.x. [DOI] [PubMed] [Google Scholar]
- 23.O'Connor WJ, Barnes L. Melanoma – a five year audit. Irish Med J. 1999;87:138–9. [PubMed] [Google Scholar]
- 24.Duff CG, Melsom D, Rigby HS, Kenealy JM, Townsend PL. A 6 year prospective analysis of the diagnosis of malignant melanoma in a pigmented-lesion clinic: even the experts miss malignant melanomas, but not often. Br J Plast Surg. 2001;54:317–21. doi: 10.1054/bjps.2000.3561. [DOI] [PubMed] [Google Scholar]
- 25.Paine SL, Cockburn J, Noy SM, Marks R. Early detection of skin cancer. knowledge, perceptions and practices of general practitioners in Victoria. Med J Aust. 1994;161:188–9. doi: 10.5694/j.1326-5377.1994.tb127380.x. [DOI] [PubMed] [Google Scholar]
- 26.Ibbotson SH, Dahl MG, Farr PM. Do we need pigmented lesion clinics? Lancet. 1995;346:1373. doi: 10.1016/s0140-6736(95)92392-6. [DOI] [PubMed] [Google Scholar]
- 27.MacKie RM, Bray CA, Leman JA. Effect of public education aimed at early diagnosis of malignant melanoma: cohort comparison study. BMJ. 2003;326:367. doi: 10.1136/bmj.326.7385.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacKie RM, Hole D. Audit of public education campaign to encourage earlier detection of malignant melanoma. BMJ. 1992;304:1012–5. doi: 10.1136/bmj.304.6833.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacKie RM, Hunter JA, Aitchison TC, Hole D, McLaren K, Rankin R, et al. Cutaneous malignant melanoma, Scotland, 1979–89. The Scottish Melanoma Group. Lancet. 1992;339:971–5. doi: 10.1016/0140-6736(92)91539-k. [DOI] [PubMed] [Google Scholar]
- 30.de Rooij MJ, Rampen FH, Schouten LJ, Neumann HA. Skin cancer screening focusing on melanoma yields more selective attendance. Arch Dermatol. 1995;131:422–5. [PubMed] [Google Scholar]
- 31.Mihm MC, Jr, Clark WH, Jr, Reed RJ. The clinical diagnosis of malignant melanoma. Semin Oncol. 1975;2:105–18. [PubMed] [Google Scholar]
- 32.Koh HK. Cutaneous melanoma. N Engl J Med. 1991;325:171–82. doi: 10.1056/NEJM199107183250306. [DOI] [PubMed] [Google Scholar]
- 33.MacKie RM. Incidence, risk factors and prevention of melanoma. Eur J Cancer. 1998;34(Suppl 3):S3–6. doi: 10.1016/s0959-8049(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 34.Langley RG, Sober AJ. Clinical recognition of melanoma and its precursors. Hematol Oncol Clin North Am. 1998;12:699–715. doi: 10.1016/s0889-8588(05)70019-8. [DOI] [PubMed] [Google Scholar]
- 35.Slominski A, Wortsman J, Carlson AJ, Matsuoka LY, Balch CM, Mihm MC. Malignant melanoma. Arch Pathol Lab Med. 2001;125:1295–306. doi: 10.5858/2001-125-1295-MM. [DOI] [PubMed] [Google Scholar]
- 36.McGovern VJ, Mihm MC, Jr, Bailly C, Booth JC, Clark WH, Jr, Cochran AJ, et al. The classification of malignant melanoma and its histologic reporting. Cancer. 1973;32:1446–57. doi: 10.1002/1097-0142(197312)32:6<1446::aid-cncr2820320623>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 37.Mallett RB, Fallowfield ME, Cook MG, Landells WN, Holden CA, Marsden RA. Are pigmented lesion clinics worthwhile? Br J Dermatol. 1993;129:689–93. doi: 10.1111/j.1365-2133.1993.tb03332.x. [DOI] [PubMed] [Google Scholar]
- 38.Southampton Melanoma Group. Effects of rapid referral on thickness of melanomas. BMJ. 1986;293:270. doi: 10.1136/bmj.293.6550.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Melia J, Moss S, Coleman D, Frost T, Graham-Brown R, Hunter JA, et al. The relation between mortality from malignant melanoma and early detection in the Cancer Research Campaign Mole Watcher Study. Br J Cancer. 2001;85:803–7. doi: 10.1054/bjoc.2001.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]