Abstract
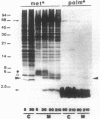
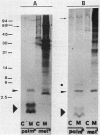
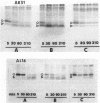
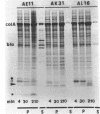
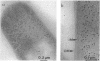
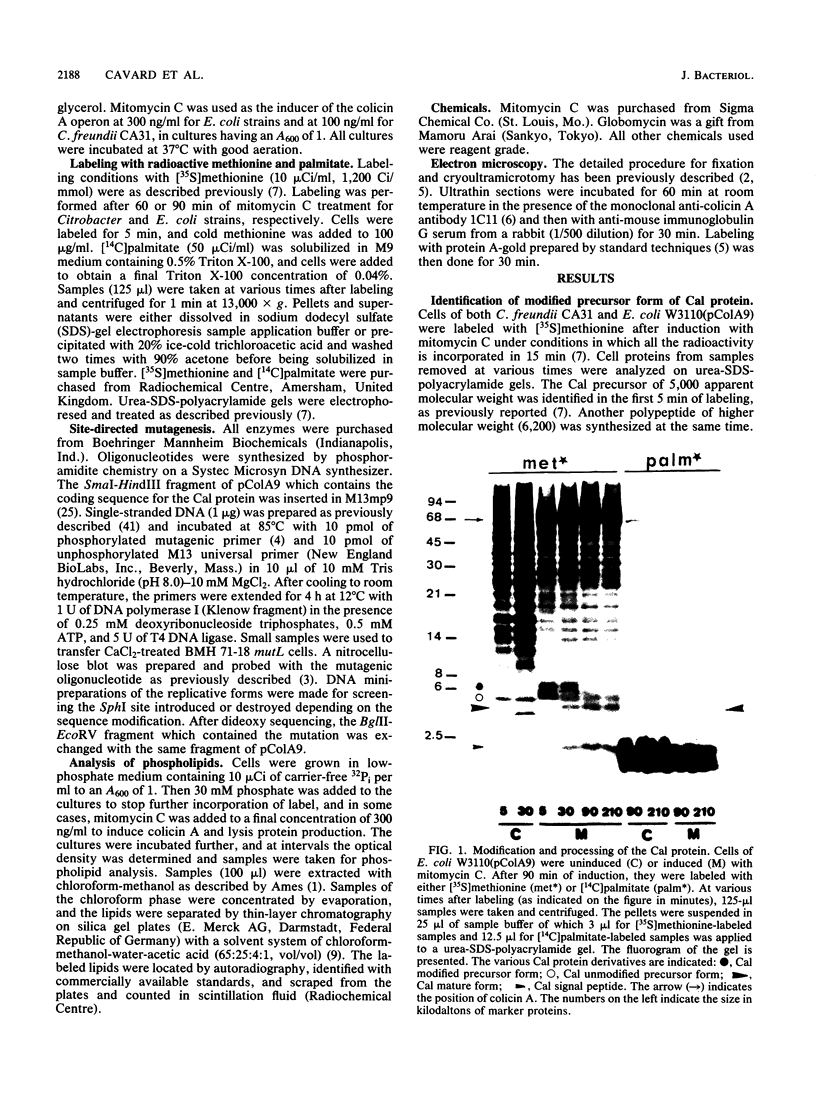
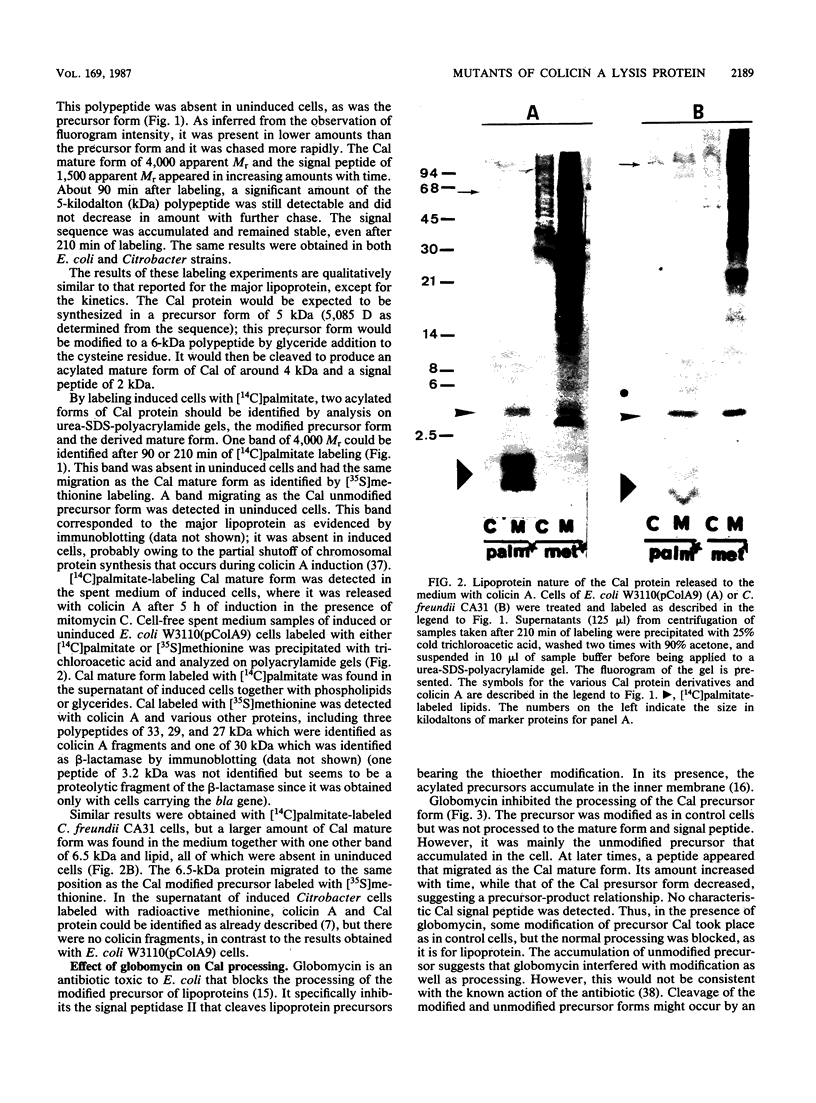
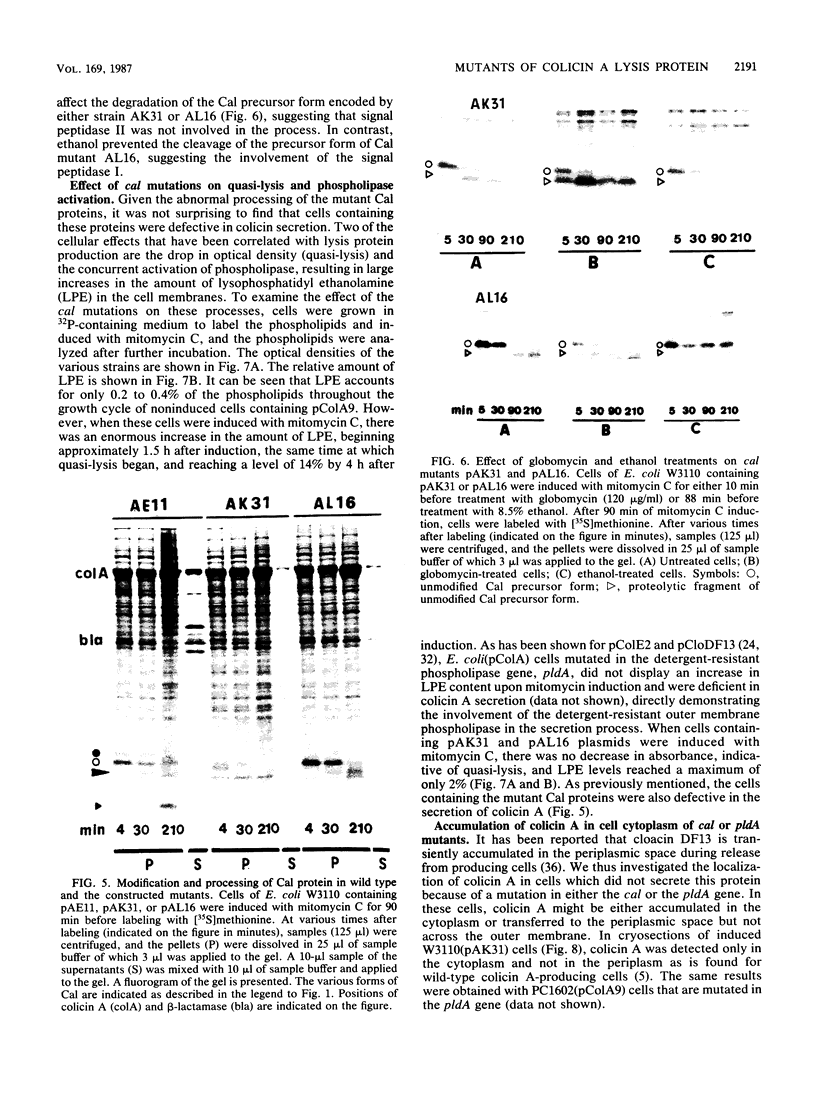
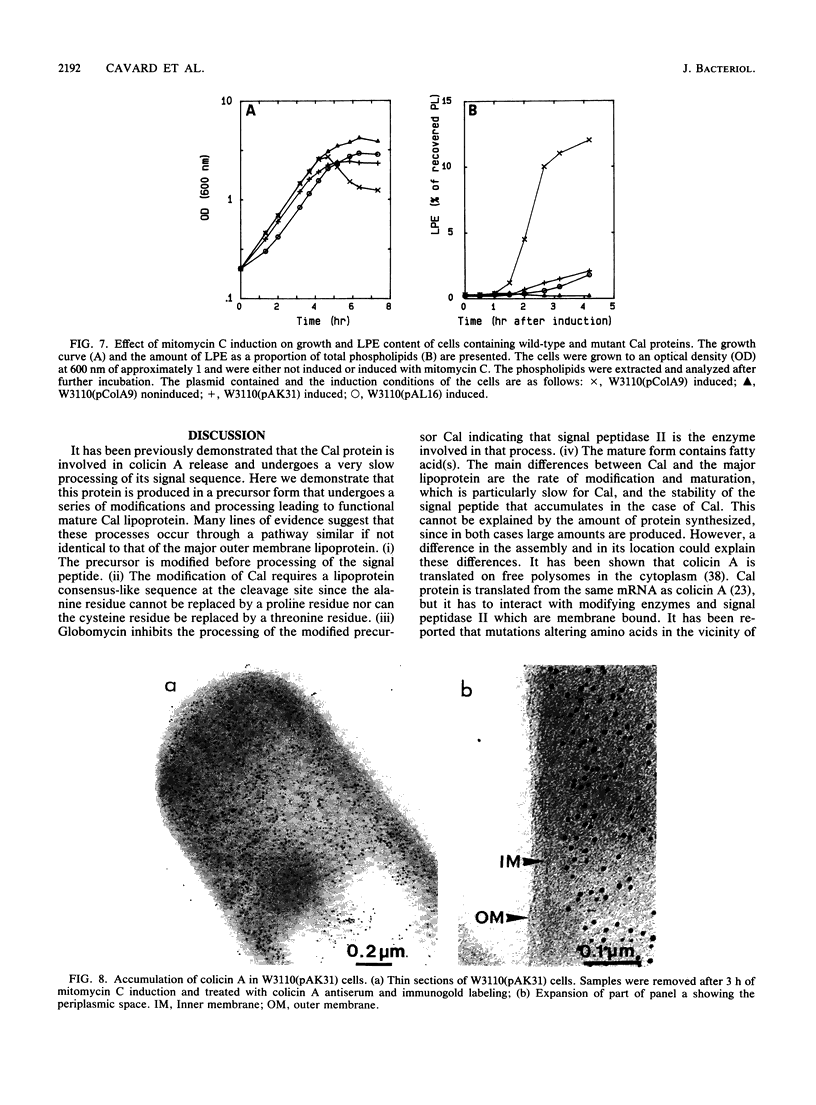
The colicin A lysis protein (Cal) is required for the release of colicin A to the medium by producing bacteria. This protein is produced in a precursor form that contains a cysteine at the cleavage site (-Leu-Ala-Ala-Cys). The precursor must be modified by the addition of lipid before it can be processed. The maturation is prevented by globomycin, an inhibitor of signal peptidase II. Using oligonucleotide-directed mutagenesis, the alanine and cystein residues in the -1 and +1 positions of the cleavage site were replaced by proline and threonine residues, respectively, in two different constructs. Both substitutions prevented the normal modification and cleavage of the protein. The marked activation of the outer membrane detergent-resistant phospholipase A observed with wild-type Cal was not observed with the Cal mutants. Both Cal mutants were also defective for the secretion of colicin A. In one mutant, the signal peptide appeared to be cleaved off by an alternative pathway involving signal peptidase I. Electron microscope studies with immunogold labeling of colicin A on cryosections of pldA and cal mutant cells indicated that the colicin remains in the cytoplasm and is not transferred to the periplasmic space. These results demonstrate that Cal must be modified and processed to activate the detergent-resistant phospholipase A and to promote release of colicin A.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P. J., Winter G., Wilkinson A. J., Fersht A. R. The use of double mutants to detect structural changes in the active site of the tyrosyl-tRNA synthetase (Bacillus stearothermophilus). Cell. 1984 Oct;38(3):835–840. doi: 10.1016/0092-8674(84)90278-2. [DOI] [PubMed] [Google Scholar]
- Carter P., Bedouelle H., Winter G. Improved oligonucleotide site-directed mutagenesis using M13 vectors. Nucleic Acids Res. 1985 Jun 25;13(12):4431–4443. doi: 10.1093/nar/13.12.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavard D., Bernadac A., Pages J. M., Lazdunski C. Colicins are not transiently accumulated in the periplasmic space before release from colicinogenic cells. Biol Cell. 1984;51(1):79–86. doi: 10.1111/j.1768-322x.1984.tb00285.x. [DOI] [PubMed] [Google Scholar]
- Cavard D., Crozel V., Gorvel J. P., Pattus F., Baty D., Lazdunski C. A molecular, genetic and immunological approach to the functioning of colicin A, a pore-forming protein. J Mol Biol. 1986 Feb 5;187(3):449–459. doi: 10.1016/0022-2836(86)90445-6. [DOI] [PubMed] [Google Scholar]
- Cavard D., Lloubès R., Morlon J., Chartier M., Lazdunski C. Lysis protein encoded by plasmid ColA-CA31. Gene sequence and export. Mol Gen Genet. 1985;199(1):95–100. doi: 10.1007/BF00327516. [DOI] [PubMed] [Google Scholar]
- Duckworth D. H., Bevers E. M., Verkleij A. J., Op den Kamp J. A., van Deenen L. L. Action of phospholipase A2 and phospholipase C on Escherichia coli. Arch Biochem Biophys. 1974 Nov;165(1):379–387. doi: 10.1016/0003-9861(74)90176-3. [DOI] [PubMed] [Google Scholar]
- Ebina Y., Nakazawa A. Cyclic AMP-dependent initiation and rho-dependent termination of colicin E1 gene transcription. J Biol Chem. 1983 Jun 10;258(11):7072–7078. [PubMed] [Google Scholar]
- Ghrayeb J., Lunn C. A., Inouye S., Inouye M. An alternate pathway for the processing of the prolipoprotein signal peptide in Escherichia coli. J Biol Chem. 1985 Sep 15;260(20):10961–10965. [PubMed] [Google Scholar]
- Giam C. Z., Chai T., Hayashi S., Wu H. C. Prolipoprotein modification and processing in Escherichia coli. A unique secondary structure in prolipoprotein signal sequence for the recognition by glyceryl transferase. Eur J Biochem. 1984 Jun 1;141(2):331–337. doi: 10.1111/j.1432-1033.1984.tb08196.x. [DOI] [PubMed] [Google Scholar]
- Hakkaart M. J., Veltkamp E., Nijkamp H. J. Protein H encoded by plasmid Clo DF13 involved in lysis of the bacterial host. I. Localisation of the gene and identification and subcellular localisation of the gene H product. Mol Gen Genet. 1981;183(2):318–325. doi: 10.1007/BF00270635. [DOI] [PubMed] [Google Scholar]
- Hayashi S., Chang S. Y., Chang S., Wu H. C. Modification and processing of Bacillus licheniformis prepenicillinase in Escherichia coli. Fate of mutant penicillinase lacking lipoprotein modification site. J Biol Chem. 1984 Aug 25;259(16):10448–10454. [PubMed] [Google Scholar]
- Hussain M., Ichihara S., Mizushima S. Accumulation of glyceride-containing precursor of the outer membrane lipoprotein in the cytoplasmic membrane of Escherichia coli treated with globomycin. J Biol Chem. 1980 Apr 25;255(8):3707–3712. [PubMed] [Google Scholar]
- Ichihara S., Hussain M., Mizushima S. Characterization of new membrane lipoproteins and their precursors of Escherichia coli. J Biol Chem. 1981 Mar 25;256(6):3125–3129. [PubMed] [Google Scholar]
- Ichihara S., Suzuki T., Suzuki M., Mizushima S. Molecular cloning and sequencing of the sppA gene and characterization of the encoded protease IV, a signal peptide peptidase, of Escherichia coli. J Biol Chem. 1986 Jul 15;261(20):9405–9411. [PubMed] [Google Scholar]
- Inouye S., Franceschini T., Sato M., Itakura K., Inouye M. Prolipoprotein signal peptidase of Escherichia coli requires a cysteine residue at the cleavage site. EMBO J. 1983;2(1):87–91. doi: 10.1002/j.1460-2075.1983.tb01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Hsu C. P., Itakura K., Inouye M. Requirement for signal peptide cleavage of Escherichia coli prolipoprotein. Science. 1983 Jul 1;221(4605):59–61. doi: 10.1126/science.6344218. [DOI] [PubMed] [Google Scholar]
- Inukai M., Ghrayeb J., Nakamura K., Inouye M. Apolipoprotein, an intermediate in the processing of the major lipoprotein of the Escherichia coli outer membrane. J Biol Chem. 1984 Jan 25;259(2):757–760. [PubMed] [Google Scholar]
- Jakes K. S., Zinder N. D. Plasmid ColE3 specifies a lysis protein. J Bacteriol. 1984 Feb;157(2):582–590. doi: 10.1128/jb.157.2.582-590.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer B., Kramer W., Fritz H. J. Different base/base mismatches are corrected with different efficiencies by the methyl-directed DNA mismatch-repair system of E. coli. Cell. 1984 Oct;38(3):879–887. doi: 10.1016/0092-8674(84)90283-6. [DOI] [PubMed] [Google Scholar]
- Lloubes R., Baty D., Lazdunski C. The promoters of the genes for colicin production, release and immunity in the ColA plasmid: effects of convergent transcription and Lex A protein. Nucleic Acids Res. 1986 Mar 25;14(6):2621–2636. doi: 10.1093/nar/14.6.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luirink J., van der Sande C., Tommassen J., Veltkamp E., De Graaf F. K., Oudega B. Effects of divalent cations and of phospholipase A activity on excretion of cloacin DF13 and lysis of host cells. J Gen Microbiol. 1986 Mar;132(3):825–834. doi: 10.1099/00221287-132-3-825. [DOI] [PubMed] [Google Scholar]
- Morlon J., Lloubès R., Varenne S., Chartier M., Lazdunski C. Complete nucleotide sequence of the structural gene for colicin A, a gene translated at non-uniform rate. J Mol Biol. 1983 Oct 25;170(2):271–285. doi: 10.1016/s0022-2836(83)80148-x. [DOI] [PubMed] [Google Scholar]
- Nakata A., Peterson G. R., Brooks E. L., Rothman F. G. Location and orientation of the phoA locus on the Escherichia coli K-12 linkage map. J Bacteriol. 1971 Sep;107(3):683–689. doi: 10.1128/jb.107.3.683-689.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva E. T., Hirst T. R., Hardy S. J., Holmgren J., Randall L. Synthesis of a precursor to the B subunit of heat-labile enterotoxin in Escherichia coli. J Bacteriol. 1981 Apr;146(1):325–330. doi: 10.1128/jb.146.1.325-330.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollitt S., Inouye S., Inouye M. Effect of amino acid substitutions at the signal peptide cleavage site of the Escherichia coli major outer membrane lipoprotein. J Biol Chem. 1986 Feb 5;261(4):1835–1837. [PubMed] [Google Scholar]
- Pugsley A. P., Schwartz M. Colicin E2 release: lysis, leakage or secretion? Possible role of a phospholipase. EMBO J. 1984 Oct;3(10):2393–2397. doi: 10.1002/j.1460-2075.1984.tb02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P., Schwartz M. Expression of a gene in a 400-base-pair fragment of colicin plasmid ColE2-P9 is sufficient to cause host cell lysis. J Bacteriol. 1983 Oct;156(1):109–114. doi: 10.1128/jb.156.1.109-114.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabik J. F., Suit J. L., Luria S. E. cea-kil operon of the ColE1 plasmid. J Bacteriol. 1983 Mar;153(3):1479–1485. doi: 10.1128/jb.153.3.1479-1485.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toba M., Masaki H., Ohta T. Primary structures of the ColE2-P9 and ColE3-CA38 lysis genes. J Biochem. 1986 Feb;99(2):591–596. doi: 10.1093/oxfordjournals.jbchem.a135515. [DOI] [PubMed] [Google Scholar]
- Tokunaga M., Tokunaga H., Wu H. C. Post-translational modification and processing of Escherichia coli prolipoprotein in vitro. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2255–2259. doi: 10.1073/pnas.79.7.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varenne S., Cavard D., Lazdunski C. Biosynthesis and export of colicin A in Citrobacter freundii CA31. Eur J Biochem. 1981 Jun 1;116(3):615–620. doi: 10.1111/j.1432-1033.1981.tb05380.x. [DOI] [PubMed] [Google Scholar]
- Wu H. C., Tokunaga M., Tokunaga H., Hayashi S., Giam C. Z. Posttranslational modification and processing of membrane lipoproteins in bacteria. J Cell Biochem. 1983;22(3):161–171. doi: 10.1002/jcb.240220305. [DOI] [PubMed] [Google Scholar]
- Yamada M., Miki T., Nakazawa A. Translocation of colicin E1 through cytoplasmic membrane of Escherichia coli. FEBS Lett. 1982 Dec 27;150(2):465–468. doi: 10.1016/0014-5793(82)80790-4. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]
- van Tiel-Menkveld G. J., Veltkamp E., De Graaf F. K. Mitomycin C-induced synthesis of cloacin DF13 and lethality in cloacinogenic Escherichia coli cells. J Bacteriol. 1981 Apr;146(1):41–48. doi: 10.1128/jb.146.1.41-48.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]