Abstract
The importance of extracellular H2O2 in lignin degradation has become increasingly apparent with the recent discovery of H2O2-requiring ligninases produced by white-rot fungi. Here we describe a new H2O2-producing activity of Phanerochaete chrysosporium that involves extracellular oxidases able to use simple aldehyde, alpha-hydroxycarbonyl, or alpha-dicarbonyl compounds as substrates. The activity is expressed during secondary metabolism, when the ligninases are also expressed. Analytical isoelectric focusing of the extracellular proteins, followed by activity staining, indicated that minor proteins with broad substrate specificities are responsible for the oxidase activity. Two of the oxidase substrates, glyoxal and methylglyoxal, were also identified, as their quinoxaline derivatives, in the culture fluid as secondary metabolites. The significance of these findings is discussed with respect to lignin degradation and other proposed systems for H2O2 production in P. chrysosporium.
Full text
PDF

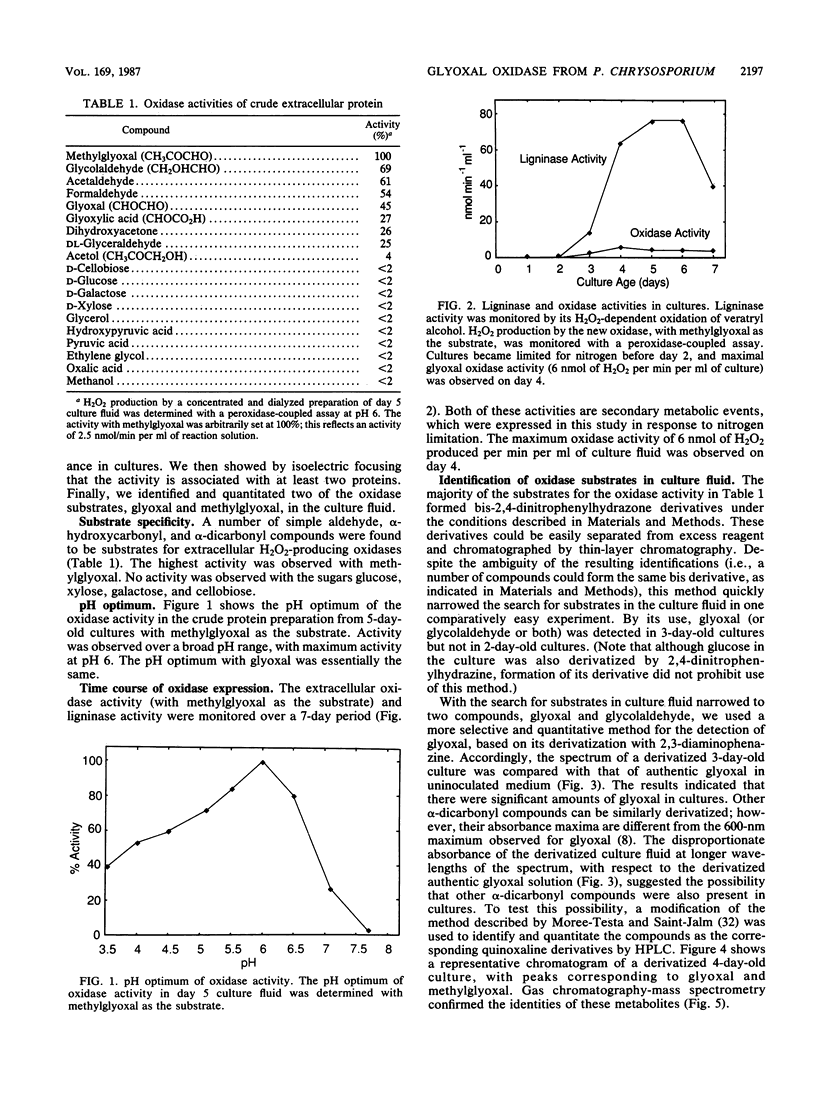
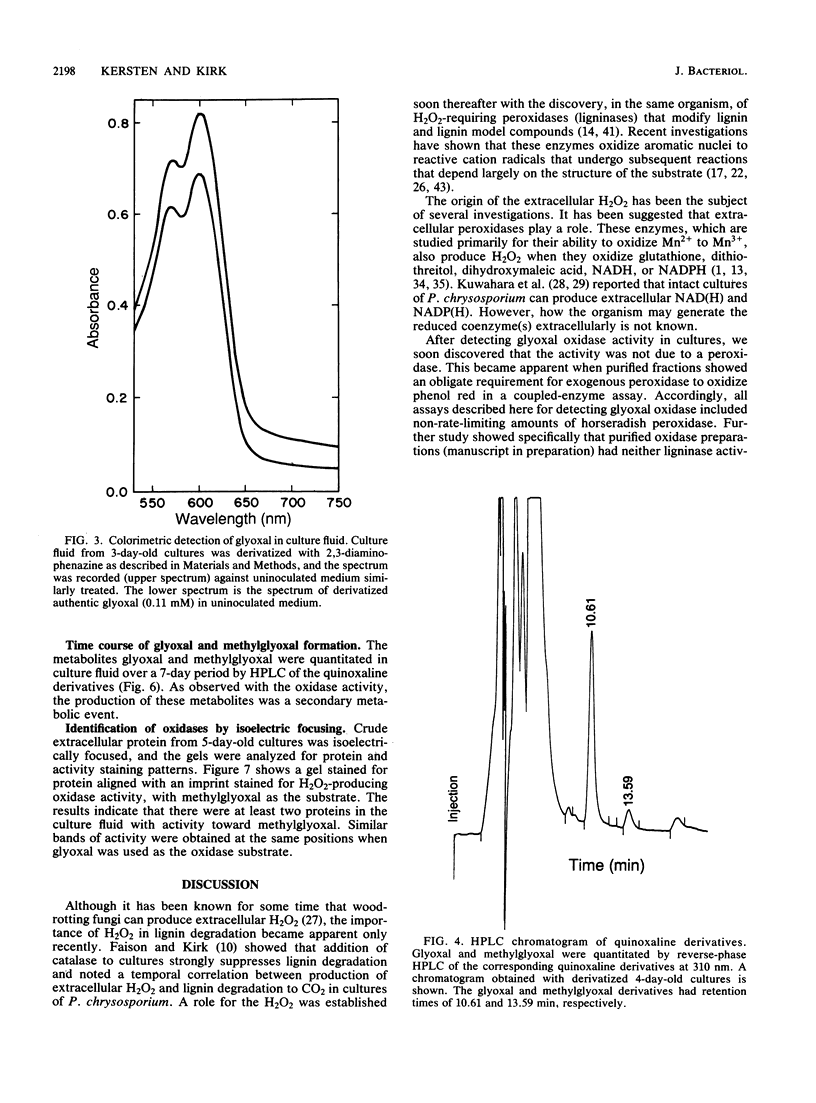
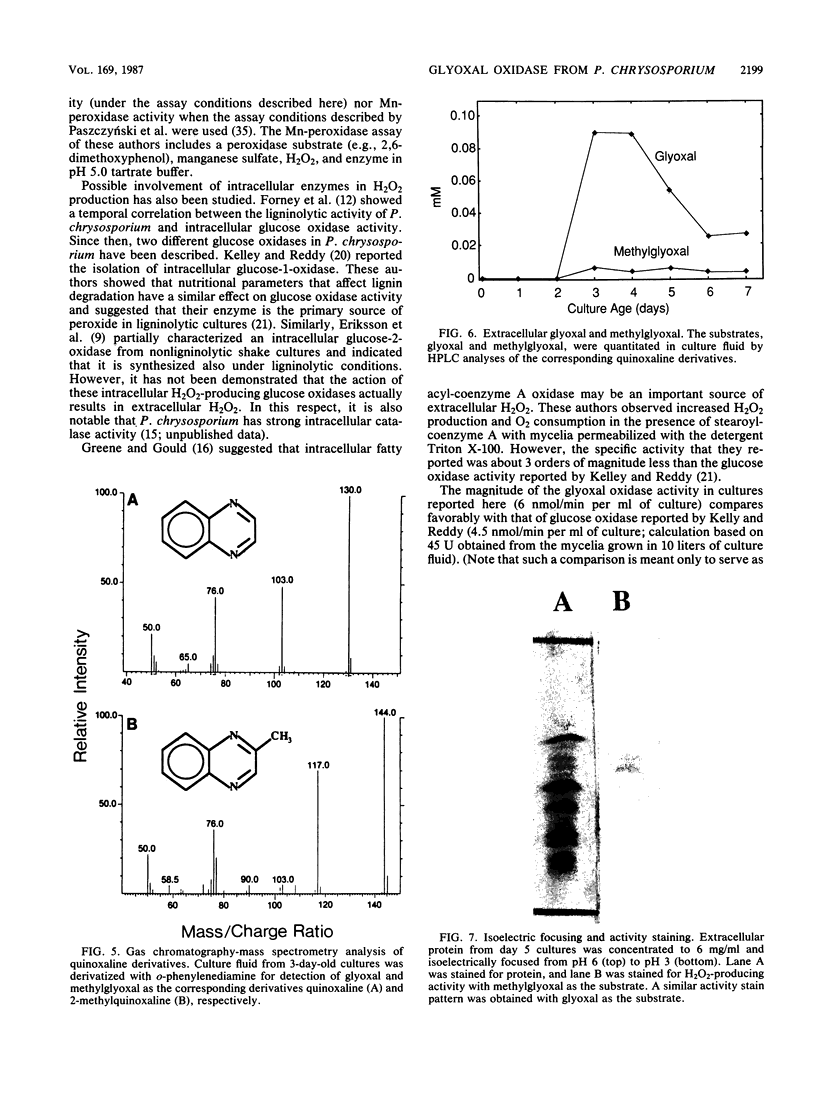


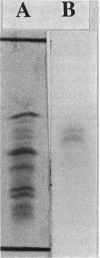
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar-Lev S. S., Kirk T. K. Effects of molecular oxygen on lignin degradation by Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1981 Mar 31;99(2):373–378. doi: 10.1016/0006-291x(81)91755-1. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Byrne G. A. The separation of 2,4-dinitrophenylhydrazones by thin-layer chromatography. J Chromatogr. 1965 Dec;20(3):528–540. doi: 10.1016/s0021-9673(01)97455-2. [DOI] [PubMed] [Google Scholar]
- Cohen H. J. The use of diaminobenzidine for spectrophotometric and acrylamide gel detection of sulfite oxidase and its applicability to hydrogen peroxide-generating enzymes. Anal Biochem. 1973 May;53(1):208–222. doi: 10.1016/0003-2697(73)90423-5. [DOI] [PubMed] [Google Scholar]
- Cooper R. A. Metabolism of methylglyoxal in microorganisms. Annu Rev Microbiol. 1984;38:49–68. doi: 10.1146/annurev.mi.38.100184.000405. [DOI] [PubMed] [Google Scholar]
- DAGLEY S., TRUDGILL P. W., CALLELY A. G. Synthesis of cell constituents from glycine by a Pseudomonas. Biochem J. 1961 Dec;81:623–631. doi: 10.1042/bj0810623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faison B. D., Kirk T. K. Relationship Between Lignin Degradation and Production of Reduced Oxygen Species by Phanerochaete chrysosporium. Appl Environ Microbiol. 1983 Nov;46(5):1140–1145. doi: 10.1128/aem.46.5.1140-1145.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forney L. J., Reddy C. A., Tien M., Aust S. D. The involvement of hydroxyl radical derived from hydrogen peroxide in lignin degradation by the white rot fungus Phanerochaete chrysosporium. J Biol Chem. 1982 Oct 10;257(19):11455–11462. [PubMed] [Google Scholar]
- Glenn J. K., Gold M. H. Purification and characterization of an extracellular Mn(II)-dependent peroxidase from the lignin-degrading basidiomycete, Phanerochaete chrysosporium. Arch Biochem Biophys. 1985 Nov 1;242(2):329–341. doi: 10.1016/0003-9861(85)90217-6. [DOI] [PubMed] [Google Scholar]
- Glenn J. K., Morgan M. A., Mayfield M. B., Kuwahara M., Gold M. H. An extracellular H2O2-requiring enzyme preparation involved in lignin biodegradation by the white rot basidiomycete Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1983 Aug 12;114(3):1077–1083. doi: 10.1016/0006-291x(83)90672-1. [DOI] [PubMed] [Google Scholar]
- Greene R. V., Gould J. M. Fatty acyl-coenzyme A oxidase activity and H2O2 production in Phanerochaete chrysosporium mycelia. Biochem Biophys Res Commun. 1984 Jan 30;118(2):437–443. doi: 10.1016/0006-291x(84)91322-6. [DOI] [PubMed] [Google Scholar]
- Greene R. V., Gould J. M. Substrate-induced H2O2 production in mycelia from the lignin-degrading fungus Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1983 Nov 30;117(1):275–281. doi: 10.1016/0006-291x(83)91571-1. [DOI] [PubMed] [Google Scholar]
- Hammel K. E., Tien M., Kalyanaraman B., Kirk T. K. Mechanism of oxidative C alpha-C beta cleavage of a lignin model dimer by Phanerochaete chrysosporium ligninase. Stoichiometry and involvement of free radicals. J Biol Chem. 1985 Jul 15;260(14):8348–8353. [PubMed] [Google Scholar]
- Jeffries T. W., Choi S., Kirk T. K. Nutritional Regulation of Lignin Degradation by Phanerochaete chrysosporium. Appl Environ Microbiol. 1981 Aug;42(2):290–296. doi: 10.1128/aem.42.2.290-296.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley R. L., Reddy C. A. Purification and characterization of glucose oxidase from ligninolytic cultures of Phanerochaete chrysosporium. J Bacteriol. 1986 Apr;166(1):269–274. doi: 10.1128/jb.166.1.269-274.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten P. J., Tien M., Kalyanaraman B., Kirk T. K. The ligninase of Phanerochaete chrysosporium generates cation radicals from methoxybenzenes. J Biol Chem. 1985 Mar 10;260(5):2609–2612. [PubMed] [Google Scholar]
- Keyser P., Kirk T. K., Zeikus J. G. Ligninolytic enzyme system of Phanaerochaete chrysosporium: synthesized in the absence of lignin in response to nitrogen starvation. J Bacteriol. 1978 Sep;135(3):790–797. doi: 10.1128/jb.135.3.790-797.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk T. K., Tien M., Kersten P. J., Mozuch M. D., Kalyanaraman B. Ligninase of Phanerochaete chrysosporium. Mechanism of its degradation of the non-phenolic arylglycerol beta-aryl ether substructure of lignin. Biochem J. 1986 May 15;236(1):279–287. doi: 10.1042/bj2360279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszczyński A., Huynh V. B., Crawford R. Comparison of ligninase-I and peroxidase-M2 from the white-rot fungus Phanerochaete chrysosporium. Arch Biochem Biophys. 1986 Feb 1;244(2):750–765. doi: 10.1016/0003-9861(86)90644-2. [DOI] [PubMed] [Google Scholar]
- Pick E., Keisari Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J Immunol Methods. 1980;38(1-2):161–170. doi: 10.1016/0022-1759(80)90340-3. [DOI] [PubMed] [Google Scholar]
- Renganathan V., Miki K., Gold M. H. Multiple molecular forms of diarylpropane oxygenase, an H2O2-requiring, lignin-degrading enzyme from Phanerochaete chrysosporium. Arch Biochem Biophys. 1985 Aug 15;241(1):304–314. doi: 10.1016/0003-9861(85)90387-x. [DOI] [PubMed] [Google Scholar]
- Tien M., Kirk T. K. Lignin-Degrading Enzyme from the Hymenomycete Phanerochaete chrysosporium Burds. Science. 1983 Aug 12;221(4611):661–663. doi: 10.1126/science.221.4611.661. [DOI] [PubMed] [Google Scholar]
- Tien M., Kirk T. K. Lignin-degrading enzyme from Phanerochaete chrysosporium: Purification, characterization, and catalytic properties of a unique H(2)O(2)-requiring oxygenase. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2280–2284. doi: 10.1073/pnas.81.8.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zancan G. T., Amaral D. New substrate for galactose oxidase. Biochim Biophys Acta. 1970 Jan 14;198(1):146–147. doi: 10.1016/0005-2744(70)90043-4. [DOI] [PubMed] [Google Scholar]