Abstract
INTRODUCTION
Patients undergoing colorectal surgical resections have a high incidence of surgical site infection (SSI). Many patient-specific risk factors have been recognised in association with SSI in such patients, but environmental contamination is increasingly recognised as a contributor to hospital-acquired infection (HAI). This study set out to describe the bacterial contamination of the patient environment, using hospital bed-control handsets, as they are frequently handled by both staff and patients and represent a marker of environmental contamination.
PATIENTS AND METHODS
On two unannounced sampling events, 1 week apart, 140 bacteriological assessments were made of 70 hospital bed control handsets within a specialist colorectal surgical unit.
RESULTS
Of the handsets examined, 67 (95.7%) demonstrated at least one bacterial species (52.9% grew 1, 30% grew 2 and 12.9% grew 3 or more bacterial species). Of these, 29 (41.4%) bed-control handsets grew bacteria known to cause nosocomial infection, including 22 (31.4%) handsets which grew Enterococcus spp., 9 (12.9%) which grew MRSA, 2 (2.9%) which grew MSSA, 2 (2.9%) which grew coliforms, and 1 (1.4%) handset which grew anaerobes. At 1-week follow-up, 31 bed-control handsets showed evidence of contamination by the same bacterial species.
CONCLUSIONS
This study revealed high levels of bacteria known to cause HAI, contaminating hospital bed-control handsets in a surgical setting. Further study is now required to confirm whether hospital environmental contamination is causally involved in SSI.
Keywords: Hospital, Surgical, Handset, MRSA, Infection, Bacteria
Colorectal surgical patients have one of the highest incidences of surgical site infection (SSI), second only to limb amputation surgery. Some 10% of operative procedures in the UK involving the large or small bowel will result in a SSI1 These rates are higher still in those undergoing operative resection for rectal tumours2–3 and an independent effect on the 5-year survival rates in patients with colon cancer has also been reported,4 although this association has been disputed.5
SSI is the third most commonly reported hospital-acquired infection (HAI) and accounts for 14–16% of all HAIs amongst hospital in-patients.6 Of all general surgery patients, 2–5% will develop a SSI1 and recent reports cite increasing evidence of a relationship between the presence of bacteria known to cause nosocomial infection in the patient's healthcare environment and subsequent development of HAI.7–9
We undertook a prospective, cross-sectional study to examine bacterial contamination in the healthcare environment in the proximity of colorectal surgical patients. In order to control for potential variations achieved from a variety of hospital surfaces, materials and devices, we aimed to examine a uniform surface that was identical and present in all individual patient environments in our colorectal surgery ward setting.
A previous study by Young et al.,10 in an American healthcare institution, reported that electronic hospital bed-control handsets are a high-touch surface, which have the potential to be contaminated by bacteria known to cause nosocomial infection. These devices are regularly handled by both healthcare personnel and patients, are permanently present on all in-patient beds in our unit and provided a potential surface for the hand-transfer of bacteria between individuals and surfaces. Whilst our study involved sampling of a very specific inanimate object in the clinical setting, the hospital bed-control handsets also provided a sampling frame of general healthcare environmental contamination.
Patients and Methods
All hospital bed-control handsets present within the colorectal surgical unit of the Western General Hospital, Edinburgh were first marked with an anonymous permanent identifier number. During unannounced sampling events, the control panels of the bed-control handsets (which are permanently attached to each individual hospital bed) were sampled by standardised swabbing in two directions at right angles with a cotton-tipped swab moistened with sterile water. The swabs were inoculated within 1 h of collection onto two Blood agar plates (Columbia Agar with Horse Blood; Oxoid Ltd, Basingstoke, UK) and were incubated, one aerobically and the other anaerobically, at 37°C for 48 h.
The seeded swabs were then additionally inoculated into a single Fastidious Anaerobic broth (LAB M, Lancashire, UK) and incubated at 37°C for 24 h. Following incubation, broths were sub-cultured onto two Blood agar plates and incubated as described above. All plates were inspected daily for visible growth and any micro-organisms present were identified using standard laboratory procedures.
To compare the reproducibility of the results, and to assess temporal change of contamination, a repeat sampling of all bed-control handsets was performed as previous described, 7 days later. The results were subsequently matched, by the permanent and unique bed-control identifier number, to those obtained from the individual bed-control handsets a week previously.
A daily survey process of monitoring bed occupancy was performed over 3 weeks to include both bed-control sampling periods.
Results
A total of 77 bed-control handsets were originally permanently identified for inclusion in the study during week 1; however, 7 bed-control handsets were lost to follow-up by week 2 (i.e. bed transferred from the ward or handset removed for maintenance). These handsets were excluded from later analysis. Therefore, 70 bed-control handsets were included over the 2-week sampling period.
Of these 70 bed-control handsets, 67 (95.7%) demonstrated bacterial growth with an average of 1.5 different bacterial species identified per hospital bed-control handset. Only 3 (4.3%) handsets did not demonstrate bacterial growth during the 2-week sampling period. On analysis, 37 (52.9%) handsets grew 1 species, 21 (30%) handsets grew 2 bacterial species and 9 (12.9%) handsets grew 3 or more bacterial species. The specific breakdown of bacterial species recovered during separate bed-control sampling events is recorded in Table 1.
Table 1.
Breakdown of individual bacteriological species results from sampling events
| Bacterial species | No. of samples, week 1 (%) | No. of samples, week 2 (%) | No. of handsets. both weeks 1 and 2 (%) | No. of handsets, either week 1 or week 2 (%) |
|---|---|---|---|---|
| No growth | 23 (32.9) | 13 (18.5) | 3 (4.3) | 35 (51.4) |
| Coagulase-negative | ||||
| Staphylococcus spp. | 41 (58.5) | 54 (77.1) | 29 (41.4) | 66 (94.3) |
| Enterococcus spp. | 10 (14.3) | 14 (20%) | 2 (2.9) | 22 (31.4) |
| Bacillus spp. | 3 (4.3) | 1 (1.4) | 0 (0) | 4 (5.7) |
| MRSA | 3 (4.3) | 7 (10) | 1 (1.4) | 9 (12.9) |
| MSSA | 1 (1.4) | 1 (1.4) | 1 (1.4) | 2 (2.9) |
| Coliforms | 0 (0) | 2 (2.9) | 0 | 2 (2.9) |
| Anaerobes | 0 (0) | 1 (1.4) | 0 (0) | 1 (1.4) |
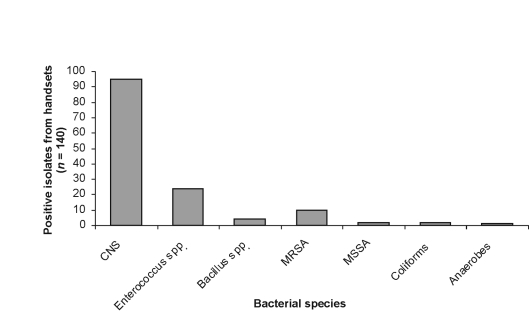
Twenty-nine (41.4%) bed control handsets grew bacteria known to cause nosocomial infection, including 22 (31.4%) handsets that grew enterococcal species, 9 (12.9%) that grew MRSA, 2 (2.9%) that grew MSSA, 2 (2.9%) that grew coliforms, and 1 (1.4%) handset that grew anaerobic bacteria. A representation of the overall bed-control bacteriology results during the whole sampling period is shown in Figure 1.
Figure 1.
The number of bacteria isolated from all samples relative to species.
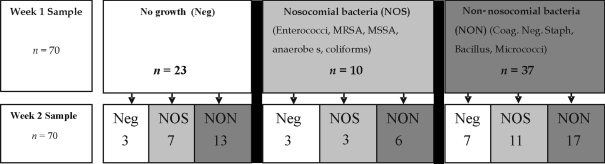
Figure 2.
Follow-up results of individual handsets from week 1 and week 2
Thirty-one (44.2%) bed control handsets demonstrated a similar bacterial species present on both bacteriological sampling events. Of these, 29 were positive for coagulase-negative Staphylococcus spp., 2 for Enterococcus spp. and 1 for MRSA with one of the bed-control handsets demonstrating both coagulase-negative Staphylococcus spp. and Enterococcus spp. on the same handset, one week later.
During the execution of the sampling events, 14 beds (9.5%) were identified as being not assigned to a particular in-patient (i.e. empty), following the previous discharge of the occupant. Nine (64.3%) of the bed-control handsets attached to these empty beds grew coagulase-negative Staphylococcus spp., 5 (35.7%) had no growth identified and 1 (7.1%) grew bacillus. The remaining beds had current inpatients at the time of sampling; no bed was empty during both sampling events.
During the occupancy sampling period, 277 in-patient episodes were recorded with an average in-patient stay of 5.55 days. Each bed had an average of 3.6 different in-patients during the 3-week monitoring period. The beds were monitored as empty on 160 occasions during the 1610 bed monitoring events.
Discussion
Staphylococcus aureus is the most common bacterial species implicated as a cause of SSI in the UK and more than half are methicillin-resistant.11 Methicillin-resistant S. aureus (MRSA) has been shown to survive on dry surfaces for prolonged periods12 and surfaces which are commonly touched by healthcare workers and patients may act as sources of hand transfer of bacteria known to cause nosocomial infection.13
HAI costs the NHS £1 billion/year and postoperative SSI has been estimated to cost an extra £1594 per SSI, with an average extra length of stay for infected patients of an additional 7.1 days.14 A number of pre-operative and operative risk factors for the development of a SSI have been identified in association with colorectal surgical patients.3,15–22 However, evidence is now emerging of the relationship between healthcare environmental bacterial contamination and resultant HAI.
In the study by Hardy et al.7 of intensive care MRSA bacteraemia, patients developed MRSA infections during their stay in ICU and there was evidence to suggest that the MRSA was directly acquired from the surrounding healthcare environment. Dancer et al.8 have also recently confirmed patient MRSA acquisition in a Scottish ICU department, which was temporally associated with reduced numbers of trained nurses and hygiene failures predominantly involving hand-touch sites. In addition, Rampling et al.9 reported the successful reduction of an MRSA outbreak through aggressively addressing environmental contamination in the surgical ward by introducing an intensive environmental cleaning intervention.
In relating contamination of the patient environment to specific patient outcomes there is a need to ensure that the object sampled is uniform and in the proximity of the patient in question. Patients and medical and nursing staff commonly touch hospital bed-control handsets and they are relatively permanently attached to the bed frames of all inpatient beds. These characteristics suggest their use as a potential marker of general healthcare environmental contamination.
The percentage of hospital bed-control handsets in our study demonstrating evidence of MRSA colonisation was 12.9%. Young et al.10 reported 1% of bed-control handsets to be contaminated by MRSA, in their study in an American hospital, although no mention was recorded of contemporary regional MRSA bacteraemia rates or the incidence within the host institution. The high levels of environmental contamination are likely to be a reflection of both poor hand-hygiene practices and compromised hygiene measures.
Many authors have previously reported on the poor levels of staff compliance with hand-hygiene practices23,24 and the recorded levels of contamination in this study could reflect a need to re-iterate the importance of basic hand-hygiene measures. In addition, both Boyce et al.13 and Bhalla et al.25 have previously reported the acquisition of MRSA on the hands or gloves of healthcare staff following contact with contaminated environmental surfaces surrounding a patient emphasising the importance of hand-washing before and after contact with patients in preventing cross-contamination of surfaces.
Local policy26 dictates that the patient beds and bed frames are formally cleaned by nursing staff, using detergent and water, following the discharge of an in-patient, with obvious visual debris removed on a regular ad-hoc basis in the interim. The higher bacterial contamination rates in our study could suggest that the current method of cleaning may not be fit for purpose and consideration should be given to use of disinfectant (e.g. hypochlorite) or other agents, as a routine practice to reduce bioburden in addition to more intensive cleaning regimens. However, even with the introduction of such disinfectants, recent studies have demonstrated that this is, in itself, ineffective in reducing MRSA colonisation of the patient environment and that a more considered approach, involving more effective or novel cleaning and decontamination measures, may be required.27
In the Young et al.10 report, a number of novel potential solutions to combat bed-control contamination were described, including disposable bed-control covers, regular routine cleaning of removable handsets at a specialist facility, and disposable handsets for individual patients. Further evaluation of these cleaning methodologies and technological adjuncts may be beneficial but hospital bed-control contamination represents a sentinel marker of healthcare environmental bacterial contamination as a whole; therefore, a wider approach, addressing the general hospital environment and process of cleaning and disinfection, may be more appropriate.
Conclusions
This study provides evidence of a high incidence of bacterial contamination of bed-control handsets in a colorectal ward setting. The high incidence of bacteria known to cause nosocomial infections in the close proximity to surgical patients are concerning given the higher risk of surgical site infection and HAI in this surgical population. This study also identifies a potential inanimate object, ubiquitously present in many patient environments, which could be used as a marker of environmental contamination. This should allow the further study of the effectiveness of interventions and the causal relationship to clinical outcomes.
Acknowledgments
The authors would like to thank the medical, nursing, laboratory and management staff of the Western General Hospital for their support. The bacteriology assessment was funded by the WGH Colorectal Surgery Endowment Fund. This research has been previously reported at the Surgical Infection Society-Europe 2007, Association of Surgeons of Great Britain and Ireland, 2006 Annual Scientific Meeting, and at the NHS Health Protection Scotland, October 2005 ‘HAI: focusing on Patient Safety’ Conference. The authors declare no conflict of interest.
References
- 1.Coello R, Charlett A, Wilson J, Ward V, Pearson A, Borriello P. Adverse impact of surgical site infections in English hospitals. J Hosp Infect. 2005;60:93–103. doi: 10.1016/j.jhin.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 2.Horzic M, Kopljar M. Postoperative infections in colorectal cancer patients. Hepatogastroenterology. 2005;52:101–4. [PubMed] [Google Scholar]
- 3.Yoshida J, Shinohara M, Ishikawa M, Matsuo K. Surgical site infection in general and thoracic surgery: surveillance of 2663 cases in a Japanese teaching hospital. Surg Today. 2006;36:114–8. doi: 10.1007/s00595-005-3120-6. [DOI] [PubMed] [Google Scholar]
- 4.Nespoli A, Gianotti L, Bovo G, Brivio F, Nespoli L, Totis M. Impact of postoperative infections on survival in colon cancer patients. Surg Infect (Larchmt) 2006;7(Suppl 2):s41–3. doi: 10.1089/sur.2006.7.s2-41. [DOI] [PubMed] [Google Scholar]
- 5.Varty PP, Linehan IP, Boulos PB. Intra-abdominal sepsis and survival after surgery for colorectal cancer. Br J Surg. 1994;81:915–8. doi: 10.1002/bjs.1800810641. [DOI] [PubMed] [Google Scholar]
- 6.Smyth ET, Emmerson AM. Surgical site infection surveillance. J Hosp Infect. 2000;45:173–84. doi: 10.1053/jhin.2000.0736. [DOI] [PubMed] [Google Scholar]
- 7.Hardy KJ, Oppenheim BA, Gossain S, Gao F, Hawkey PM. A study of the relationship between environmental contamination with methicillin-resistant Staphylococcus aureus (MRSA) and patients' acquisition of MRSA. Infect Control Hosp Epidemiol. 2006;27:127–32. doi: 10.1086/500622. [DOI] [PubMed] [Google Scholar]
- 8.Dancer SJ, Coyne M, Speekenbrink A, Samavedam S, Kennedy J, Wallace PG. MRSA acquisition in an intensive care unit. Am J Infect Control. 2006;34:10–7. doi: 10.1016/j.ajic.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Rampling A, Wiseman S, Davis L, Hyett AP, Walbridge AN, Payne GC, et al. Evidence that hospital hygiene is important in the control of methicillin-resistant Staphylococcus aureus. J Hosp Infect. 2001;49:109–16. doi: 10.1053/jhin.2001.1013. [DOI] [PubMed] [Google Scholar]
- 10.Young JM, Naqvi M, Richards L. Microbial contamination of hospital bed handsets. Am J Infect Control. 2005;33:170–4. doi: 10.1016/j.ajic.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Nosocomial Infection National Surveillance Service (NINSS) Surveillance of surgical site infections in English Hospitals 1997–2001. London: Public Health Laboratory Service, Central Public Health Laboratory Service; 2003. [Google Scholar]
- 12.Neely AN, Maley MP. Survival of enterococci and staphylococci on hospital fabrics and plastic. J Clin Microbiol. 2000;38:724–6. doi: 10.1128/jcm.38.2.724-726.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyce JM, Potter-Bynoe G, Chenevert C, King T. Environmental contamination due to methicillin-resistant Staphylococcus aureus: possible infection control implications. Infect Control Hosp Epidemiol. 1997;18:622–7. [PubMed] [Google Scholar]
- 14.Plowman R, Graves N, Griffin MA, Roberts JA, Swan AV, Cookson B, et al. The rate and cost of hospital-acquired infections occurring in patients admitted to selected specialties of a district general hospital in England and the national burden imposed. J Hosp Infect. 2001;47:198–209. doi: 10.1053/jhin.2000.0881. [DOI] [PubMed] [Google Scholar]
- 15.Jesus Hernandez-Navarrete M, Arribas-Llorente JL, Solano-Bernad VM, Misiego-Peral A, Rodriguez-Garcia J, Fernandez-Garcia JL, et al. [Quality improvement program of nosocomial infection in colorectal cancer surgery] Med Clin (Barc) 2005;125:521–4. doi: 10.1157/13080450. [DOI] [PubMed] [Google Scholar]
- 16.Ford CD, VanMoorleghem G, Menlove RL. Blood transfusions and postoperative wound infection. Surgery. 1993;113:603–7. [PubMed] [Google Scholar]
- 17.Tang R, Chen HH, Wang YL, Changchien CR, Chen JS, Hsu KC, et al. Risk factors for surgical site infection after elective resection of the colon and rectum: a single-center prospective study of 2,809 consecutive patients. Ann Surg. 2001;234:181–9. doi: 10.1097/00000658-200108000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith RL, Bohl JK, McElearney ST, Friel CM, Barclay MM, Sawyer RG, et al. Wound infection after elective colorectal resection. Ann Surg. 2004;239:599–605. doi: 10.1097/01.sla.0000124292.21605.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song F, Glenny AM. Antimicrobial prophylaxis in colorectal surgery: a systematic review of randomized controlled. Br J Surg. 1998;85:1232–41. doi: 10.1046/j.1365-2168.1998.00883.x. [DOI] [PubMed] [Google Scholar]
- 20.Tartter PI, Quintero S, Barren DM. Perioperative blood transfusion associated with infectious complications after colorectal cancer operations. Am J Surg. 1986;152:479–82. doi: 10.1016/0002-9610(86)90207-2. [DOI] [PubMed] [Google Scholar]
- 21.Quintiliani L, Pescini A, Di Girolamo M, Ludicone P, Martini F, Guglielmetti M, et al. Relationship of blood transfusion, post-operative infections and immunoreactivity in patients undergoing surgery for gastrointestinal cancer. Haematologica. 1997;82:318–23. [PubMed] [Google Scholar]
- 22.Manian FA, Meyer PL, Setzer J, Senkel D. Surgical site infections associated with methicillin-resistant Staphylococcus aureus: do postoperative factors play a role? Clin Infect Dis. 2003;36:863–8. doi: 10.1086/368195. [DOI] [PubMed] [Google Scholar]
- 23.Albert RK, Condie F. Hand-washing patterns in medical intensive-care units. N Engl J Med. 1981;304:1465–6. doi: 10.1056/NEJM198106113042404. [DOI] [PubMed] [Google Scholar]
- 24.Pittet D, Mourouga P, Perneger TV. Compliance with handwashing in a teaching hospital. Infection Control Program. Ann Intern Med. 1999;130:126–30. doi: 10.7326/0003-4819-130-2-199901190-00006. [DOI] [PubMed] [Google Scholar]
- 25.Bhalla A, Pultz NJ, Gries DM, Ray AJ, Eckstein EC, Aron DC, et al. Acquisition of nosocomial pathogens on hands after contact with environmental surfaces near hospitalised patients. Infect Control Hosp Epidemiol. 2004;25:164–7. doi: 10.1086/502369. [DOI] [PubMed] [Google Scholar]
- 26.Lothian University Hospitals Trust Infection Control Team. Lothian University Hospitals Trust. Infection Control Policy Manual. Edinburgh: Lothian University Hospitals Trust; 2005. Section 1.7, page 6. [Google Scholar]
- 27.Sexton T, Clarke P, O'Neill E, Dillane T, Humphreys H. Environmental reservoirs of methicillin-resistant Staphylococcus aureus in isolation rooms: correlation with patient isolates and implications for hospital hygiene. J Hosp Infect. 2006;62:187–94. doi: 10.1016/j.jhin.2005.07.017. [DOI] [PubMed] [Google Scholar]