Abstract
Rationale
Nicotine and other agonists of nicotinic cholinergic receptors (nAChR) have been shown to improve performance in specific memory domains in rodents and monkeys. Such beneficial effects are observed in preclinical models of age-related cognitive decline, stimulating interest in nAChR ligands as possible therapeutics. Prior work has typically focused on assays of spatial working memory in rodent studies and visual recognition memory in monkey studies.
Objective
The current study was conducted to determine the role nAChRs play in multiple types of memory in monkeys.
Methods
Rhesus monkeys (N = 6) were trained to perform a battery of 6 behavioral tasks and then serially challenged with acute doses of nicotine (3.2–56 μg/kg, i.m.) and the nAChR antagonist mecamylamine (0.32–1.78 mg/kg, i.m).
Results
Nicotine improved performance on tests designed to assay visual recognition memory, spatial working memory and visuo-spatial associative memory while mecamylamine impaired visuo-spatial associative memory. Ballistic and fine motor performance was not significantly improved by nicotine but fine motor performance was impaired by mecamylamine.
Conclusions
Although nicotine may improve performance in multiple domains, effects on visuo-spatial associative memory is the most specifically attributable to nAChR signaling.
Keywords: Attention, Memory, Cholinergic, CANTAB, Alzheimer's Disease, Parkinson's Disease, Primate, Nicotinic
Introduction
Nicotine, the primary psychoactive ingredient in smoked tobacco, acts as an agonist at nicotinic subtypes of acetylcholine receptors (nAChRs) which are widely distributed in the central nervous system (Court et al. 2000a). Cholinergic mechanisms have long been demonstrated to be crucial for learning and memory, however the majority of research efforts over past decades has been devoted to investigation of signaling through the muscarinic subtype cholinergic receptors (see Bartus 2000 for review). Recent attention has been focused on the involvement of nAChRs in mnemonic functions in part because of observations that nicotinic receptor binding is reduced in the cortex and hippocampus of Alzheimer’s Disease (AD) patients (Kellar et al. 1987; Nordberg et al. 1988; Nordberg and Winblad 1986) and in the striatum of Parkinson's Disease (PD) patients (Aubert et al. 1992; Court et al. 2000b; Perry et al. 1998). Consistent with the finding for postmortem AD brain tissue, transdermal administration of nicotine can improve learning rates and attentional processing in AD patients (Min et al. 2001; White and Levin 1999; Wilson et al. 1995). While intravenous, transdermal or smoked nicotine may improve symptoms in PD patients (Ishikawa and Miyatake 1993; Kelton et al. 2000), other studies suggest that transdermal, smoked or buccal nicotine does not improve, and may worsen, PD symptoms (Ebersbach et al. 1999; Vieregge et al. 2001). Additional evidence suggests that chronic exposure to nicotine may be neuroprotective since the use of tobacco may reduce the incidence of AD (Brenner et al. 1993; Graves et al. 1991; Lee 1994; van Duijn et al. 1995; van Duijn and Hofman 1991) and PD (Hernan et al. 2001; Ross and Petrovitch 2001). Finally, nAChR alterations may be involved in cognitive disturbance associated with a variety of other conditions including Down syndrome, autism, schizophrenia, Tourette syndrome and Lewy body dementia (see Court et al. 2000a for review). Given the involvement of nAChRs in a range of cognitive disorders, much effort has been devoted to the development of nAChR ligands (see Lloyd and Williams 2000; Rezvani and Levin 2001 for review) which are not limited by the substantial adverse health risks, including addiction, associated with nicotine. In addition, the nicotinic receptor agonist ABT-418 has been shown to have beneficial cognitive effects in Alzheimer’s disease patients (Potter et al. 1999), without the adverse health effects of nicotine. Further exploration of the relationships between nAChR signaling and aspects of cognitive or behavioral function in preclinical models will therefore contribute substantially to both understanding of the etiology of cognitive disruption and the development of potential therapeutics.
Preclinical evidence also confirms that stimulation of nAChRs can improve a range of cognitive functions both in the intact animal and under conditions of experimental or developmental brain compromise. For example nicotine reverses attentional and memory impairments in rats caused by basal forebrain lesions (Grigoryan et al. 1996; Muir et al. 1995) or lesions of the septohippocampal pathway (Decker et al. 1992; Levin et al. 1993). The nAChR agonist SIB-1508Y reverses cognitive deficits associated with chronic low dose exposure to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in monkeys (Schneider et al. 1999) and acts synergistically with levodopa to improve cognitive and motor performance in monkeys exposed to higher doses of MPTP (Schneider et al. 1998a; Schneider et al. 1998b). Nicotine also enhances visual recognition memory as assessed with the delayed match-to-sample (DMS) task in adult (Buccafusco and Jackson 1991; Elrod et al. 1988; Terry et al. 1993) and aged (Buccafusco and Jackson 1991; Buccafusco et al. 1999) macaques. Similarly, other nAChR ligand such as ABT-089, ABT-418, GTS-21 and SIB1553A can also improve monkeys' performance on DMS (Bontempi et al. 2001; Briggs et al. 1997; Buccafusco et al. 1995; Decker et al. 1997; Prendergast et al. 1998). Prior studies in the nonhuman primate have frequently been limited to single behavioral assay (Buccafusco and Jackson 1991; Elrod et al. 1988; Terry et al. 1993); although see (Schneider et al. 1999), thus the generality or specificity of nicotine-related cognitive improvement in nonhuman primates is not well established. Given the diversity of behavioral impairments which have been associated with nAChR signaling alterations it is of substantial interest to develop nonhuman primate models which can assess the function of nAChRs in multiple cognitive domains.
Previous preclinical research has focused on the involvement of muscarinic cholinergic systems in cognitive behavior. The muscarinic cholinergic receptor antagonists (mAChR) scopolamine and atropine have been used extensively to model age-related cognitive deficits, particularly in mnemonic domains. Scopolamine has been shown to impair performance on a wide variety of memory tasks in several species, including rats (Deutsch 1971; Kirk et al. 1988), pigeons (Santi and Weise 1995), nonhuman primates (Aigner and Mishkin 1986; Bartus and Johnson 1976; Ridley et al. 1984; Rupniak et al. 1991; Taffe et al. 1999) and humans (Broks et al. 1988; Drachman and Leavitt 1974; Flicker et al. 1990; Ghoneim and Mewaldt 1975; Robbins et al. 1997; Safer and Allen 1971).
In comparison with such studies of the cognitive effects of mAChR blockade, the effects of nAChR blockade on cognitive behavior have not been examined as extensively. Nevertheless, the available data suggest that cholinergic signaling mediated by nAChRs is also important for memory function. In particular, the noncompetitive nAChR antagonist mecamylamine has been shown to impair learning and memory processes in rodents, nonhuman primates and humans. In rodents, mecamylamine has been shown to disrupt the acquisition of spatial information in the Morris water maze and impair inhibitory avoidance learning (Bammer 1982; Decker and Majchrzak 1992; Dilts and Berry 1967; Riekkinen et al. 1993; Riekkinen et al. 1992). Mecamylamine also impaired performance in a five-choice serial reaction task in rats (Grottick and Higgins 2000), a finding in agreement with Jones et al. (Jones et al. 1995) who reported task-specific disruptive effects of mecamylamine in middle aged rats. However, Mirza and Stolerman (Mirza and Stolerman 1998) and Ruotsalainen and colleagues (Ruotsalainen et al. 2000) only reported decrements in reaction time, not accuracy following mecamylamine administration in the five-choice serial reaction time task. In nonhuman primates, mecamylamine has been demonstrated to impair delayed matching-to-sample performance (Elrod et al. 1988). Similarly, mecamylamine has also been found to impair the acquisition of new information, but not the retrieval of previously learned information in humans (Newhouse et al. 1992). The cognitive impairing effects of mecamylamine suggest that tonic nicotinic cholinergic activity is necessary for normal cognitive functioning.
The present investigation was therefore designed to further determine the role nAChR signaling plays in multiple aspects of cognitive function of rhesus monkeys using a neuropsychological test battery approach. In this model, monkeys are trained to concurrently perform a number of behavioral tests including ones designed to assess the mnemonic domains of visual recognition memory (Delayed Match-to-Sample; DMS), spatial working memory (Self-Ordered Spatial Search; SOSS) and visuo-spatial associative learning (visuo-spatial Paired Associates Learning; vsPAL). Additional tests of motor function (Reaction Time, RT; Bimanual Motor Skill, BMS) and reinforcer efficacy/sustained attention (Progressive Ratio; PR) are also included. Monkeys' performance on tests from this battery have been shown to be selectively affected by a number of acute pharmacological challenges in rhesus monkeys (Taffe et al. 2002a; Taffe et al. 2002b; Taffe et al. 2003b; Taffe et al. 1999; Taffe et al. 2002c; Weed and Gold 1998). One working hypothesis for the current investigation was that nicotine would have a broad range of beneficial effects including improving performance on all memory tasks and speeding response latencies in the RT and BMS procedures. Nicotinic agonists can reverse the cognitive deficits associated with Alzheimer’s disease (Brenner et al. 1993; Ishikawa and Miyatake 1993; Lee 1994; Newhouse et al. 1997; van Duijn et al. 1994; van Duijn et al. 1995) and mecamylamine impairs DMS performance in nonhuman primates (Elrod et al. 1988) and disrupts the acquisition of spatial information in the Morris water maze in rodents (Bammer 1982; Decker and Majchrzak 1992; Dilts and Berry 1967; Riekkinen et al. 1993; Riekkinen et al. 1992). Furthermore, the vsPAL task is particularly sensitive to the cognitive deficits observed in Alzheimer’s disease patients (Fowler et al. 2002; Swainson et al. 2001). Therefore, it is hypothesized that blockade of nicotinic cholinergic receptors with mecamylamine will impair vsPAL, SOSS and DMS performance.
Finally, it should be recognized that a broadly cognitive-enhancing substance (or even one limited to learning and memory) would be of substantial utility to many individuals in important vocational and educational settings at the least. As discussed briefly by Heishman (Heishman 1999), this raises the possibility that cognition enhancing effects of nicotine may be partially responsible for supporting the use of tobacco products, particularly early in an individuals' use history prior to the development of significant addiction. Thus further study of the manner in which nAChRs contribute to cognition may result in increased understanding of the public health concerns related to the compulsive use of tobacco products. In summary, the present investigation will further knowledge of the role nAChRs play in cognition which may facilitate understanding of a range of concerns from neurodegenerative disease to drug abuse.
Materials and methods
Animals
Six male rhesus monkeys (Macaca mulatta) served as subjects. The monkeys were approximately 6 years of age and weighed 8–12 kg at the beginning of the study. Animals were individually housed and fed in the home cage after completion of the daily testing session. The animals' normal diet (Lab Diet 5038, PMI Nutrition International) was supplemented with fruit or vegetables four days per week and water was available ad libitum in the home cage at all times. The animals' diet was modestly restricted 5 days per week to ensure consistent behavioral responding while maintaining adequate growth rates. Laboratory experience with this food restriction protocol produces a mean growth rate of 0.06 kg/month prior to puberty and 0.14 kg/month thereafter for animals 2–8 years of age. The United States National Institutes of Health guidelines for laboratory animal care (Clark et al. 1997) were followed, and all protocols were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute. The monkeys had previously been trained on components of a behavioral test battery and had participated in prior acute drug challenge studies with ketamine (Taffe et al. 2002a; Taffe et al. 2002c), scopolamine (Taffe et al. 2002c) and Δ9-tetrahydrocannabinol; these drugs were administered at least 3 months prior to the current study. One monkey (#289) participated in the nicotine study but not the mecamylamine study. In addition, all animals were immobilized with ketamine in doses of 5–10 mg/kg (i.m.) no less than semiannually for the purposes of routine care and health monitoring prior to the present study.
Apparatus
Animals were tested in transport cages modified by the removal of several bar sections to allow the animal to easily reach out of the cage. The transport cage was placed in front of a computer monitor fitted with a touch-sensitive screen on which visual stimuli were presented. Animals were previously trained to reach out of the cage to touch the location on the screen at which stimuli are presented to obtain a food pellet reward. Stimulus presentation and response detection were controlled by a microcomputer equipped with a version of the CAmbridge Neuropsychological Test Automated Battery (CANTAB; Cambridge Cognition, Ltd.) designed for use with non-human primates. Following correct responses, a dispenser delivered 190 mg flavored pellets (P.J. Noyes Co., Lancaster, NH) to a bin mounted on the front of the cage. The majority of animals were tested in small experimental rooms where a white-noise generator remained on and the subject was left alone during each behavioral session. One animal (#302) was tested using the apparatus described but sessions took place in the colony room. The training procedures and normative performance for these tasks have been previously described (Taffe et al. 2004; Weed et al. 1999).
Delayed Match-to-Sample (DMS)
The DMS task is a recognition memory task involving sequential sets of independent visual discriminations. A sample stimulus is presented in the center of the screen and the animal must make an observing touch to its location within 30 seconds otherwise the trial is scored as a sample omission error and a 4 sec inter-trial delay is initiated. After a touch, the screen is blanked and following a variable retention interval (2, 60 or 90 sec) four choice stimuli are presented in the corners of the screen. One stimulus is identical to the sample stimulus and the others are novel. A touch directed to the choice stimulus that is identical to the sample is followed by reinforcer delivery and scored as a correct choice. In addition to the three retention interval conditions, a simultaneous condition is included in which the sample stimulus remains present after the observing touch and while the choice stimuli are presented. A session consists of 10 trials at each retention interval (plus 10 simultaneous trials) presented in randomly intermixed fashion for a total of 40 trials. Monkeys typically complete the DMS session within 40 minutes. One animal (#325) was not trained to final testing contingencies on DMS, thus nicotine challenge was omitted for this task in this monkey and for the mecamylamine study, this animal was tested on simultaneous, 2 sec and 60 sec trials only. The software utilizes 469 shapes and 7 colors to ensure that discriminations are unique for approximately 120,000 trials. Performance accuracy is measured as a proportion of correct choices to all choices (i.e., both sample and choice omission errors are excluded from the accuracy calculation).
Self-Ordered Spatial Search (SOSS)
In each trial of the SOSS task, two, four, six or eight small colored rectangles (boxes) are displayed on the screen in positions randomly allocated from 16 possible locations. The animal must touch a box within 30 seconds of stimulus onset. After each successful touch, the color of the touched box is briefly (100 ms) changed and then the screen is blanked and a reinforcer is delivered. After a 2 second delay, the boxes are re-displayed and the animal must touch a box which has not previously been touched in the trial. The trial is completed when the animal has either touched all boxes without a repetition (correct), touched a box that had previously been selected in that trial (error) or failed to touch a box within 30 seconds of stimulus presentation (omission). Errors and omissions are followed by a tone and a 4 second screen blank. After an inter-trial interval of 5 seconds, another trial is presented with stimuli in new (randomly allocated) positions. A session consisted of 40 trials grouped into 8 blocks by trial type as follows: 5 (2 boxes), 5 (4 boxes), 5 (6 boxes), 5 (8 boxes), 5 (4 boxes), 5 (6 boxes), 5 (8 boxes), 5 (2 boxes). Monkeys typically complete the SOSS session within 20–30 minutes. Accuracy scores are calculated for each trial type by dividing the number of correctly completed trials by the number of trials in which there was at least one response (i.e., errors of omission are excluded from the calculation). One animal (#333) was not trained on SOSS, thus nicotine challenge was omitted for this task in this monkey.
Visuo-Spatial Paired Associates Learning (vsPAL)
The vsPAL task involves learning to associate visual patterns with specific spatial locations on a trial by trial basis. To begin a trial, one sample stimulus is presented in one of four possible target locations (center of the left, right, top or bottom edge of the screen) and the animal must make an observing touch to its location within 30 sec. For the more difficult trials, second, third and/or fourth sample stimuli are presented (a 0.5 sec screen blank follows each observing response) in unique locations prior to the choice phase of the trial. After each sample stimulus has been presented, and following a 1 sec screen blank, one of the sample stimuli (pseudo-randomly selected) is simultaneously presented in 2–4 target locations (choice presentation).The animal is then required to touch the target location in which that stimulus was originally presented to obtain a reinforcer pellet. The next choice is then presented following a 0.5 sec screen blank. Touches directed to the stimulus in an incorrect location (error) or a failure to touch within 30 sec (omission) instantiate an additional 4 sec screen blank prior to presentation of the subsequent choice. A successful trial completion requires the accurate selection of each of the 1–4 stimulus-location associations presented in the sample phase. If a subject fails to successfully complete a given trial on the first attempt, the same set of stimulus-location associations are presented again, in a new sample- and choice-order. Animals are allowed up to 5 additional attempts to successfully complete the set of stimulus-location associations in a given trial. If the subject does not succeed after 6 total attempts the trial is terminated and a next trial is initiated, (i.e., a new set of stimulus-location associations is presented). Task performance is measured by the initial-attempt trial completion success (the proportion of trials successfully completed on the first attempt) and the overall trial completion success (the proportion of trials successfully completed within 6 attempts). Each session consisted of 35 trials blocked as follows: 5 × 1-stimulus trials, 10 × 2-, 3- and 4-stimuli trials which monkeys typically complete within 40–50 minutes. The number of choice locations was identical to the number of stimuli in each trial type except for 1-stimulus trials in which 3 choice locations are presented.
Reaction Time (RT)
For the RT task, a response lever (BRS/LVE, Laurel, MD) is mounted below the monitor and in front of the transport cage. For each trial a grid of five circles, or target locations, connected by lines is presented in white on the screen. The monkey initiates the trial by holding down the lever. After a pseudorandomly-variable delay lasting between 0.75 and 2.5 seconds, a yellow circle appears within one of the five target locations and is removed after 20, 100 or 1000 msec. Touching the appropriate circle within 2 seconds results in reinforcer delivery. A session consists of 45 trials evenly divided in terms of the 5 target locations as well as the 3 stimulus durations. Animals typically complete the RT session within 7 minutes. The interval between target onset and the monkey's release of the lever (release latency) and the interval between target onset and the monkey's touch of the target location (response latency) is recorded in milliseconds. The interval required to move from lever to the target location (movement time) is calculated by subtracting the release latency from the response latency. One animal (#320) was not trained to final testing contingencies on RT for the nicotine challenge but participated in the mecamylamine study. One animal (#302) failed to perform RT in a consistent fashion prior to the initiation of the mecamylamine study and thus this task was not evaluated in this monkey.
Progressive-Ratio (PR) Schedule of Reinforcement
In the PR task a large colored rectangle is presented in the center of the screen and the animal must touch the rectangle for reinforcer delivery. The response requirement starts at 1 and increments by arithmetic progression within blocks of 8 reinforcers and by geometric progression between blocks of 8 (i.e., the first successive 8 ratios increase by 1, the second successive 8 increase by 2, the third successive 8 increase by 4, etc.) The session is terminated after 10 minutes, or earlier if 3 minutes elapse following a response. The interval between session start and the last response emitted is recorded as the "time-to-last-response" measure. The last completed response ratio, the number of reinforcers earned, the total number of responses made and the response rate are also recorded.
Bimanual Motor Skill Task (BMS)
A transparent polycarbonate board drilled with 15 holes is filled with raisins and mounted perpendicular to the door of the transport cage. The hole diameter is such that for efficient retrieval of raisins, the animal must push the raisin partially out of the hole with one finger before retrieving it. With training, animals universally adopt a strategy of pushing the raisin with one hand while retrieving it with the other hand, thus entailing bimanual dexterity. The time required to retrieve all 15 raisins is recorded by stopwatch.
Acute Drug Challenge Procedure
The challenge studies were initiated in monkeys trained to perform the battery tasks in a standard 3-day alternation in which the following combinations of battery tests were completed on sequential days: PR/SOSS/BMS one day, DMS/RT/BMS on the following day and either PR/vsPAL/BMS or vsPAL/RT/BMS on the third day. During the drug challenge studies, non-injection baseline sessions were administered twice (Mon, Wed), drug sessions were administered twice (Tue, Fri) and a vehicle (physiological saline) session was administered once (Thur) per week. The behavioral testing and drug challenge schedule is outlined in Table 1. To summarize, each task was evaluated at least twice under baseline conditions, twice under drug challenge and once following vehicle administration within each 3 week period. Prior to initiating a challenge study, vehicle was injected three days per week (Tue, Thur, Fri) for a minimum of two weeks or until performance following injections was equivalent to non-injection performance. Doses of nicotine bitartrate (3.2–56 μg/kg, i.m.; 15 min prior to session; doses expressed as the salt) or mecamylamine HCl (0.32, 1.0, 1.78 mg/kg, i.m.; 15 min prior to session; doses expressed as the salt) were evaluated twice for each SOSS, DMS or PAL task combination in an ascending-descending order per combination (each of the two “vsPAL” combinations, i.e., PR/vsPAL/BMS and vsPAL/RT/BMS, was evaluated once at each dose). Each compound was dissolved in physiological saline to obtain a constant injection volume of 0.1 ml/kg. Thus the effect of a given dose was double-determined for the memory tasks, triple determined for PR and RT and quadruple-determined for BMS. The mean performance of an individual for all sessions run under a given treatment condition was used for all analyses, including “best dose”. The nicotine and mecamylamine studies were conducted sequentially with a minimum inter-study interval of 6 weeks. Some individual monkeys were not trained to final testing contingencies on all tasks and one animal was not available for the mecamylamine study (detailed above), thus the number of animals contributing to each analysis ranges from N = 4 to N = 6.
TABLE 1. Schedule for drug challenge study.
Schedules such as this were used to determine the effects of nicotine and mecamylamine (individual animals were at different points in the task rotation upon initiation of the drug challenges). Animals completed one of three memory tasks (vsPAL, DMS, SOSS), and either PR or RT on alternating days. The BMS task was completed at the end of each session. Thus the behavioral schedule repeated every 3 weeks. Drug challenges were performed Tue and Fri in an ascending-descending order for each memory task combination. Only the ascending schedule is outlined here for nicotine however for mecamylamine Doses 4–6 reflect the three doses in descending order. DMS, Delayed Match to Sample; RT, Reaction Time; BMS, Bimanual Motor Skill; vsPAL, visuo-spatial Paired Associates Learning; SOSS, Self-Ordered Spatial Search.
| Monday | Tuesday | Wednesday | Thursday | Friday | |
|---|---|---|---|---|---|
| Week 1 | DMS/RT/BMS
Baseline |
vsPAL/RT/BMS
Dose 1 |
PR/SOSS/BMS
Baseline |
DMS/RT/BMS
Vehicle |
PR/vsPAL/BMS
Dose 2 |
| Week 2 | PR/SOSS/BMS
Baseline |
DMS/RT/BMS
Dose 1 |
vsPAL/RT/BMS
Baseline |
PR/SOSS/BMS
Vehicle |
DMS/RT/BMS
Dose 2 |
| Week 3 | PR/vsPAL/BMS
Baseline |
PR/SOSS/BMS
Dose 1 |
DMS/RT/BMS
Baseline |
vsPAL/RT/BMS
Vehicle |
PR/SOSS/BMS
Dose 2 |
| Week 4 | DMS/RT/BMS
Baseline |
vsPAL/RT/BMS
Dose 3 |
PR/SOSS/BMS
Baseline |
DMS/RT/BMS
Vehicle |
PR/vsPAL/BMS
Dose 4 |
| Week 5 | PR/SOSS/BMS
Baseline |
DMS/RT/BMS
Dose 3 |
PR/ vsPAL/BMS
Baseline |
PR/SOSS/BMS
Vehicle |
DMS/RT/BMS
Dose 4 |
| Week 6 | vsPAL/RT/BMS
Baseline |
PR/SOSS/BMS
Dose 3 |
DMS/RT/BMS
Baseline |
PR/vsPAL/BMS
Vehicle |
PR/SOSS/BMS
Dose 4 |
| Week 7 | DMS/RT/BMS
Baseline |
vsPAL/RT/BMS
Dose 5 |
PR/SOSS/BMS
Baseline |
DMS/RT/BMS
Vehicle |
PR/vsPAL/BMS
Dose 6 |
| Week 8 | PR/SOSS/BMS
Baseline |
DMS/RT/BMS
Dose 5 |
PR/ vsPAL/BMS
Baseline |
PR/SOSS/BMS
Vehicle |
DMS/RT/BMS
Dose 6 |
| Week 9 | vsPAL/RT/BMS
Baseline |
PR/SOSS/BMS
Dose 5 |
DMS/RT/BMS
Baseline |
PR/vsPAL/BMS
Vehicle |
PR/SOSS/BMS
Dose 6 |
Data Analysis
Nicotine Challenge
The data for each task were analyzed in two ways. One set of randomized block analysis of variance (ANOVA) tests were conducted including repeated measures factors of trial difficulty (DMS, retention interval; SOSS, number of boxes per trial; vsPAL, number of stimuli per trial), and of treatment condition (baseline, vehicle, nicotine doses) to determine if any significant group effects were detected at a given dose of nicotine. A second set of analyses were also conducted which included repeated measures factors of trial difficulty and a treatment condition factor including only the vehicle condition and an individually-determined most-efficacious, or "best dose", of nicotine. Similar "best dose" analyses have been employed previously to demonstrate improved performance on memory, and other cognitive, tasks in monkeys treated with putative cognitive enhancing compounds including nAChR ligands (Arnsten and Cai 1993; Arnsten and Contant 1992; Arnsten and Goldman-Rakic 1990; Buccafusco and Jackson 1991; Elrod et al. 1988; Franowicz and Arnsten 1998; Schneider et al. 1999; Terry et al. 1993). Such analyses are of particular use when challenge compounds are expected to consistently produce biphasic dose-response functions (e.g., inverted "U") with significant individual variability in the left-right shift of the curve relative to the mean. Here, the "best dose" was selected for each task for each individual subject based on the maximum improvement observed over vehicle in percent correct choices for 60 and 90 sec delay trials (DMS); percent correct trials for 6-box and 8-box trial (SOSS); initial-attempt trial completion success for 3-stimulus and 4-stimulus trials (vsPAL); release latency for all trial types (RT); number of reinforcers acquired (PR) and retrieval latency (BMS). Statistical analysis of the DMS (choice accuracy), SOSS (trial completion) and vsPAL (trial completion success) data was conducted by randomized block analysis of variance (ANOVA) with repeated measures factors of drug treatment condition and trial difficulty (DMS, retention interval; SOSS, number of boxes per trial; vsPAL, number of stimuli per trial). A third repeated measures factor was included in the vsPAL analysis to compare initial-attempt with overall trial completion success.
Mecamylamine Challenge
Statistical analysis of the vsPAL (trial completion) data was conducted by a randomized block three-way analysis of variance (ANOVA) with repeated measures factors of initial/overall trial completion success, trial difficulty and drug treatment condition. Statistical analysis of the SOSS (trial completion, response latency and strategy score) and DMS (choice accuracy and choice latency) data was conducted by randomized block two-way ANOVA with repeated measures factors of drug treatment condition and trial difficulty (DMS, retention interval; SOSS, number of boxes). The RT (release latency, movement time), PR (the number of reinforcers acquired, the time to the last response, total number of responses, response rate) and BMS (retrieval latency) data were analyzed by repeated measures ANOVA with the single factor of drug treatment condition
General
Post-hoc analyses of any significant main effects or interactions found in the randomized block ANOVAs were conducted with the Tukey-Kramer procedure. The RT (response latency, movement time; collapsed across trial type), PR (the number of reinforcers acquired, the time to last response, response rate) and BMS (retrieval latency) data were analyzed using repeated measures ANOVAs with the single factor of drug treatment condition. Post-hoc analyses of any significant main effects in these 1-way analyses were conducted using the Dunnett procedure with vehicle as the control comparison condition. All analyses were conducted using the GB-STAT statistical software package (v7.0; Dynamic Microsystems, Silver Spring MD) and in all tests the criterion for significance was p < 0.05.
Results
Nicotine Challenge
DMS / SOSS / vsPAL
As with prior studies, performance on each of the memory tasks depended on trial difficulty (Figures 1–3, upper panels). Specifically, there was a significant main effect of retention interval in the DMS task [F3,12 = 30.03, p < 0.05], and of the number of boxes per trial in the SOSS task [F3,12 = 159.66, p < 0.05]. Similarly, the percent of trials completed successfully in vsPAL depended on the number of stimuli per trial [F3,15 = 40.06, p < 0.05] and monkeys exhibited within-trial learning, achieving significantly higher overall-attempt scores in comparison with performance on the initial attempt at a trial [F1,5 = 449.71, p < 0.05]. In the overall dose-response analysis, there were no mean effects of drug treatment condition on DMS, SOSS, or vsPAL performance, as is illustrated in upper panels of Figures 1–3. The monkeys unchallenged performance levels on the remaining tasks (Table 3) were also consistent with observations from prior studies, however no significant effects of drug treatment condition were noted.
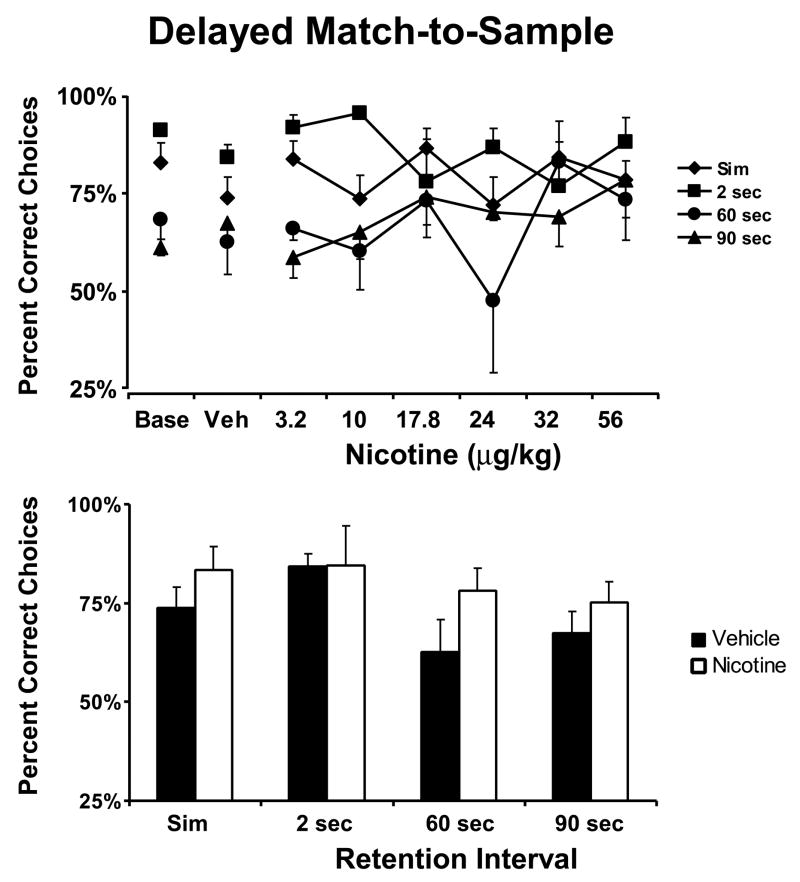
Figure 1. Nicotine Effect on Delayed Match-to-Sample.
The mean (N = 5; ± SEM) effect of acute doses of nicotine on performance of each trial type of the DMS task are presented in the upper panel. The lower panel reflects the effect of the most beneficial dose selected on an individual basis (see text). Nicotine improved overall DMS choice accuracy as indicated by a significant main effect in the best dose analysis. A complete description of the statistical analysis is provided in the Methods. Base indicates noninjection baseline sessions; Veh indicates vehicle injection sessions.
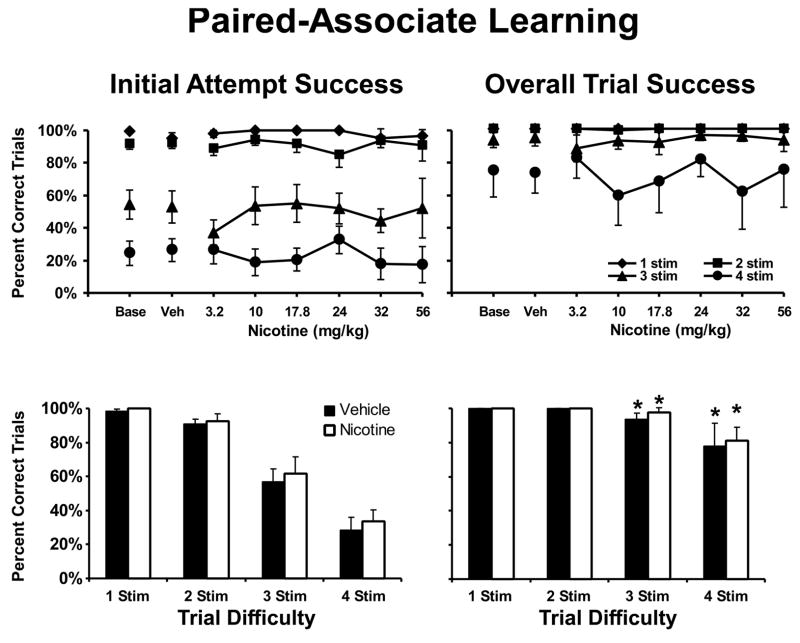
Figure 3. Nicotine Effect on visuo-spatial Paired Associates Learning.
The mean (N =6; ± SEM) effect of acute doses of nicotine on performance of the vsPAL task are presented in the upper panels. The lower panels reflect the effect of the most beneficial dose selected on an individual basis. For each analysis, the trial completion success on the initial attempt is presented in the left panel and the right panel presents the overall trial completion success. Nicotine improved vsPAL trial completion success as indicated by a significant main effect in the best dose analysis. A significant improvement in overall trial completion success compared with the initial attempt completion success is indicated by *. A complete description of the statistical analysis is provided in the Methods. Base indicates noninjection baseline sessions; Veh indicates vehicle injection sessions.
Table 3. Effect of nicotine on performance of the BMS, RT and PR tasks.
Mean scores for the Bimanual Motor Skill (N = 6), Reaction Time (N = 5) and Progressive Ratio (N = 6) tasks are listed; values in parenthesis indicate SEM. No significant group effects of nicotine were observed for these tasks at any dose. Animals did not generally exhibit similar curves at different points of the dose-response function, as with the memory tasks, therefore the best-dose analyses did not identify any consistent effects of nicotine.
| Task | Measure | Treatment Condition | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Vehicle | Nicotine (μg/kg) | |||||||
| 3.2 | 10 | 17.8 | 24 | 32 | 56 | ||||
| BMS | Percent of baseline latency | 20.4 (2.4) | 20.4 (2.6) | 20.5 (2.6) | 19.8 (2.5) | 19.3 (2.4) | 18.6 (2.3) | 18.7 (1.1) | 20.1 (1.9) |
| RT | Release latency (ms) | 302.2 (34.5) | 302.5 (29.9) | 317.2 (34.3) | 299.9 (33.7) | 305.6 (38.2) | 302.3 (35.9) | 255.4 (10.1) | 303.2 (39.1) |
| Movement time (ms) | 475.8 (44.3) | 470.9 (38.7) | 497.2 (48.3) | 481.9 (47.7) | 475.6 (45.5) | 475.1 (40.5) | 419.7 (137.5) | 442.1 (32.6) | |
| PR | Reinforcers acquired | 20.6 (1.8) | 20.3 (1.6) | 20.5 (1.4) | 21.5 (2.2) | 21.1 (2.4) | 20.6 (3.0) | 21.4 (1.2) | 19.4 (0.9) |
| Time to last response (min) | 6.8 (0.8) | 7.0 (0.7) | 6.6 (1.2) | 7.5 (0.6) | 7.1 (1.1) | 6.0 (1.1) | 6.1 (1.0) | 7.1 (0.9) | |
| Response rate (touches/min) | 48.2 (8.2) | 44.2 (6.8) | 40.8 (7.5) | 48.2 (12.5) | 49.5 (14.0) | 50.1 (17.6) | 47.2 (7.1) | 34.0 (5.6) | |
The "best dose" analyses, in contrast, demonstrated that nicotine administration significantly improved performance on all three of the memory procedures (Figures 1–3, lower panels); this improvement was observed at different doses for individual animals (see Table 2).
Table 2. Most efficacious nicotine doses for each task.
The doses of nicotine which resulted in the greatest improvement in performance are listed for individual subjects.
| Monkey | DMS | SOSS | PAL | RT | PR | BMS |
|---|---|---|---|---|---|---|
| Nicotine (μg/kg) | ||||||
| 289 | 24.0 | 3.2 | 17.8 | 10.0 | 3.2 | 24.0 |
| 302 | 56.0 | 56.0 | 17.8 | 24.0 | 24.0 | 24.0 |
| 320 | 56.0 | 24.0 | 32.0 | 32.0 | 24.0 | |
| 325 | 3.2 | 32.0 | 24.0 | 17.8 | 10.0 | 24.0 |
| 329 | 56.0 | 24.0 | 24.0 | 24.0 | 32.0 | 24.0 |
| 333 | 32.0 | 24.0 | 24.0 | 10.0 | 24.0 | |
The most efficacious dose of nicotine significantly improved choice accuracy in the DMS procedure (Figure 1; lower panel), as confirmed by a significant main effect of drug condition [F1,4 = 9.36, p < 0.05]. Choice accuracy did not depend on retention interval in this analysis nor was any significant interaction between the effects of retention interval and drug condition observed.
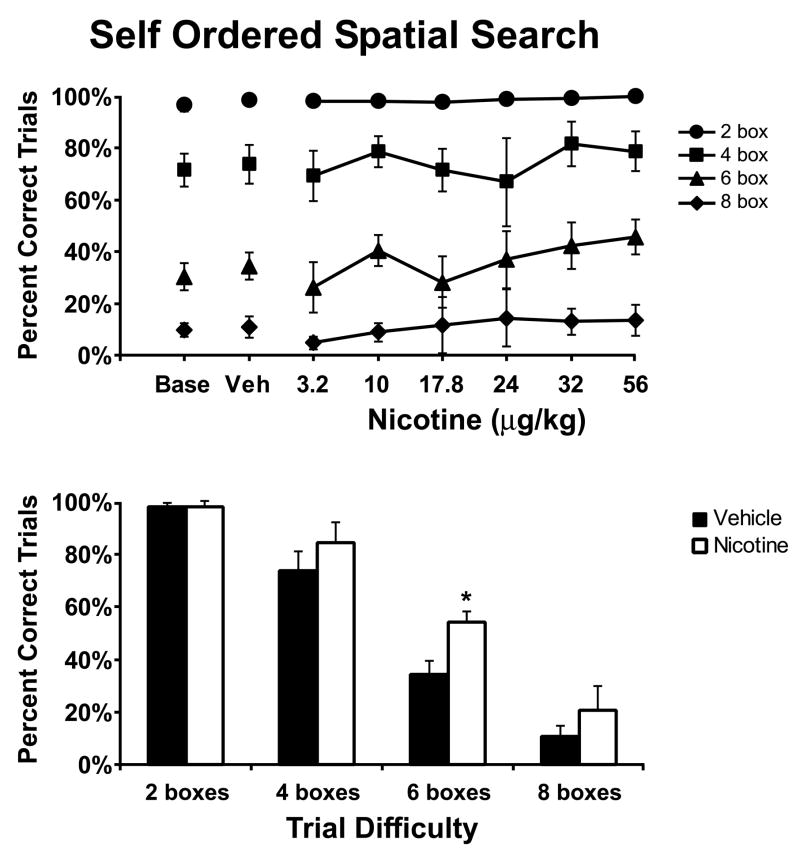
The most efficacious dose of nicotine also significantly improved the percent of trials successfully completed in the SOSS task (Figure 2; lower panel) as confirmed by a main effect of drug condition [F1,5 = 16.99, p < 0.05]. Performance depended on the number of boxes per trial (significant main effect of trial difficulty [F3,15 = 131.89, p < 0.05]) and nicotine was more likely to improve performance on the more-difficult trials (significant interaction between trial difficulty and drug condition [F3,15 = 5.09, p < 0.05]). Post hoc analysis of this interaction (including all pairwise comparisons) confirmed a significant nicotine-related improvement relative to vehicle for performance for 6 box trials; a trend for similar improvement on 8 box trials failed to reach significance, possibly due to a performance floor effect. Overall however, this dose × difficulty related effect of the drug suggests a highly specific effect on the spatial working memory aspects of the task.
Figure 2. Nicotine Effect on Self-Ordered Spatial Search.
The mean (N = 5; ± SEM) effect of acute doses of nicotine on performance of the SOSS task are presented in the upper panel. The lower panel reflects the effect of the most beneficial dose selected on an individual basis. Nicotine improved overall SOSS trial completion success as indicated by a significant main effect in the best dose analysis. A significant difference between vehicle and nicotine conditions for a given trial type is indicated by *. A complete description of the statistical analysis is provided in the Methods. Base indicates noninjection baseline sessions; Veh indicates vehicle injection sessions.
Similar to the outcome for the DMS and SOSS procedures, the most efficacious dose of nicotine significantly improved performance on vsPAL (Figure 3; lower panel) as confirmed by a significant main effect of drug condition [F1,5 = 9.52, p < 0.05]. Performance on this task depended on trial difficulty as confirmed by a significant main effect of trial type [F3,15 = 31.76, p < 0.05] and monkeys were able to improve their performance on a given trial, achieving significantly greater overall trial-completion scores in comparison with the initial-attempt completion scores [F1,5 = 243.62, p < 0.05]. The only significant interaction for this three-way ANOVA was between trial difficulty and the initial attempt/overall trial-completion factor [F3,15 = 25.03, p < 0.05]. This interaction reflects the fact that monkeys' initial attempt was near perfect for 1-stimulus and 2-stimuli trials and thus significant improvements with practice were only be observed for the more-difficult trials as confirmed by the post hoc analysis. In summary, nicotine produced improvements in the performance of DMS, SOSS and vsPAL confirming a general mnemonic enhancement.
RT / PR / BMS
In contrast to the effects on memory task performance, even the most-efficacious doses of nicotine failed to produce any significant improvement in performance on the remaining battery tasks. Specifically, the mean release latencies and movement times generated in the RT procedure were comparable under vehicle and nicotine conditions and the mean number of reinforcers acquired, time to last response and response rate in the PR task were unchanged by nicotine. Although the mean latency to retrieve all 15 raisins in the BMS task was shorter following the most efficacious dose of nicotine (18.6 sec; SEM 2.4) in comparison with the vehicle condition (20.4 sec; SEM 2.6), this effect was not statistically reliable [F1,5 = 5.14, p = 0.07]. Thus the beneficial effects of nicotine were limited to the tasks which are designed to access mnemonic cognitive domains.
Mecamylamine Challenge
vsPAL
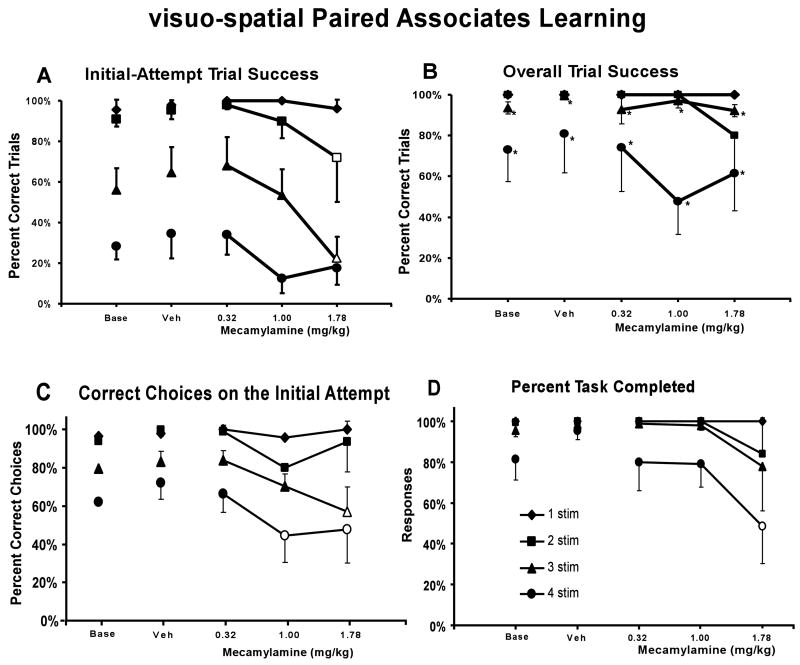
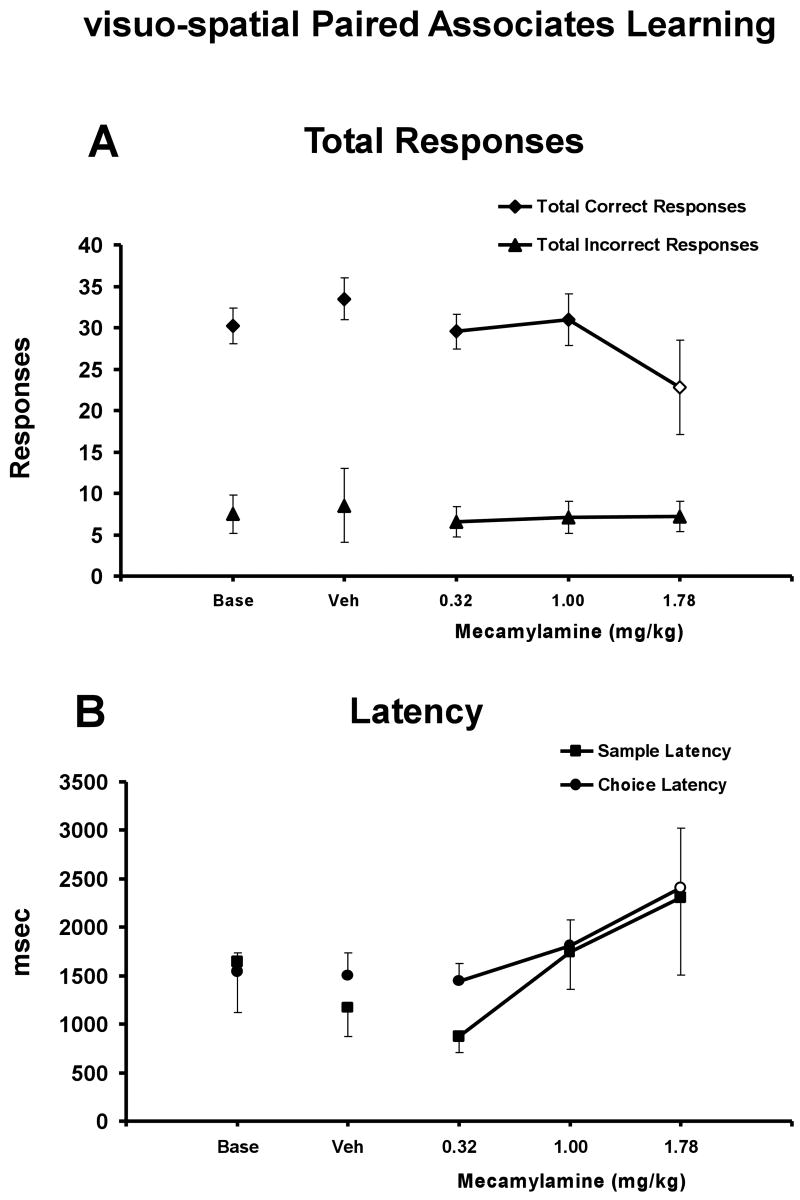
The monkeys' performance depended on trial difficulty and improved with repeated attempts at a given trial (main effects of completion measure, F1,4 =128.29, p < .05; trial type, F3,12 = 18.60, p < .05), as illustrated in Figure 4. Post-hoc analyses determined that overall attempt trial success was signifcantly higher than initial-attempt trial success for a given drug treatment condition for the 3- and 4-stimulus trials (Figures 4A and B). Acute challenge with mecamylamine interfered with trial completion success (main effect of drug condition; (F4,16 = 3.36; p < .05) as is illustrated in Figure 4. This detrimental effect of mecamylamine challenge impacted initial-attempt trial completion success (Figure 4A), while not greatly affecting overall trial completion success (Figure 4B) (interaction between completion measure and drug condition; F4,16 = 9.15; p < .05). Post hoc exploration of the main effects confirmed that for the 3-stimulus trial condition, the 1.78 mg/kg dose of mecamylamine significantly impaired initial-attempt success compared to the vehicle and all other drug treatments (Figure 4A). Furthermore, the 1.78 mg/kg dose of mecamylamine significantly impaired initial-attempt success relative to performance after the 0.32 mg/kg dose of mecamylamine for the 2-stimulus trial condition. The lack of a mecamylamine effect on the initial-attempt success for 4-stimuli trials is most likely related to a floor effect at this condition. Specifically, the percentage of correct initial responses for the 4-stimulus baseline condition was 28 ± 7 % and the highest dose of mecamylmine resulted in values of 18 ± 9 %. In particular, one subject had very few correct trials on the initial attempt under baseline or vehicle conditions. Thus, under these low baseline levels of performance it may be difficult to detect differences in performance due to drug treatment or other insult. This interpretation is supported by the analysis of the percent of correct choices on the initial attempt (Figure 4C). A two-way ANOVA confirmed a significant main effect of task difficulty [F3,12 = 34.95; p < .05] and treatment condition [F4,16 = 4.56; p < .05], as well as the interaction of the two factors [F12,48 = 3.06; p < .05] on the percent of correct choices on the initial attempt. Post hoc analysis confirmed that the 1.78 mg/kg dose of mecamylamine decreased the percent of correct choices on the initial attempt compared to the vehicle condition and the 0.32 mg/kg dose of mecamylamine for the 3-stimulus trial condition, and that each of the 1.0 and 1.78 mg/kg doses of mecamylamine reduced the percent of correct choices on the intitial attempt compared to the vehicle conditon for the 4-stimulus trial condition.
Figure 4. Mecamylamine Effect on visuo-spatial Paired Associates Learning.
The mean (N = 5; ± SEM) proportion of successfully completed trials (A: initial-attempt success, B: overall trial completion success), the percent of correct choices on the initial attempt (C), and the percent task completed (D) are presented by trial type (i.e., difficulty) for monkeys challenged acutely with doses of mecamylamine. In Panel A, the open symbols indicate significant differences from the 0.32 mg/kg dose for the 2-stimulus trial and from all other drug treatments for the 3-stimulus trial. In Panel B, * indicates significant differences between initial-attempt success and overall trial completion success for a given drug treatment for the 3- and 4-stimulus trials. In Panel C, the open symbols indicate significant differences from the vehicle condition and 0.32 mg/kg dose for the 3-stimuls trial and from the vehicle condition for the 4-stimulus trial. In Panel D, the open symbol indicates a significant difference from the vehicle condition for the 4-stimulus trial. A complete description of the statistical analysis is provided in the Methods. Base indicates noninjection baseline sessions; Veh indicates vehicle injection sessions.
There was a significant interaction between task difficulty and mecamylamine on the percent of task completed (F12,48 = 2.21; p < 0.05), and post hoc analysis confirmed that the 1.78 mg/kg dose of mecamylamine reduced the percent of task completed compared to the vehicle condition for the 4-stimulus trial condition (Figure 4D). Furthermore, there was a significant interaction between task difficulty and mecamylamine on the number of total correct responses (F12,48 = 2.02; p < .05), and post hoc analysis confirmed that the 1.78 mg/kg dose of mecamylamine reduced the number of correct responses compared to vehicle for the 4-stimulus trial condition (Figure 5A). Finally, mecamylamine produced a significant increase in choice latency [F4,64 = 6.40; p < .05], without an effect on sample latency. Post hoc analysis confirmed that choice latency was significantly increased by the 1.78 mg/kg dose of mecamylamine compared to the baseline, vehicle and 0.32 mg/kg drug treatment conditions (Figure 5B).
Figure 5. Mecamylamine Effect on Ancillary vsPAL Measures.
Group means (N = 5; ± SEM) are presented for the total number of correct and incorrect responses (Panel A) and sample and choice latency (Panel B) collapsed across stimulus trials. In Panel A, the open symbol indicates a significant difference from the vehicle condition for total correct responses and in Panel B, the open symbol indicates a significant difference from the vehicle condition and the 0.32 mg/kg dose for choice latency. A complete description of the statistical analysis is provided in the Methods. Base indicates noninjection baseline sessions; Veh indicates vehicle injection sessions.
DMS
No significant effects of treatment condition or retention interval were observed on choice accuracy in the DMS task (Table 3). There was a significant interaction between drug dose and retention interval [F12,48 = 2.37; p < 0.05] for choice accuracy for the DMS task, however, post hoc analysis did not confirm significant differences between relevant comparisons and no systematic relationships between drug dose and difficulty were apparent. There was a significant main effect of retention interval [F3,12 = 23.48; p < 0.05], but not of treatment condition on choice latency (Table 3). Post hoc analysis of the retention interval effect confirmed that choice latency was greater for the 60 and 90 sec retention intervals compared to the simultaneous and 2 sec retention intervals.
SOSS
Successful trial completion in the SOSS task depended on task difficulty [F3,9 = 158.59; p < 0.05], but was unimpaired by the administration of mecamylamine (Table 3). Post hoc analysis of the task difficulty effect confirmed that trial completion success was lower for the 6 and 8 box conditions compared to either the 2 or 4 box conditions. There were no significant effects of trial difficulty or treatment condition on latency.
RT
No significant effects of mecamylamine or stimulus duration were observed on reaction time, however, there was a trend for mecamylamine to increase release latency (Table 3). Monkeys achieved a mean release latency of 310 milliseconds (SEM = 41 milliseconds) and movement time of 489 milliseconds (SEM = 94 milliseconds) under baseline conditions.
PR
Mecamylamine had no significant effects on PR performance (Table 3).
BMS
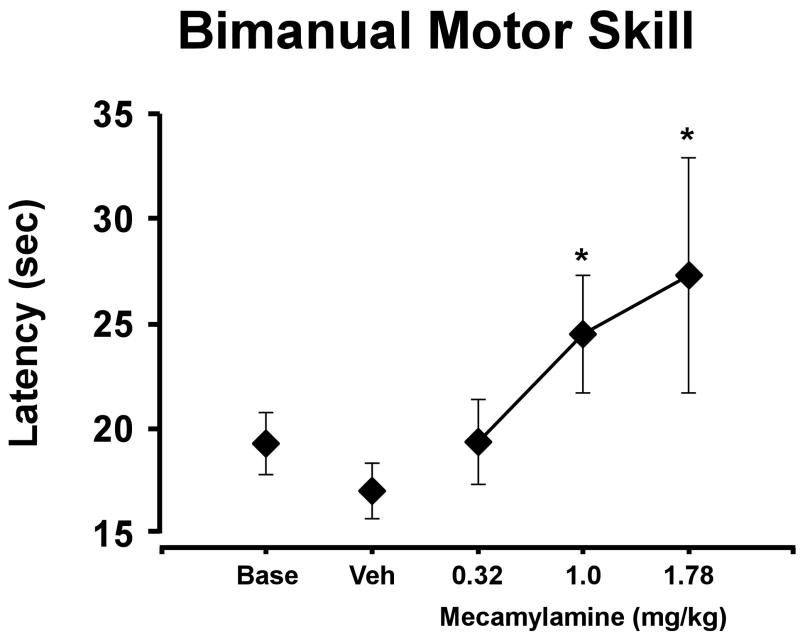
Mecamylamine slowed raisin-retrieval performance as illustrated in Figure 6 [F3,12 = 5.027; p < 0.05]. Post hoc analysis confirmed that the time required to retrieve all 15 raisins after the 1.0 and 1.78 mg/kg doses of mecamylamine was significantly longer than in the vehicle condition. This result indicates that mecamylamine interferes with fine motor coordination.
Figure 6. Mecamylamine Effect on BMS Performance.
The mean (±SEM; N=5) time required to retrieve 15 raisins was significantly slowed by mecamylamine. * indicates a significant difference from vehicle performance. A complete description of the statistical analysis is provided in the Methods. Base indicates noninjection baseline sessions; Veh indicates vehicle injection sessions.
Discussion
The present study demonstrates that nicotine can significantly improve the performance of young adult monkeys on tasks assessing multiple mnemonic domains including spatial working memory, visual recognition memory and visuo-spatial associative learning. This confirms and extends previous observations of improved DMS performance in either young adult or aged monkeys following nicotine administration (Buccafusco and Jackson 1991; Buccafusco et al. 1999; Elrod et al. 1988; Hironaka et al. 1992). Although perhaps unsurprising given the wide distribution of nAChRs in the primate brain (see (Court et al. 2000a) for review) the present results clearly demonstrate that the memory enhancing effects of nicotine extend beyond visual recognition memory for stimulus hue in the macaque. These data provide support for a hypothesis that nAChR signaling may be critically involved in lower-level, or general, mechanisms which support the storage of information. In contrast, mecamylamine impaired visuo-spatial associative learning while leaving other memory domains unaffected. Interestingly, preliminary work with aged rhesus macaques suggests that vsPAL acquisition is more impaired than SOSS or DMS acquisition in comparison with young monkeys (Taffe et al. 2003a). In addition, mecamylamine impaired fine motor performance while leaving ballistic motor performance intact. Thus these data suggest that tonic activity of nAChRs may be important for some but not all aspects of memory.
Nicotine significantly improved performance on three memory tasks featuring divergent cognitive demands. Visual recognition memory was affected since choice accuracy in the DMS task was increased, albeit in a manner independent of retention interval. This pattern of results is slightly different from prior studies which employed the two-color alternation version of DMS in which nicotine preferentially improved performance on longer retention intervals (Buccafusco and Jackson 1991; Elrod et al. 1988); but also see (Buccafusco et al. 1999). Nevertheless, the present result confirm that nicotine can improve visual recognition memory in a (nearly) unique-trials version of DMS (presumed to be less subject to proactive interference) in addition to prior evidence that it can improve performance on a limited-stimulus-set version of DMS (which features significant proactive interference and working memory demand). Performance on SOSS was also improved significantly with minimal evidence for a specific effect on the more-difficult trial types. Finally, nicotine significantly increased the proportion of successfully completed trials on the vsPAL procedure, however this effect was not specific for the incremental acquisition aspects of the task (i.e., the overall-success versus the initial-attempt success differential was not affected by nicotine). The degree of improvement on vsPAL was modest in comparison with the effects noted for the DMS and SOSS procedures. Therefore, given evidence that the vsPAL task is the most sensitive of these three tests in the detection of AD-related cognitive disruption in questionably demented human populations (Fowler et al. 2002; Swainson et al. 2001), the present findings suggest that nAChR agonists may not be the best pharmacotherapeutic for cognitive improvement early in disease course. Such interpretations should be qualified, of course, since the present work was conducted in young rather than aged animals. Still it should be noted that where behavioral effects of nicotinic ligands have been evaluated in young and old monkeys in the same assays, results are qualitatively similar. The present nicotine data do, however, stand somewhat in contrast to the effects of the nAChR antagonist mecamylamine which significantly impaired vsPAL performance without affecting DMS and SOSS performance.
The present data may possibly be interpreted in terms of attentional effects. Indeed it may be impossible to design a memory storage task which does not include an attentional requirement. In specific terms, Buccafusco and colleagues have shown that nicotine and other nicotinic agonists make monkeys more resistant to accuracy-decreasing effects of distracting stimuli presented during the retention interval in the DMS procedure (Prendergast et al. 1998; Terry et al. 2002) although they have also shown that performance on the standard version of the task is enhanced. Marrocco and colleagues have shown that nicotine enhances disengagement from an invalid cue in the Posner task (Witte et al. 1997), whereas scopolamine slows attentional orientation to valid or invalid spatial cues (Davidson et al. 1999). Similarly, nicotine also enhances disengagement from invalid cues (Phillips et al. 2000) and enhances sustained attention (Bizarro and Stolerman 2003; Hahn et al. 2003; Mirza and Stolerman 1998) in the rat. Sahakian and colleagues have shown that subcutaneous nicotine can improve measures of attention in Alzheimer’s Disease (AD) patients although unfortunately they do not report other CANTAB measures in this study (Jones et al. 1992). In related work (Sahakian et al. 1993) they show that the cholinesterase inhibitor tetrahydroaminoacradine improves AD patients’ release latency and movement time in an analog of our RT procedure without affecting performance on analogs of our DMS and vsPAL procedures. Interestingly while some studies in human volunteers report improved sustained attention with nicotine (Mumenthaler et al. 2003), others report improved memory performance in the absence of attentional improvement (Howe and Price 2001; Min et al. 2001). Similar to this latter work, nicotine failed to significantly improve the battery measures which are most consistent with vigilance and sustained attention, namely release latency in the RT procedure and the time of last response in the PR procedure. Thus neither a strong hypothesis of non-attentional mnemonic effects, nor a strong hypothesis of non-mnemonic attentional effects is specifically supported by the data obtained.
Mecamylamine produced a dose × difficulty dependent impairment of performance as measured by initial-attempt, but not overall completion success, in the vsPAL task. For example following the 1.78 mg/kg dose, performance on the 1-stimulus trial was not significantly decreased, whereas performance on the 2- and 3-stimulus trials was significantly impaired for initial-attempt success. Mecamylamine also produced a dose × difficulty dependent impairment of performance as measured by percent correct choices on the initial attempt in the vsPAL task. The dose × difficulty effect for this measure was more consistent than for initial-attempt trial success for the vsPAL task, which may have been due to a floor effect for the 4-stimulus condition for the initial attempt trial success measure. The difficulty-dependent dose-effect suggests a highly specific effect of mecamylamine on the memory load aspects of the task. In contrast, overall trial completion success was unaffected by mecamylamine as monkeys were able to approximate baseline performance following all doses. This suggests that incremental acquisition, or learning, in this task does not depend on nAChR signaling. These results demonstrate that mecamylamine interferes with the formation of a stimulus-location association (initial attempt success) and not the incremental strengthening of that association (overall success). Mecamylamine also reduced task persistence as measured by percent task completed, however, a significant impairment was only observed at the highest dose of mecamylamine for the 4-stimulus condition. Thus nonspecific effects are unlikely to explain the dose-dependent pattern of effects.
One available study on the mnemonic effects of mecamylamine in young adult monkeys reported a significant decrement in DMS performance limited to short retention interval trials (Elrod et al. 1988). In the present study there was a numerical reduction in DMS accuracy following the highest dose of mecamylamine which was most pronounced for the short-delay and simultaneous trials (Table 4). Since Elrod and colleagues failed to control for multiple comparisons in their statistical analysis (Elrod et al. 1988) it is possible that this difference simply reflects a difference in the selected analysis technique. As with any negative result it is possible that our finding reflects a Type II error. Thus the possibility that the vsPAL task is only most-sensitive to, rather than selective for, mecamylamine effects cannot be ruled out. Additional cautions in interpreting the effects of mecamylamine challenge are warranted. While the primary mechanism of action in the CNS is a blockade of the open nAChR ionophore, evidence suggests that mecamylamine can affect activity of a number of other neurotransmitter systems (see Young et al. 2001 for review). With particular relevance to mnemonic function mecamylamine may inhibit function of the N-methyl-D-aspartate (NMDA) glutamate receptor. Thus, although most non-nAChR effects of mecamylamine appear to occur at higher doses than employed here it is always possible that the observed effects were not a result of nAChR blockade. Finally, it should be noted that mecamylamine has been shown to improve DMS performance of aged monkeys (Terry et al. 1999), albeit at doses much lower (i.e., 0.01–0.25 mg/kg) than used here.
Table 4. Effect of mecamylamine on performance of the DMS, SOSS, RT and PR tasks.
Mean (± SEM) performance of animals on each of the Delayed Match-to-Sample, Self Ordered Spatial Search, Reaction Time and Progressive Ratio tasks is presented for baseline conditions and following challenge with vehicle or three doses of mecamylamine.
| Task | Trial Difficulty | Baseline | Vehicle | 0.32 mg/kg | 1.00 mg/kg | 1.78 mg/kg |
|---|---|---|---|---|---|---|
| DMS Accuracy (% Correct Choices) | Simultaneous | 72 ± 11 | 76 ± 8 | 77 ± 8 | 76 ± 13 | 59 ± 9 |
| 2 seconds | 80 ± 12 | 80 ± 11 | 76 ± 8 | 75 ± 14 | 73 ± 11 | |
| 60 seconds | 56 ± 11 | 71 ± 12 | 57 ± 12 | 67 ± 14 | 70 ± 17 | |
| 90 seconds | 54 ± 8 | 66 ± 4 | 63 ± 6 | 50 ± 14 | 62 ± 17 | |
| DMS Latency (msec) | Simultaneous | 1918 ± 236 | 1970 ± 288 | 2048 ± 265 | 2082 ± 314 | 2863 ± 979 |
| 2 seconds | 2060 ± 279 | 2082 ± 237 | 2959 ± 998 | 2404 ± 424 | 2170 ± 218 | |
| 60 seconds | 3775 ± 255 | 5522 ± 1269 | 4812 ± 770 | 4436 ± 449 | 4021 ± 388 | |
| 90 seconds | 4844 ± 839 | 3774 ± 833 | 4063 ± 329 | 6775 ± 1950 | 5732 ± 2577 | |
| SOSS Accuracy (% Correct Trials) | 2 box | 99 ± 1 | 99 ± 1 | 100 ± 0 | 98 ± 3 | 98 ± 3 |
| 4 box | 82 ± 5 | 83 ± 3 | 84 ± 7 | 88 ± 5 | 76 ± 15 | |
| 6 box | 42 ± 8 | 38 ± 6 | 26 ± 9 | 45 ± 10 | 21 ± 16 | |
| 8 box | 19 ± 7 | 18 ± 7 | 16 ± 5 | 6 ± 7 | 6 ± 7 | |
| SOSS Latency (msec) | 2 box | 1125 ± 203 | 1092 ± 150 | 1435 ± 485 | 1121 ± 206 | 1704 ± 365 |
| 4 box | 763 ± 83 | 848 ± 109 | 870 ± 110 | 795 ± 99 | 1485 ± 646 | |
| 6 box | 842 ± 88 | 907 ± 87 | 902 ± 141 | 877 ± 135 | 1627 ± 625 | |
| RT Release Latency (msec) | 310 ± 41 | 323 ± 35 | 314 ± 39 | 336 ± 33 | 338 ± 41 | |
| RT Movement Time (msec) | 489 ± 94 | 505 ± 82 | 528 ± 117 | 489 ± 74 | 501 ± 117 | |
| PR (# of Reinforcers) | 21 ± 2 | 22 ± 3 | 22 ± 3 | 25 ± 3 | 22 ± 3 |
Choice latency was affected by mecamylamine in the vsPAL task, however, latencies in the other memory tasks (SOSS, DMS) and RT were unaffected. These observations suggest that the increased choice latency observed with mecamylamine in the vsPAL task was not due to non-specific motor effects, but rather may reflect potential shifts in the speed/accuracy tradeoff bias (Osman et al. 2000). Specifically, the significant increase in choice latency at the highest dose of mecamylamine may reflect an attempt to preserve accuracy by slowing the response speed. Interestingly, Alzheimer’s disease patients have been found to compensate for impairments in information processing by slowing down their response time (Koss et al. 1984) and basal forebrain cholinergic lesions in rats have also been shown to decrease processing capacity and preserve response accuracy at the cost of response latency (Turchi and Sarter 1997). Thus, nAChR signaling may be mechanistically involved in adjustments of speed/accuracy tradeoff bias. The slowing of performance in the BMS task associated with mecamylamine suggests that this compound produces alterations in fine motor coordination. However, this does not explain the aforementioned effects of mecamylamine on vsPAL performance, since mecamylamine produced a dose × difficulty effect on vsPAL performance, no impairment of RT task performance (which is a measure more similar to choice latency for all tasks) and no impairments on other tasks in the test battery. Interestingly a previous report found no effect on mecamylamine on force-lever performance in rhesus monkeys (Preston et al. 1985), also suggesting that the BMS result may illustrate a highly selective motor effect.
The contrast between the relatively specific memory effects of mecamylamine and the general effects of nicotine observed here may be attributable to differing affinities of the compounds for various nAChR subtypes with different regional distributions. Alternately it may point to non-opposing differences between stimulation of a receptor and inhibition of tonic activity. Theoretically, some of these differences may be attributed to pharmacokinetic effects since the halflife of both nicotine and mecamylamine are relatively short thus significant differences in CNS drug levels may have been obtained across the ~60 min testing sessions. This is somewhat unlikely to explain the present results. First, because of the differing session types and individual differences there is considerable randomization/overlap in the per-task pre-treatment interval for both drug challenges. In the case of the mecamylamine challenge the BMS task run at the end of sessions was significantly affected thus behaviorally active drug levels persisted. Between the three memory tasks, the vsPAL trials are run in order of difficulty and lasts the longest and thus might be expected to be most affected by declining CNS drug levels; here it was the most sensitive. Similar analyses hold true for the nicotine study. In any case, this comparison of nAChR agonist/antagonist effects cautions that interpretation of the behavioral effects of systemic administration of compounds is complex, even when compounds are presumed to affect similar brain circuits. Thus the comprehensive behavioral evaluation of cognition and the rigorous validation of a given pharmacological model of cognitive disruption within a common preclinical model are necessary for the development of potential pharmacotherapies for cognitive enhancement.
Apart from the possible mnemonic benefits of nicotine, the present results also have important implications for aspects of nicotine which are detrimental to health. Nicotine is the major psychoactive ingredient in smoked tobacco and is presumed to be a primary reason for the development of dependence on tobacco. Such dependence remains a significant public health concern with long-term smokers suffering from a variety of health problems including many that are terminal (USCDC 1996), leading to substantial societal costs (Keeler et al. 2002; Schauffler et al. 2001; Stapleton et al. 1999). The observation here that acute doses of nicotine can improve memory in a number of domains presents the possibility that implicit or explicit seeking of such beneficial effects may support use of tobacco, particularly early in the use pattern prior to the development of substantial dependence. Certainly improved memory, learning, sustained attention (or concentration) and speeded motor performance would appear to offer benefits to most occupational and educational endeavors. Thus it is possible that the pan-mnemonic beneficial properties of nicotine (such as were demonstrated here) may help to drive early exposure to tobacco products. Such a possibility is particularly troubling given evidence that use of tobacco in the early- to mid-teen years, a time of substantial pressure for academic success, is associated with increased risk for the development of tobacco dependence (Everett et al. 1999; Giovino 1999).
In conclusion this study demonstrates that nicotine is a broadly acting performance enhancer in monkeys, since it increased accuracy on three separate memory tasks. These tasks span the mnemonic domains of visual recognition memory, spatial working memory and visuo-spatial associative memory thus it is likely that nAChR signaling plays a critical role in many, if not all, types of memory. Since there were no significant effects here on the non-mnemonic tests it is at least possible that nicotine (and possibly other nAChR ligands) may be titrated to improve memory selectively while minimizing nonspecific effects, e.g., on motor function. Thus the present results support the further development of nAChR ligands as broadly acting memory enhancers.
Acknowledgments
This work was supported by USPHS grant DA13390. This is publication #15351-NP from The Scripps Research Institute.
References
- Aigner TG, Mishkin M. The effects of physostigmine and scopolamine on recognition memory in monkeys. Behav Neural Biol. 1986;45:81–87. doi: 10.1016/s0163-1047(86)80008-5. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Cai JX. Postsynaptic alpha-2 receptor stimulation improves memory in aged monkeys: indirect effects of yohimbine versus direct effects of clonidine. Neurobiol Aging. 1993;14:597–603. doi: 10.1016/0197-4580(93)90044-c. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Contant TA. Alpha-2 adrenergic agonists decrease distractibility in aged monkeys performing the delayed response task. Psychopharmacology (Berl) 1992;108:159–169. doi: 10.1007/BF02245302. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Goldman-Rakic PS. Analysis of alpha-2 adrenergic agonist effects on the delayed nonmatch-to-sample performance of aged rhesus monkeys. Neurobiol Aging. 1990;11:583–590. doi: 10.1016/0197-4580(90)90021-q. [DOI] [PubMed] [Google Scholar]
- Aubert I, Araujo DM, Cecyre D, Robitaille Y, Gauthier S, Quirion R. Comparative alterations of nicotinic and muscarinic binding sites in Alzheimer's and Parkinson's diseases. J Neurochem. 1992;58:529–541. doi: 10.1111/j.1471-4159.1992.tb09752.x. [DOI] [PubMed] [Google Scholar]
- Bammer G. Pharmacological investigations of neurotransmitter involvement in passive avoidance responding: a review and some new results. Neurosci Biobehav Rev. 1982;6:247–296. doi: 10.1016/0149-7634(82)90041-0. [DOI] [PubMed] [Google Scholar]
- Bartus RT. On neurodegenerative diseases, models, and treatment strategies: lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp Neurol. 2000;163:495–529. doi: 10.1006/exnr.2000.7397. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Johnson HR. Short-term memory in the rhesus monkey: disruption from the anti-cholinergic scopolamine. Pharmacol Biochem Behav. 1976;5:39–46. doi: 10.1016/0091-3057(76)90286-0. [DOI] [PubMed] [Google Scholar]
- Bizarro L, Stolerman IP. Attentional effects of nicotine and amphetamine in rats at different levels of motivation. Psychopharmacology (Berl) 2003:271–277. doi: 10.1007/s00213-003-1543-6. [DOI] [PubMed] [Google Scholar]
- Bontempi B, Whelan KT, Risbrough VB, Rao TS, Buccafusco JJ, Lloyd GK, Menzaghi F. SIB-1553A, (+/−)-4-[[2-(1-methyl-2-pyrrolidinyl)ethyl]thio]phenol hydrochloride, a subtype-selective ligand for nicotinic acetylcholine receptors with putative cognitive-enhancing properties: effects on working and reference memory performances in aged rodents and nonhuman primates. PG J Pharmacol Exp Ther. 2001;299:297–306. [PubMed] [Google Scholar]
- Brenner DE, Kukull WA, van Belle G, Bowen JD, McCormick WC, Teri L, Larson EB. Relationship between cigarette smoking and Alzheimer's disease in a population-based case-control study. Neurology. 1993;43:293–300. doi: 10.1212/wnl.43.2.293. [DOI] [PubMed] [Google Scholar]
- Briggs CA, Anderson DJ, Brioni JD, Buccafusco JJ, Buckley MJ, Campbell JE, Decker MW, Donnelly-Roberts D, Elliott RL, Gopalakrishnan M, Holladay MW, Hui YH, Jackson WJ, Kim DJ, Marsh KC, O'Neill A, Prendergast MA, Ryther KB, Sullivan JP, Arneric SP. Functional characterization of the novel neuronal nicotinic acetylcholine receptor ligand GTS-21 in vitro and in vivo. Pharmacol Biochem Behav. 1997;57:231–41. doi: 10.1016/s0091-3057(96)00354-1. [DOI] [PubMed] [Google Scholar]
- Broks P, Preston GC, Traub M, Poppleton P, Ward C, Stahl SM. Modelling dementia: effects of scopolamine on memory and attention. Neuropsychologia. 1988;26:685–700. doi: 10.1016/0028-3932(88)90004-8. [DOI] [PubMed] [Google Scholar]
- Buccafusco JJ, Jackson WJ. Beneficial effects of nicotine administered prior to a delayed matching-to-sample task in young and aged monkeys. Neurobiol Aging. 1991;12:233–8. doi: 10.1016/0197-4580(91)90102-p. [DOI] [PubMed] [Google Scholar]
- Buccafusco JJ, Jackson WJ, Jonnala RR, Terry AV., Jr Differential improvement in memory-related task performance with nicotine by aged male and female rhesus monkeys. Behav Pharmacol. 1999;10:681–690. doi: 10.1097/00008877-199911000-00015. [DOI] [PubMed] [Google Scholar]
- Buccafusco JJ, Jackson WJ, Terry AV, Jr, Marsh KC, Decker MW, Arneric SP. Improvement in performance of a delayed matching-to-sample task by monkeys following ABT-418: a novel cholinergic channel activator for memory enhancement. Psychopharmacology (Berl) 1995;120:256–266. doi: 10.1007/BF02311172. [DOI] [PubMed] [Google Scholar]
- Clark JD, Gebhart GF, Gonder JC, Keeling ME, Kohn DF. Special Report: The 1996 Guide for the Care and Use of Laboratory Animals. Ilar J. 1997;38:41–48. doi: 10.1093/ilar.38.1.41. [DOI] [PubMed] [Google Scholar]
- Court JA, Martin-Ruiz C, Graham A, Perry E. Nicotinic receptors in human brain: topography and pathology. J Chem Neuroanat. 2000a;20:281–298. doi: 10.1016/s0891-0618(00)00110-1. [DOI] [PubMed] [Google Scholar]
- Court JA, Piggott MA, Lloyd S, Cookson N, Ballard CG, McKeith IG, Perry RH, Perry EK. Nicotine binding in human striatum: elevation in schizophrenia and reductions in dementia with Lewy bodies, Parkinson's disease and Alzheimer's disease and in relation to neuroleptic medication. Neuroscience. 2000b;98:79–87. doi: 10.1016/s0306-4522(00)00071-3. [DOI] [PubMed] [Google Scholar]
- Davidson MC, Cutrell EB, Marrocco RT. Scopolamine slows the orienting of attention in primates to cued visual targets. Psychopharmacology (Berl) 1999;142:1–8. doi: 10.1007/s002130050855. [DOI] [PubMed] [Google Scholar]
- Decker MW, Bannon AW, Curzon P, Gunther KL, Brioni JD, Holladay MW, Lin NH, Li Y, Daanen JF, Buccafusco JJ, Prendergast MA, Jackson WJ, Arneric SP. ABT-089 [2-methyl-3-(2-(S)-pyrrolidinylmethoxy)pyridine dihydrochloride]: II. A novel cholinergic channel modulator with effects on cognitive performance in rats and monkeys. J Pharmacol Exp Ther. 1997;283:247–258. [PubMed] [Google Scholar]
- Decker MW, Majchrzak MJ. Effects of systemic and intracerebroventricular administration of mecamylamine, a nicotinic cholinergic antagonist, on spatial memory in rats. Psychopharmacology (Berl) 1992;107:530–534. doi: 10.1007/BF02245267. [DOI] [PubMed] [Google Scholar]
- Decker MW, Majchrzak MJ, Anderson DJ. Effects of nicotine on spatial memory deficits in rats with septal lesions. Brain Res. 1992;572:281–285. doi: 10.1016/0006-8993(92)90485-r. [DOI] [PubMed] [Google Scholar]
- Deutsch JA. The cholinergic synapse and the site of memory. Science. 1971;174:788–794. doi: 10.1126/science.174.4011.788. [DOI] [PubMed] [Google Scholar]
- Dilts SL, Berry C. Effect of cholinergic drugs on passive avoidance in the mouse. J Pharmacol Exp Ther. 1967;158:279–285. [PubMed] [Google Scholar]
- Drachman DA, Leavitt J. Human memory and the cholinergic system. A relationship to aging? Arch Neurol. 1974;30:113–121. doi: 10.1001/archneur.1974.00490320001001. [DOI] [PubMed] [Google Scholar]
- Ebersbach G, Stock M, Muller J, Wenning G, Wissel J, Poewe W. Worsening of motor performance in patients with Parkinson's disease following transdermal nicotine administration. Mov Disord. 1999;14:1011–1013. doi: 10.1002/1531-8257(199911)14:6<1011::aid-mds1016>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Elrod K, Buccafusco JJ, Jackson WJ. Nicotine enhances delayed matching-to-sample performance by primates. Life Sci. 1988;43:277–287. doi: 10.1016/0024-3205(88)90318-9. [DOI] [PubMed] [Google Scholar]
- Everett SA, Warren CW, Sharp D, Kann L, Husten CG, Crossett LS. Initiation of cigarette smoking and subsequent smoking behavior among U.S. high school students. Prev Med. 1999;29:327–333. doi: 10.1006/pmed.1999.0560. [DOI] [PubMed] [Google Scholar]
- Flicker C, Serby M, Ferris SH. Scopolamine effects on memory, language, visuospatial praxis and psychomotor speed. Psychopharmacology (Berl) 1990;100:243–250. doi: 10.1007/BF02244414. [DOI] [PubMed] [Google Scholar]
- Fowler KS, Saling MM, Conway EL, Semple JM, Louis WJ. Paired associate performance in the early detection of DAT. J Int Neuropsychol Soc. 2002;8:58–71. [PubMed] [Google Scholar]
- Franowicz JS, Arnsten AF. The alpha-2a noradrenergic agonist, guanfacine, improves delayed response performance in young adult rhesus monkeys. Psychopharmacology (Berl) 1998;136:8–14. doi: 10.1007/s002130050533. [DOI] [PubMed] [Google Scholar]
- Ghoneim MM, Mewaldt SP. Effects of diazepam and scopolamine on storage, retrieval and organizational processes in memory. Psychopharmacologia. 1975;44:257–262. doi: 10.1007/BF00428903. [DOI] [PubMed] [Google Scholar]
- Giovino GA. Epidemiology of tobacco use among US adolescents. Nicotine Tob Res. 1999:S31–40. doi: 10.1080/14622299050011571. [DOI] [PubMed] [Google Scholar]
- Graves AB, van Duijn CM, Chandra V, Fratiglioni L, Heyman A, Jorm AF, Kokmen E, Kondo K, Mortimer JA, Rocca WA, et al. Alcohol and tobacco consumption as risk factors for Alzheimer's disease: a collaborative re-analysis of case-control studies. EURODEM Risk Factors Research Group. Int J Epidemiol. 1991;20(Suppl 2):S48–57. doi: 10.1093/ije/20.supplement_2.s48. [DOI] [PubMed] [Google Scholar]
- Grigoryan G, Hodges H, Mitchell S, Sinden JD, Gray JA. 6-OHDA lesions of the nucleus accumbens accentuate memory deficits in animals with lesions to the forebrain cholinergic projection system: effects of nicotine administration on learning and memory in the water maze. Neurobiol Learn Mem. 1996;65:135–153. doi: 10.1006/nlme.1996.0016. [DOI] [PubMed] [Google Scholar]
- Grottick AJ, Higgins GA. Effect of subtype selective nicotinic compounds on attention as assessed by the five-choice serial reaction time task. Behav Brain Res. 2000;117:197–208. doi: 10.1016/s0166-4328(00)00305-3. [DOI] [PubMed] [Google Scholar]
- Hahn B, Sharples CG, Wonnacott S, Shoaib M, Stolerman IP. Attentional effects of nicotinic agonists in rats. Neuropharmacology. 2003;44:1054–1067. doi: 10.1016/s0028-3908(03)00099-6. [DOI] [PubMed] [Google Scholar]
- Heishman SJ. Behavioral and cognitive effects of smoking: relationship to nicotine addiction. Nicotine Tob Res. 1999;1(Suppl 2):S143–147. S165–166. doi: 10.1080/14622299050011971. [DOI] [PubMed] [Google Scholar]
- Hernan MA, Zhang SM, Rueda-deCastro AM, Colditz GA, Speizer FE, Ascherio A. Cigarette smoking and the incidence of Parkinson's disease in two prospective studies. Ann Neurol. 2001;50:780–786. doi: 10.1002/ana.10028. [DOI] [PubMed] [Google Scholar]
- Hironaka N, Miyata H, Ando K. Effects of psychoactive drugs on short-term memory in rats and rhesus monkeys. Jpn J Pharmacol. 1992;59:113–120. doi: 10.1254/jjp.59.113. [DOI] [PubMed] [Google Scholar]
- Howe MN, Price IR. Effects of transdermal nicotine on learning, memory, verbal fluency, concentration, and general health in a healthy sample at risk for dementia. Int Psychogeriatr. 2001;13:465–475. doi: 10.1017/s1041610201007888. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Miyatake T. Effects of smoking in patients with early-onset Parkinson's disease. J Neurol Sci. 1993;117:28–32. doi: 10.1016/0022-510x(93)90150-w. [DOI] [PubMed] [Google Scholar]
- Jones DN, Barnes JC, Kirkby DL, Higgins GA. Age-associated impairments in a test of attention: evidence for involvement of cholinergic systems. J Neurosci. 1995;15:7282–7292. doi: 10.1523/JNEUROSCI.15-11-07282.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GM, Sahakian BJ, Levy R, Warburton DM, Gray JA. Effects of acute subcutaneous nicotine on attention, information processing and short-term memory in Alzheimer's disease. Psychopharmacology (Berl) 1992;108:485–494. doi: 10.1007/BF02247426. [DOI] [PubMed] [Google Scholar]
- Keeler TE, Hu TW, Keith A, Manning R, Marciniak MD, Ong M, Sung HY. The benefits of switching smoking cessation drugs to over-the-counter status. Health Econ. 2002;11:389–402. doi: 10.1002/hec.677. [DOI] [PubMed] [Google Scholar]
- Kellar KJ, Whitehouse PJ, Martino-Barrows AM, Marcus K, Price DL. Muscarinic and nicotinic cholinergic binding sites in Alzheimer's disease cerebral cortex. Brain Res. 1987;436:62–68. doi: 10.1016/0006-8993(87)91556-3. [DOI] [PubMed] [Google Scholar]
- Kelton MC, Kahn HJ, Conrath CL, Newhouse PA. The effects of nicotine on Parkinson's disease. Brain Cogn. 2000;43:274–282. [PubMed] [Google Scholar]
- Kirk RC, White KG, McNaughton N. Low dose scopolamine affects discriminability but not rate of forgetting in delayed conditional discrimination. Psychopharmacology (Berl) 1988;96:541–546. doi: 10.1007/BF02180037. [DOI] [PubMed] [Google Scholar]
- Koss E, Ober BA, Delis DC, Friedland RP. The Stroop color-word test: indicator of dementia severity. Int J Neurosci. 1984;24:53–61. doi: 10.3109/00207458409079534. [DOI] [PubMed] [Google Scholar]
- Lee PN. Smoking and Alzheimer's disease: a review of the epidemiological evidence. Neuroepidemiology. 1994;13:131–144. doi: 10.1159/000110372. [DOI] [PubMed] [Google Scholar]
- Levin ED, Christopher NC, Briggs SJ, Rose JE. Chronic nicotine reverses working memory deficits caused by lesions of the fimbria or medial basalocortical projection. Brain Res Cogn Brain Res. 1993;1:137–143. doi: 10.1016/0926-6410(93)90021-v. [DOI] [PubMed] [Google Scholar]
- Lloyd GK, Williams M. Neuronal nicotinic acetylcholine receptors as novel drug targets. J Pharmacol Exp Ther. 2000;292:461–467. [PubMed] [Google Scholar]
- Min SK, Moon IW, Ko RW, Shin HS. Effects of transdermal nicotine on attention and memory in healthy elderly non-smokers. Psychopharmacology (Berl) 2001;159:83–88. doi: 10.1007/s002130100899. [DOI] [PubMed] [Google Scholar]
- Mirza NR, Stolerman IP. Nicotine enhances sustained attention in the rat under specific task conditions. Psychopharmacology (Berl) 1998;138:266–274. doi: 10.1007/s002130050671. [DOI] [PubMed] [Google Scholar]
- Muir JL, Everitt BJ, Robbins TW. Reversal of visual attentional dysfunction following lesions of the cholinergic basal forebrain by physostigmine and nicotine but not by the 5-HT3 receptor antagonist, ondansetron. Psychopharmacology (Berl) 1995;118:82–92. doi: 10.1007/BF02245253. [DOI] [PubMed] [Google Scholar]
- Mumenthaler MS, Yesavage JA, Taylor JL, O'Hara R, Friedman L, Lee H, Kraemer HC. Psychoactive drugs and pilot performance: a comparison of nicotine, donepezil, and alcohol effects. Neuropsychopharmacology. 2003;28:1366–1373. doi: 10.1038/sj.npp.1300202. [DOI] [PubMed] [Google Scholar]
- Newhouse PA, Potter A, Corwin J, Lenox R. Acute nicotinic blockade produces cognitive impairment in normal humans. Psychopharmacology (Berl) 1992;108:480–484. doi: 10.1007/BF02247425. [DOI] [PubMed] [Google Scholar]
- Newhouse PA, Potter A, Levin ED. Nicotinic system involvement in Alzheimer's and Parkinson's diseases. Implications for therapeutics. Drugs Aging. 1997;11:206–228. doi: 10.2165/00002512-199711030-00005. [DOI] [PubMed] [Google Scholar]
- Nordberg A, Adem A, Hardy J, Winblad B. Change in nicotinic receptor subtypes in temporal cortex of Alzheimer brains. Neurosci Lett. 1988;86:317–321. doi: 10.1016/0304-3940(88)90503-4. [DOI] [PubMed] [Google Scholar]
- Nordberg A, Winblad B. Reduced number of [3H]nicotine and [3H]acetylcholine binding sites in the frontal cortex of Alzheimer brains. Neurosci Lett. 1986;72:115–119. doi: 10.1016/0304-3940(86)90629-4. [DOI] [PubMed] [Google Scholar]
- Osman A, Lou L, Muller-Gethmann H, Rinkenauer G, Mattes S, Ulrich R. Mechanisms of speed- accuracy tradeoff: evidence from covert motor processes. Biol Psychol. 2000;51:173–199. doi: 10.1016/s0301-0511(99)00045-9. [DOI] [PubMed] [Google Scholar]
- Perry E, Court J, Goodchild R, Griffiths M, Jaros E, Johnson M, Lloyd S, Piggott M, Spurden D, Ballard C, McKeith I, Perry R. Clinical neurochemistry: developments in dementia research based on brain bank material. J Neural Transm. 1998;105:915–933. doi: 10.1007/s007020050102. [DOI] [PubMed] [Google Scholar]
- Phillips JM, McAlonan K, Robb WG, Brown VJ. Cholinergic neurotransmission influences covert orientation of visuospatial attention in the rat. Psychopharmacology (Berl) 2000;150:112–116. doi: 10.1007/s002130000437. [DOI] [PubMed] [Google Scholar]
- Potter A, Corwin J, Lang J, Piasecki M, Lenox R, Newhouse PA. Acute effects of the selective cholinergic channel activator (nicotinic agonist) ABT-418 in Alzheimer's disease. Psychopharmacology (Berl) 1999;142:334–342. doi: 10.1007/s002130050897. [DOI] [PubMed] [Google Scholar]
- Prendergast MA, Jackson WJ, Terry AV, Jr, Decker MW, Arneric SP, Buccafusco JJ. Central nicotinic receptor agonists ABT-418, ABT-089, and (-)-nicotine reduce distractibility in adult monkeys. Psychopharmacology (Berl) 1998;136:50–58. doi: 10.1007/s002130050538. [DOI] [PubMed] [Google Scholar]
- Preston KL, Schuster CR, Seiden LS. Methamphetamine, physostigmine, atropine and mecamylamine: effects on force lever performance. Pharmacol Biochem Behav. 1985;23:781–788. doi: 10.1016/0091-3057(85)90072-3. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Levin ED. Cognitive effects of nicotine. Biol Psychiatry. 2001;49:258–267. doi: 10.1016/s0006-3223(00)01094-5. [DOI] [PubMed] [Google Scholar]
- Ridley RM, Bowes PM, Baker HF, Crow TJ. An involvement of acetylcholine in object discrimination learning and memory in the marmoset. Neuropsychologia. 1984;22:253–263. doi: 10.1016/0028-3932(84)90073-3. [DOI] [PubMed] [Google Scholar]
- Riekkinen P, Jr, Riekkinen M, Sirvio J. Cholinergic drugs regulate passive avoidance performance via the amygdala. J Pharmacol Exp Ther. 1993;267:1484–1492. [PubMed] [Google Scholar]
- Riekkinen P, Jr, Riekkinen M, Sirvio J, Riekkinen P. Effects of concurrent nicotinic antagonist and PCPA treatments on spatial and passive avoidance learning. Brain Res. 1992;575:247–250. doi: 10.1016/0006-8993(92)90086-o. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Semple J, Kumar R, Truman MI, Shorter J, Ferraro A, Fox B, McKay G, Matthews K. Effects of scopolamine on delayed-matching-to-sample and paired associates tests of visual memory and learning in human subjects: comparison with diazepam and implications for dementia. Psychopharmacology (Berl) 1997;134:95–106. doi: 10.1007/s002130050430. [DOI] [PubMed] [Google Scholar]
- Ross GW, Petrovitch H. Current evidence for neuroprotective effects of nicotine and caffeine against Parkinson's disease. Drugs Aging. 2001;18:797–806. doi: 10.2165/00002512-200118110-00001. [DOI] [PubMed] [Google Scholar]
- Ruotsalainen S, Miettinen R, MacDonald E, Koivisto E, Sirvio J. Blockade of muscarinic, rather than nicotinic, receptors impairs attention, but does not interact with serotonin depletion. Psychopharmacology (Berl) 2000;148:111–123. doi: 10.1007/s002130050032. [DOI] [PubMed] [Google Scholar]
- Rupniak NM, Samson NA, Tye SJ, Field MJ, Iversen SD. Evidence against a specific effect of cholinergic drugs on spatial memory in primates. Behav Brain Res. 1991;43:1–6. doi: 10.1016/s0166-4328(05)80047-6. [DOI] [PubMed] [Google Scholar]
- Safer DJ, Allen RP. The central effects of scopolamine in man. Biol Psychiatry. 1971;3:347–355. [PubMed] [Google Scholar]
- Sahakian BJ, Owen AM, Morant NJ, Eagger SA, Boddington S, Crayton L, Crockford HA, Crooks M, Hill K, Levy R. Further analysis of the cognitive effects of tetrahydroaminoacridine (THA) in Alzheimer's disease: assessment of attentional and mnemonic function using CANTAB. Psychopharmacology (Berl) 1993;110:395–401. doi: 10.1007/BF02244644. [DOI] [PubMed] [Google Scholar]
- Santi A, Weise L. The effects of scopolamine on memory for time in rats and pigeons. Pharmacol Biochem Behav. 1995;51:271–277. doi: 10.1016/0091-3057(94)00376-t. [DOI] [PubMed] [Google Scholar]
- Schauffler HH, McMenamin S, Olson K, Boyce-Smith G, Rideout JA, Kamil J. Variations in treatment benefits influence smoking cessation: results of a randomised controlled trial. Tob Control. 2001;10:175–180. doi: 10.1136/tc.10.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Pope-Coleman A, Van Velson M, Menzaghi F, Lloyd GK. Effects of SIB-1508Y, a novel neuronal nicotinic acetylcholine receptor agonist, on motor behavior in parkinsonian monkeys. Mov Disord. 1998a;13:637–642. doi: 10.1002/mds.870130405. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Tinker JP, Van Velson M, Menzaghi F, Lloyd GK. Nicotinic acetylcholine receptor agonist SIB-1508Y improves cognitive functioning in chronic low-dose MPTP-treated monkeys. J Pharmacol Exp Ther. 1999;290:731–739. [PubMed] [Google Scholar]
- Schneider JS, Van Velson M, Menzaghi F, Lloyd GK. Effects of the nicotinic acetylcholine receptor agonist SIB-1508Y on object retrieval performance in MPTP-treated monkeys: comparison with levodopa treatment. Ann Neurol. 1998b;43:311–317. doi: 10.1002/ana.410430308. [DOI] [PubMed] [Google Scholar]
- Stapleton JA, Lowin A, Russell MA. Prescription of transdermal nicotine patches for smoking cessation in general practice: evaluation of cost-effectiveness. Lancet. 1999;354:210–215. doi: 10.1016/s0140-6736(99)90001-6. [DOI] [PubMed] [Google Scholar]
- Swainson R, Hodges JR, Galton CJ, Semple J, Michael A, Dunn BD, Iddon JL, Robbins TW, Sahakian BJ. Early detection and differential diagnosis of Alzheimer's disease and depression with neuropsychological tasks. Dement Geriatr Cogn Disord. 2001;12:265–280. doi: 10.1159/000051269. [DOI] [PubMed] [Google Scholar]
- Taffe M, Davis S, Gutierrez T, Gold L. Ketamine impairs multiple cognitive domains in rhesus monkeys. Drug Alcohol Depend. 2002a;68:175–187. doi: 10.1016/s0376-8716(02)00194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA, Bernot TJ, Mckay HL, Davis SA, Roberts JA, Tuszynski MH. Adaptation of a computerized neuropsychological testing battery to the aged macaque. Society for Neuroscience Annual Meeting; New Orleans, LA. 2003a. [Google Scholar]
- Taffe MA, Davis SA, Yuan J, Schroeder R, Hatzidimitriou G, Parsons LH, Ricaurte GA, Gold LH. Cognitive performance of MDMA-treated rhesus monkeys: Sensitivity to serotonergic challenge. Neuropsychopharmacology. 2002b;27:994–1006. doi: 10.1016/S0893-133X(02)00380-9. [DOI] [PubMed] [Google Scholar]
- Taffe MA, Huitron-Resendiz S, Schroeder R, Parsons LH, Henriksen SJ, Gold LH. MDMA exposure alters cognitive and electrophysiological sensitivity to rapid tryptophan depletion in rhesus monkeys. Pharmacol Biochem Behav. 2003b;76:141–152. doi: 10.1016/s0091-3057(03)00217-x. [DOI] [PubMed] [Google Scholar]
- Taffe MA, Weed MR, Gold LH. Scopolamine alters rhesus monkey performance on a novel neuropsychological test battery. Brain Res Cogn Brain Res. 1999;8:203–212. doi: 10.1016/s0926-6410(99)00021-x. [DOI] [PubMed] [Google Scholar]
- Taffe MA, Weed MR, Gutierrez T, Davis SA, Gold LH. Differential muscarinic and NMDA contributions to visuo-spatial paired-associate learning in rhesus monkeys. Psychopharmacology (Berl) 2002c;160:253–262. doi: 10.1007/s00213-001-0954-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA, Weed MR, Gutierrez T, Davis SA, Gold LH. Modeling a task that is sensitive to dementia of the Alzheimer's type: Individual differences in acquisition of a visuo-spatial paired-associate learning task in rhesus monkeys. Behav Brain Res. 2004 doi: 10.1016/s0166-4328(03)00214-6. in press. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr, Buccafusco JJ, Jackson WJ. Scopolamine reversal of nicotine enhanced delayed matching-to-sample performance in monkeys. Pharmacol Biochem Behav. 1993;45:925–929. doi: 10.1016/0091-3057(93)90141-f. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr, Buccafusco JJ, Prendergast MA. Dose-specific improvements in memory-related task performance by rats and aged monkeys administered the nicotinic-cholinergic antagonist mecamylamine. Drug Development Research. 1999;47:127–136. [Google Scholar]
- Terry AV, Jr, Risbrough VB, Buccafusco JJ, Menzaghi F. Effects of (+/−)-4-[[2-(1-methyl-2-pyrrolidinyl)ethyl]thio]phenol hydrochloride (SIB-1553A), a selective ligand for nicotinic acetylcholine receptors, in tests of visual attention and distractibility in rats and monkeys. J Pharmacol Exp Ther. 2002;301:284–292. doi: 10.1124/jpet.301.1.284. [DOI] [PubMed] [Google Scholar]
- Turchi J, Sarter M. Cortical acetylcholine and processing capacity: effects of cortical cholinergic deafferentation on crossmodal divided attention in rats. Brain Res Cogn Brain Res. 1997;6:147–158. doi: 10.1016/s0926-6410(97)00027-x. [DOI] [PubMed] [Google Scholar]
- USCDC. Projected smoking-related deaths among youth--United States. Morb Mortal Wkly Rep. 1996;45:971–974. [PubMed] [Google Scholar]
- van Duijn CM, Clayton DG, Chandra V, Fratiglioni L, Graves AB, Heyman A, Jorm AF, Kokmen E, Kondo K, Mortimer JA, et al. Interaction between genetic and environmental risk factors for Alzheimer's disease: a reanalysis of case-control studies. EURODEM Risk Factors Research Group. Genet Epidemiol. 1994;11:539–551. doi: 10.1002/gepi.1370110609. [DOI] [PubMed] [Google Scholar]
- van Duijn CM, Havekes LM, Van Broeckhoven C, de Knijff P, Hofman A. Apolipoprotein E genotype and association between smoking and early onset Alzheimer's disease. Bmj. 1995;310:627–631. doi: 10.1136/bmj.310.6980.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duijn CM, Hofman A. Relation between nicotine intake and Alzheimer's disease. Bmj. 1991;302:1491–1494. doi: 10.1136/bmj.302.6791.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieregge A, Sieberer M, Jacobs H, Hagenah JM, Vieregge P. Transdermal nicotine in PD: a randomized, double-blind, placebo-controlled study. Neurology. 2001;57:1032–1035. doi: 10.1212/wnl.57.6.1032. [DOI] [PubMed] [Google Scholar]
- Weed MR, Gold LH. The effects of dopaminergic agents on reaction time in rhesus monkeys. Psychopharmacology (Berl) 1998;137:33–42. doi: 10.1007/s002130050590. [DOI] [PubMed] [Google Scholar]
- Weed MR, Taffe MA, Polis I, Roberts AC, Robbins TW, Koob GF, Bloom FE, Gold LH. Performance norms for a rhesus monkey neuropsychological testing battery: Acquisition and long-term performance. Cogn Brain Res. 1999;8:184–201. doi: 10.1016/s0926-6410(99)00020-8. [DOI] [PubMed] [Google Scholar]
- White HK, Levin ED. Four-week nicotine skin patch treatment effects on cognitive performance in Alzheimer's disease. Psychopharmacology (Berl) 1999;143:158–165. doi: 10.1007/s002130050931. [DOI] [PubMed] [Google Scholar]
- Wilson AL, Langley LK, Monley J, Bauer T, Rottunda S, McFalls E, Kovera C, McCarten JR. Nicotine patches in Alzheimer's disease: pilot study on learning, memory, and safety. Pharmacol Biochem Behav. 1995;51:509–514. doi: 10.1016/0091-3057(95)00043-v. [DOI] [PubMed] [Google Scholar]
- Witte EA, Davidson MC, Marrocco RT. Effects of altering brain cholinergic activity on covert orienting of attention: comparison of monkey and human performance. Psychopharmacology (Berl) 1997;132:324–334. doi: 10.1007/s002130050352. [DOI] [PubMed] [Google Scholar]
- Young JM, Shytle RD, Sanberg PR, George TP. Mecamylamine: new therapeutic uses and toxicity/risk profile. Clin Ther. 2001;23:532–565. doi: 10.1016/s0149-2918(01)80059-x. [DOI] [PubMed] [Google Scholar]