Abstract
Cocaine addiction appears to be associated with a drug-induced dysregulation of stressor responsiveness that may contribute to further cocaine use. The present study examined alterations in stressor-induced activation of the hypothalamic-pituitary-adrenal (HPA) axis in rats provided daily access to cocaine for self-administration (SA) under long-access conditions (1.0 mg/kg/inf; 6 hrs × 14 days). Cocaine self-administering rats displayed reduced basal plasma corticosterone (CORT) levels but showed an augmented restraint-induced percent increase response from baseline compared to saline self-administering controls when measured 24 days after SA testing. This augmented CORT response may have been attributable to impaired glucocorticoid receptor (GR)-mediated feedback regulation of HPA function, since cocaine self-administering rats were also less susceptible to dexamethasone (0.01 mg/kg, ip) suppression of plasma CORT levels. GR protein expression measured using Western blot analysis was significantly reduced in the dorsomedial hypothalamus (including the paraventricular nucleus [PVN]) but not in the pituitary gland, ventromedial hypothalamus, dorsal hippocampus, ventral subiculum, medial prefrontal cortex or amygdala in cocaine self-administering rats. Surprisingly, basal corticotropin-releasing hormone (CRH) mRNA or post-restraint increases in CRH mRNA measured at a single (90-min) time-point in the PVN using in situ hybridization did not differ between groups. The findings suggest that cocaine use produces persistent changes in individual responsiveness to stressors that may contribute to the addiction process.
Keywords: Cocaine, corticosterone, addiction, stress, glucocorticoid receptor, self-administration
1. INTRODUCTION
A role for stress in cocaine addiction has been established (Sinha et al 2001). In addition to findings that stress promotes cocaine-seeking behavior, it has been reported that stress responses emerge or are exaggerated as a result of prior cocaine exposure, suggesting that the relationship between stress and cocaine abuse represents a self-perpetuating cycle within which stress serves as both a precipitating factor for and consequence of drug use.
This complex relationship between stress and cocaine addiction likely involves the hypothalamic pituitary adrenal (HPA) axis. Increases in circulating glucocorticoid levels as a consequence of stressor-induced activation of the HPA axis appear to promote cocaine-seeking behavior (Goeders, 2002; Marinelli and Piazza, 2005). The HPA axis is also stimulated by cocaine in rats (Moldow and Fischman, 1987; Rivier and Vale, 1987), monkeys (Sarnyai et al., 1996; Broadbear et al., 1999) and humans (Mendelson et al., 1992; Baumann et al., 1995) through a mechanism dependent on the release of the peptide corticotropin releasing hormone (CRH) from the terminals of parvocellular neurons originating in the paraventricular nucleus (PVN) of the hypothalamus (Rivier and Vale, 1987; Sarnyai et al., 1992). The effects of cocaine on the HPA axis are dependent upon the contingency of drug delivery. When cocaine is self-administered, its effects on plasma adrenocorticotropic hormone (ACTH) and cortisol in monkeys (Broadbear et al., 1999) or corticosterone (CORT) in rats (Galici et al., 2000) are different than those produced by non-self-administered cocaine delivered under otherwise identical conditions. For this reason, the use of self-administration (SA) procedures is likely more appropriate for examination of alterations in HPA function associated with cocaine use.
With repeated cocaine administration, adaptive changes in the HPA axis emerge and can be observed as disrupted basal HPA function during drug withdrawal in cocaine-dependent individuals (Vescovi et al., 1992; Buydens-Branchey et al., 2002; Contoreggi et al., 2003) and in rats (Sarnyai et al., 1998; Zorilla et al., 2001; Zhou et al., 2003). Less is known about how the response of the HPA axis to stressors is altered as a consequence of prior cocaine use. Preclinical studies examining withdrawal from repeated experimenter-delivered psychomotor stimulant drug administration have reported either no change in (Levy et al., 1994; Sarnyai et al., 1998) or an augmentation of (Mantsch et al., 2007; Barr et al., 2002) the stressor-induced CORT and/or ACTH response. These effects appear to depend on the pattern and/or amount of drug exposure as well as the duration of withdrawal. Notably, similar discrepancies have been found when examining the effects of repeated drug exposure on the HPA response to cocaine, with no changes in (Borowsky and Kuhn, 1991; Levy et al., 1992), augmentation of (Schmidt et al., 1995), or attenuation of (Zhou et al., 1996) the HPA response reported, depending on the treatment parameters used.
A clearer understanding how HPA reactivity is altered in cocaine addiction will likely be facilitated by examination of stressor-induced HPA activation in rats self-administering cocaine under conditions that produce levels and patterns of drug exposure as well as behavioral profiles that better resemble those associated with use by human addicts. For this reason, we chose to examine the impact of cocaine SA on stressor-induced HPA activation using rats provided extended (i.e., 6-h) daily access to cocaine. We and others have shown that rats self-administering under long-access conditions display a number of behavioral responses thought to be related to human cocaine addiction, including a progressive escalation of cocaine intake (Ahmed and Koob, 1998) and a persistently heightened susceptibility to reinstatement in response to a cocaine challenge (Mantsch et al., 2004; Ahmed and Cador, 2006), cocaine-associated cues (Kippin et al., 2006), and stressors (unpublished data). Repeated extended-access (i.e., 10-h) cocaine SA also produces persistent changes in basal HPA function, including a reduction in basal CORT levels and anterior pituitary POMC mRNA expression (Mantsch et al., 2003). Although it is possible that the observed reductions in basal HPA function may reflect a general attenuation of HPA responsiveness that would also include reduced activity in response to stressors, it is also possible that changes in basal activity may augment HPA reactivity to stressors by removing negative feedback exerted by basal CORT and/or producing adaptations in the HPA axis that render it more sensitive to stimulation. This possibility is highlighted by recent findings by Fox et al. (2005) who demonstrated that stressor-induced craving, anxiety, and physiological responses are magnified in high-frequency cocaine users compared to individuals with a lower frequency of cocaine use. Thus, stressor responsiveness appears to increase with the severity of cocaine addiction.
The first goal of this study was to examine persistent changes in basal and stressor-induced HPA activity resulting from cocaine SA under conditions of daily extended drug access. It was hypothesized that, despite previously characterized reductions in basal CORT levels, cocaine SA would result in an intensified HPA response. The second goal of the study was to examine potential neurobiological mechanisms through which HPA responsiveness is altered as a result of cocaine SA. Since glucocorticoid receptors (GR) in the brain and pituitary gland serve as critical negative feedback regulators of HPA function, we chose to examine the effects of cocaine SA on the ability of the GR agonist, dexamethasone (DEX), to suppress plasma CORT levels and on brain and pituitary GR protein expression. Impaired GR-mediated negative feedback would remove inhibitory constraint from the HPA axis, thus augmenting the glucocorticoid response to stressors.
2. RESULTS
2.1. Effects of Chronic SA
Alterations in HPA function were examined using rats permitted to self-administer cocaine under daily long-access conditions for 14 days and saline self-administering controls. Cocaine and saline intake across the 14 days of SA testing is shown in Figure 1. Since no differences in cocaine SA were observed between rats used for Experiments #1 and #2, SA data from these experiments were combined for statistical analyses. Two-way repeated measures ANOVA showed significant effects of SA condition (cocaine vs. saline; F1,58=1213.92;P<0.001) and SA test day (1–14; F13,754=14.11; P<0.001) on the number of self-administered infusions and a significant SA condition × test day interaction (F13,754=13.76; P<0.001). Cocaine, but not saline, intake progressively increased across SA testing (one-way ANOVA; F13,433=5.89;P<0.001) with increases compared to day one emerging on day seven of testing and persisting through the remainder of the 14-day test period (P<0.05 for each comparison).
Figure 1.
SA by rats provided access to cocaine (1.0 mg/kg/inf) or saline during daily 6-h sessions for 14 days. Data represent the daily mean numbers of infusions (± S.E.). A progressive escalation of SA was observed in cocaine but not saline SA rats (*P<0.01 vs Day 1).
2.2. Experiment #1: Effects of Cocaine SA on Basal and Stressor-Induced CORT Levels and GR-Mediated Negative Feedback
The effects of repeated long-access cocaine SA on basal and stressor- (i.e., restraint-) induced CORT secretion and GR-mediated negative feedback were examined 21–24 days after the final SA test session in cocaine and saline self-administering rats. GR-mediated negative feedback was defined as sensitivity to suppression of CORT by the GR agonist DEX.
Basal, Post-Restraint and Post-DEX CORT Levels
Plasma CORT levels under basal conditions and immediately following 30 min of restraint or 60 min following administration of 0.01 mg/kg DEX measured 21–24 days after cocaine or saline SA testing are shown in Figure 2A. Two-way ANOVA showed a significant overall effect of sampling condition (BAS, DEX, RES; F2,54=155.33;P<0.001) but not SA condition (SAL vs. COC) on CORT and a significant restraint × SA interaction (F2,54=9.55;P<0.001). Overall, restraint significantly increased and DEX significantly decreased plasma CORT compared to basal levels (P<0.001). The magnitude of the plasma CORT response to restraint did not differ between saline and cocaine rats. However, since basal CORT levels were significantly reduced following cocaine SA (t27=3.07; P<0.01), we decided to also examine the magnitude of the CORT response as a % of baseline CORT in these rats (Figure 2B). A Mann-Whitney U test comparing % baseline CORT responses between groups showed that the CORT response to restraint was significantly increased following cocaine SA (U=45; Z=2.59; two-tailed asymptotic significance of P<0.05). Simple linear regression analysis was used to examine the relationship between basal CORT and the magnitude of the CORT response to restraint (post-restraint CORT minus basal CORT). A significant inverse correlation was found between basal CORT and the CORT response (R=0.465; R2 =0.216; F1,29=7.45;P<0.05), indicating that lower basal CORT predicted a higher CORT response.
Figure 2.
Plasma CORT under basal conditions and following restraint or dexamethasone (Dex) administration 21–24 days after cocaine (COC) or saline (SAL) SA. Data in 2A represent mean basal plasma CORT (ng/ml ± S.E), CORT following 30 min of restraint, and CORT one hr after administration of Dex (0.01 mg/kg ip). Restraint significantly increased (#P<0.001 vs. basal) and Dex significantly reduced (@P<0.05 vs. basal) plasma CORT in both groups. Basal plasma CORT was significantly lower (*P<0.05) and post-Dex plasma CORT was significantly higher (**P<0.001) in COC rats. Data in 2B represent the Restraint and Dex induced changes in CORT expressed as mean % basal values (% BAS). % BAS CORT was significantly higher following Restraint and Dex in COC rats (*P<0.01). All CORT measurements were performed at approximately 10 AM either 21 (DEX) or 24 (Basal and restraint) days after the final SA session.
DEX significantly reduced plasma CORT following saline (P<0.001) but not cocaine SA. Plasma CORT following DEX was significantly lower in saline compared to cocaine self-administering rats (t27=4.28; P<0.001). We also examined DEX suppression as a % of baseline CORT values (Figure 2B). A Mann-Whitney U test showed that DEX produced a significantly greater suppression of plasma CORT following cocaine SA (U=14; Z=4.03; two-tailed asymptotic significance of P<0.001).
Brain and Pituitary GR
At the time of acquisition of the final blood sample for CORT measurement, cocaine (n=11) and saline (n=9) SA rats were sacrificed for examination of GR protein expression in the pituitary gland and brain regions involved in negative feedback regulation of the HPA axis using Western blot analysis (see Figure 3 and Table 1). GR protein expression was significantly reduced at the time of sacrifice in the dorsomedial hypothalamic dissections from cocaine SA rats (Figure 3; t18=2.72; P<0.05). These dissections included the PVN. By contrast, no significant differences were found in the pituitary gland, amygdala, dorsal hippocampus, medial prefrontal cortex, ventral subiculum, or ventromedial hypothalamus (Table 1).
Figure 3.
GR protein expression in the dorsomedial hypothalamus 24 days after saline (SAL) or cocaine (COC) SA. Data represent the optical densities of immunoreactive bands expressed as the mean % of saline control bands (± S.E.) in homogenate of dissected dorsomedial hypothalamic tissue (including PVN) 24 days after the final SA session. Expression of the 98 kD GR in the dorsomedial hypothalamus was significantly decreased in COC rats (*P<0.05).
Table 1.
Glucocorticoid receptor (GR) protein levels following saline (SAL) and cocaine (COC) SA. Data represent the densities of GR immunoreactive bands presented as % of SAL controls (± S.E.) in dissected brain regions and pituitary determined using Western blot analysis.
| GR Protein Levels (% SAL Control ± S.E.)
|
||
|---|---|---|
| SAL | COC | |
| Amygdala | 100 ± 8.6 | 100.5 ± 9.9 |
| Dorsal Hippocampus | 100 ± 9.7 | 90.5 ± 8.8 |
| Medial Prefrontal Cortex | 100 ± 5.4 | 101.9 ± 14.2 |
| Pituitary Gland | 100 ± 13.7 | 114..2 ± 29.3 |
| Ventral Subiculum | 100 ± 17.2 | 95.2 ± 13.4 |
| Ventromedial Hypothalamus | 100 ± 8.3 | 75.8 ± 10.3 |
Thymus, Adrenal, and Body Weights
Thymus, adrenal, and body weights were also determined at the time of sacrifice and are shown in Table 2. Although body weight tended to be lower after cocaine SA compared to saline SA, a significant difference was not found at the time of sacrifice. Thymus and adrenal weights were analyzed as both uncorrected values and as values normalized to body weight (mg/100 kg body weight). Both adrenal weight (t24=2.53) and adrenal/body weight ratio (t24=2.52) were significantly increased following cocaine SA (P<0.05). Thymus weight (t24=2.31; P<0.05) but not thymus/body weight ratio (P=0.06) was significantly reduced following cocaine SA.
Table 2.
Thymus, adrenal, and body weights at the time of sacrifice following cocaine (COC) or saline (SAL) SA expressed as uncorrected values (i.e., mg) and as values normalized to body weight at the time of sacrifice (i.e., mg gland weight/100 g body weight). Corrected and uncorrected adrenal weights were significantly increased and uncorrected (but not corrected) thymus weight was significantly decreased in COC rats (*P < 0.05).
| Group | ||
|---|---|---|
| SAL (n=11–13) | COC (n=12) | |
| Body Weight (g) | 424.7 ± 11.1 | 406.9 ± 9.5 |
| Adrenal Weight (mg) | 33.6 ± 4.7 | 52.4 ± 5.8* |
| Adr Wt/100 g Body Wt | 0.8 ± 0.1 | 1.3 ± 0.2* |
| Thymus Weight (mg) | 242.7 ± 16.2 | 191.2 ± 14.9* |
| Thy Wt/100 g Body Wt | 5.7 ± 0.4 | 4.7 ± 0.4 |
2.3. Experiment #2: Effects of Cocaine SA on Basal and Stressor-Induced CRH mRNA Levels in the PVN
To determine if alterations in stressor-induced CORT secretion were attributable to altered hypothalamic CRH mRNA expression, CRH mRNA levels in the PVN were measured under basal conditions or 90 min after restraint 21 days after the final cocaine or saline SA test session using in situ hybridization and are shown in Figure 4. Two-way restraint × SA condition ANOVA examining mean corrected gray levels across all levels of the PVN in sections incubated in CRH anti-sense riboprobe showed a significant effect of restraint (F1,26=6.49; P<0.05) but not SA condition and no significant restraint × SA interaction.
Figure 4 .

CRH mRNA in the PVN under basal conditions and 2 hrs after restraint 21 days after saline (Sal) or cocaine (Coc) SA. Data in 4A and 4B represent the mean corrected gray levels (gray level of PVN minus gray level of non-specifically labeled background region in the same section ±S.E.) in sections incubated in CRH anti-sense riboprobe. Overall CRH mRNA levels in the PVN were increased following restraint (*P<0.05). However no differences in basal or restaint-induced CRH mRNA levels were found between Coc and Sal rats. Panels in 4C are representative dark-field photomicrographs showing CRH mRNA expression in Sal (left) and Coc (right) rats under basal conditions (No; top panels) or after restraint (Res; bottom panels).
3. DISCUSSION
The primary finding of the present study is that cocaine SA under long-access conditions produces persistent changes in the activity of the HPA axis, including a reduction in basal plasma CORT levels, an augmentation of the CORT response to restraint, and impaired negative feedback regulation of HPA function that may be partially attributable to reduced hypothalamic GR expression. Surprisingly, the exaggerated CORT response to restraint following cocaine SA was not accompanied by an augmentation of restraint-induced CRH mRNA expression in the PVN when measured 1.5 hrs after the termination of the 30-min stressor. Overall, the findings suggest that cocaine SA under conditions designed to model use patterns in human addicts produces persistent alterations in HPA function that result in an intensified hormonal stress response.
3.1. Activation of the HPA axis by cocaine SA
Cocaine-induced increases in circulating glucocorticoid levels have been reported in rats (Galici et al., 2000; Mantsch et al., 2000; 2003), monkeys (Broadbear et al., 1999) and humans (Heesch et al., 1995; Baumann et al., 1995; Mendelson et al., 2002) and depend on the release of CRH from parvocellular neurons that originate in the PVN (Rivier and Vale, 1987). We have shown that cocaine SA under extended-access conditions similar to those used in the present study results in pronounced increases in circulating CORT levels (Mantsch et al., 2007). Although increases in plasma CORT were not measured during SA in the present study, the rats showed persistent signs of chronically elevated CORT following repeated cocaine SA, including reduced thymus mass and adrenal hypertrophy, suggesting that CORT was likely elevated during the SA sessions throughout the 14-day test period and therefore may have contributed to the alterations in HPA function found after withdrawal, most notably the observed reductions in GR.
3.2 Basal CORT levels
Our finding that cocaine SA reduced basal CORT levels is in line with earlier observations (Mantsch et al., 2000; 2003). Since we failed to find a reduction in basal CRH mRNA expression in the PVN following cocaine SA, we speculate that the reduced basal CORT levels may have been the consequence of altered pituitary function, which is consistent with our earlier finding that basal anterior pituitary POMC mRNA is reduced following long-access cocaine SA (Mantsch et al., 2003). It may be that the reduced basal CORT levels at the time of restraint contributed to the augmented stressor-induced increases following cocaine SA by removing GR-mediated basal feedback inhibitory constraint on the HPA response. Consistent with this possibility, we observed a significant inverse correlation between basal CORT levels and the magnitude of the CORT response from baseline. Notably, it does not appear that human cocaine addicts experience similar reductions in HPA function, since basal cortisol and/or ACTH levels are reportedly unchanged (Mendelson et al., 1988) or elevated (Vescovi et al., 1992; Buydens-Branchey et al., 2002; Contoreggi et al., 2003) during withdrawal. This disparity between our preclinical findings and the clinical data suggests that either there are subtle differences in the effects of cocaine SA on the HPA axis between rats and humans or that our protocol does not precisely simulate the human condition, thus highlighting that, although the long-access SA approach may be useful for investigating certain aspects of cocaine addiction, it is by no means a complete model.
3.3 Stressor-induced CORT response
Despite the reduction in basal CORT levels, the CORT response to restraint was augmented in rats with a history of cocaine SA. We have reported that repeated experimenter-administered cocaine results in an increased CORT and PVN CRH mRNA response to restraint 24 hrs into drug withdrawal (Mantsch et al., 2007), while other studies using different drug administration parameters have shown no effects (Levy et al., 1994; Sarnyai et al., 1998). Self-administered cocaine has differential effects on the HPA axis than does non-self-administered cocaine delivery (Broadbear et al., 1999; Galici et al., 2000), suggesting that the use of SA procedures may be more appropriate for investigating neuroendocrine alterations associated with abuse. Furthermore, it has been reported that when measured several weeks into withdrawal from repeated amphetamine administration, an increased restraint-induced CORT response is observed (Barr et al., 2002). Notably, our findings are consistent with the clinical data of Fox et al. (2005) who reported that stressor-induced ACTH secretion is augmented in cocaine users as a function of use history, with high-frequency users showing greater responsiveness than low-frequency users.
Since rats were tested for stressor-induced CORT secretion three days after testing with DEX, we cannot rule out the possibility that treatment-specific residual effects of DEX may have contributed to the observed differences among groups. Although it is plausible that the relatively high DEX sensitivity in saline rats may have resulted in a persistent suppression in these rats and therefore a lower CORT response, we consider it to be unlikely, since basal CORT measured immediately prior to restraint was actually higher in these rats and since we have previously demonstrated that three days is sufficient to wash out any DEX effects (Mantsch et al., 1998).
3.4 DEX suppression and GR expression
Heightened stressor-induced CORT secretion could be attributable to an augmentation of stimulatory regulation of the HPA axis or the removal of feedback inhibitory constraint. We focused on the latter possibility by examining DEX suppression of plasma CORT levels and GR protein expression in the pituitary and brain regions putatively involved in feedback regulation of the HPA axis. Cocaine self-administering rats were resistant to DEX suppression compared to saline controls, likely reflecting impaired GR regulation of HPA function in these rats. Considering that GR protein expression was selectively reduced in our dorsomedial hypothalamic dissection which included the PVN, we suggest that the augmented CORT response to restraint and impaired DEX suppression of CORT levels were the consequence of reduced GR-mediated suppression of CRH mRNA expression in the PVN. However, we failed to observe differences in restraint-induced CRH mRNA levels between cocaine and saline self-administering rats, implying that any cocaine-induced alterations in HPA responsiveness may not involve GR regulation of hypothalamic CRH.
It has been reported that DEX, at the 0.01 mg/kg dose used in this study produces its inhibitory effects on plasma CORT primarily by inhibiting POMC gene expression in the anterior pituitary gland (Birnberg et al., 1983) without producing significant inhibition of CRH mRNA in the PVN (Karssen et al., 2005). Although we failed to observe effects of cocaine SA on pituitary GR in the present study, we previously found that GR mRNA was reduced specifically in the anterior lobe of the pituitary (i.e., the site of POMC/ACTH producing corticotrophs) at a similar withdrawal time in rats with a history of long-access SA compared to rats with more limited cocaine exposure (Mantsch et al., 2003). Since we used a whole pituitary preparation for the present study, it is possible that relevant reductions in anterior pituitary GR were masked or offset and were therefore undetectable. Additionally, we cannot rule out the possibility that pituitary GR affinity or function was altered without a change in GR protein expression.
3.5 Lack of effect on stressor-induced CRH mRNA
Although restraint increased CRH mRNA expression in the PVN after both saline and cocaine SA, there was no difference in the magnitude of the CRH mRNA response. In addition to the possibilities that cocaine-induced alterations emerged at the level of the pituitary or adrenal gland (e.g., altered CRH or ACTH receptor expression or function), the disparity between our CORT and PVN CRH mRNA data could be attributable to a number of factors. First, the precise relationship between CRH mRNA expression in the PVN and CRH peptide release from the median eminence is not clear. The elevation of CRH mRNA in the PVN following stress likely represents a replenishing of parvocellular neurons to compensate for earlier peptide release. It may be that the CRH peptide and mRNA responses were differentially affected by cocaine SA. Secondly, post-restraint increases in CRH mRNA in the PVN reportedly emerge very rapidly (i.e., less than one hr; Hsu et al., 1998). Thus, we may have been able to observe differences in CRH mRNA between groups had we sacrificed rats at a time-point other than the 1.5-h post restraint time that we chose for the in situ hybridization experiment. Finally, it is possible that cocaine SA altered the stressor-induced expression/activity of other hypothalamic regulators of anterior pituitary corticotroph function such as arginine vasopression or urocortin. Each of these possibilities will require further investigation.
3.6 Relevance to cocaine-seeking behavior
As we and others have previously reported (Ahmed and Koob, 1998; Mantsch et al., 2004) rats self-administering under daily long-access conditions displayed a progressive escalation of cocaine SA that may be related to the loss of control over drug use that is a central feature of human cocaine addiction. Rats provided long access to cocaine are also more susceptible to later reinstatement in response to cocaine (Mantsch et al., 2004; Ahmed and Cador, 2006), cocaine-associated cues (Kippin et al., 2006), and electric footshock stress (unpublished data).
A role for glucocorticoids in drug-seeking behavior has been proposed (Goeders, 2002; Marinelli and Piazza, 2005). However, the exact contribution of the HPA axis to stress-driven drug use remains unclear. Although acute elevations of glucocorticoids have been reported to precipitate reinstatement in rats (Deroche et al., 1997) and craving in human addicts (Elman et al., 2003), they do not appear to be necessary for cocaine or stressor-induced reinstatement in rats (Erb et al., 1998; Shalev et al., 2002) or monkeys (Lee et al., 2003) or craving in humans (Ward et al., 1999; Mendelson et al., 2002). This is not surprising considering that neither the time-course of the CORT response to stress nor the probable genomic mechanism of CORT is compatible with immediate behavioral effects. However, with repeated or chronic stress, stressor-induced increases in circulating glucocorticoids likely exert a more substantial influence on cocaine SA and appear to facilitate the acquisition of cocaine SA (Goeders and Guerin, 1996; Mantsch et al., 1998; Campbell and Carroll, 2001) as well as the escalation of cocaine intake (Mantsch and Katz, 2007) during periods of chronic stress. For this reason, we are reluctant to suggest that an elevated HPA stress response has no functional impact of the addiction process. In support, it has recently been reported that stressor-induced cortisol levels are predictive of the amount of drug use upon relapse in cocaine-dependent individuals (Sinha et al., 2006).
4. EXPERIMENTAL PROCEDURE
4.1 Subjects
Seventy-four adult male Sprague-Dawley rats (Harlan Laboratories, Inc., St. Louis, MO), approximately 90 days old (325 g) were used for the study. All rats were housed individually in a temperature- and humidity-controlled, AAALAC-accredited animal facility under a 12h/12h reversed light/dark cycle (lights on at 6:00 pm) and had access to food and water at all times, except when in the experimental chambers. All procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the NIH.
4.2 General SA Procedures
Catheterization Surgery
Rats were implanted with chronic indwelling catheters under ketamine HCl (100 mg/kg, ip) and xylazine (2 mg/kg, ip) anesthesia as previously described (Mantsch and Katz, 2007) and were allowed to recover for at least three days prior to SA testing during time they were provided acetaminophen (480 mg/L) in their drinking water. After implantation, rats were injected daily with a sterile cefazolin antibiotic solution (15 mg, iv). Catheters were filled daily with a heparin solution (83 i.u./ml) and capped whenever the leash/delivery line assembly was disconnected.
Apparatus
Twenty plastic and stainless steel operant conditioning chambers encased in sound attenuating cubicles and equipped with retractable levers with stimulus lights located above each lever were used (MED-Associates Inc., St Albans, VT). One lever and its stimulus light were mounted on the front wall of the chamber. A second lever and light were located on the back wall. Cubicles were equipped with exhaust fans that provided ventilation and white noise.
SA training/testing
Following recovery from surgery, rats were assigned to one of two groups. Cocaine SA rats were trained to self-administer cocaine (1.0 mg/kg/inf, iv, NIDA, Bethesda, MD) by pressing a lever under a FR1 schedule during daily 2-h sessions. Saline SA rats were provided access to saline (0.9% NaCl) under identical conditions. During the training sessions, the active (i.e., front) lever was extended into the chamber and the corresponding stimulus light was illuminated. Pressing this lever resulted in an iv infusion of cocaine or saline solution (200μl over 5.0 s) followed by a 25-s time-out period during which time the stimulus light was extinguished but the lever remained extended. Responding on a second, inactive (i.e., back) lever was recorded but had no programmed consequences. Once cocaine SA rats self-administered more than 10 cocaine infusions under the FR1 schedule, the response requirements for SA were increased to FR2 and then to FR4 after 10 infusions were self-administered under the FR2 schedule. After stable response patterns were observed under the FR4 schedule (total responding within 10% of the mean over 3 consecutive sessions), rats were assigned to one of the experiments described below. Saline rats were introduced into the experiments after one week of access to saline, at which time their response requirement for saline SA was increased to FR4. SA testing and training sessions began between 7:00 am and 8:00 am each day.
4.3 CORT Radioimmunoassay
In all cases, plasma CORT was measured using commercial radioimmunoassay (RIA) kits (MP Biochemicals, Irvine, CA). Blood for CORT determination was collected in tubes containing heparin, stored on ice, and centrifuged to allow separation of plasma, which was frozen at −80° C. Plasma samples were analyzed in duplicate using four RIA kits. The mean coefficient of intra-assay variation determined from replicates of randomly chosen samples analyzed in the same assay was 9.26%. The mean coefficient of inter-assay variation determined from replicates of randomly chosen samples analyzed in different assays was 20.89%.
4.5 Experiment #1: Effects of Cocaine SA on Basal and Stressor-Induced CORT, GR-Mediated Negative Feedback, and Thymus and Adrenal Masses
Thirty-two rats were used for this experiment: 16 cocaine SA rats and 16 saline SA rats. After meeting the training criteria, these rats were provided access to cocaine (1.0 mg/kg/inf) or saline for SA under a FR4 schedule during 6-h sessions for 14 days. Twenty-one days after the final SA test session, blood samples were acquired under basal conditions and following restraint or dexamethasone DEX administration over a 3-day period prior to sacrifice for GR protein analysis and measurement of thymus and adrenal masses.
Effects on Basal, Post-Restraint, and Post-DEX Plasma CORT
Three blood samples were collected from each rat: one sample following administration of DEX, one basal sample, and one sample following 30 min of restraint. The sequence of sampling conditions was the same for all rats: the post-DEX sample was acquired 21 days after the final SA session and the basal and post-restraint samples were collected three days later (i.e., 24 days after the final SA session). During the first sampling period, blood (200 μl) for plasma CORT determination was collected from the tail 60 min after an ip injection of 0.01 mg/kg DEX. It has been previously demonstrated that this dose of DEX produces a sub-maximal suppression of plasma CORT levels (Mantsch et al., 1998). Three days later, tail blood was collected for basal plasma CORT determination. We have previously shown that the effects of DEX on CORT are no longer evident three days after administration of the 0.01 mg/kg dose (Mantsch et al., 1998). Immediately after collection of the basal blood sample, rats were placed into acrylic restraining devices (21.6 cm length × 6.4 cm diameter) and subjected to 30 min of restraint. Upon removal from the restraining devices, rats were rapidly decapitated and trunk blood was collected for measurement of stressor-induced CORT levels using RIA. Both post-DEX and basal blood samples were collected at 10:00 am (i.e., three hrs into the dark phase) while the post-restraint blood sample was collected at 10:30 am.
Tissue dissection
Following decapitation immediately after restraint, the brain was rapidly excised and frozen in a −30° C isopentane solution. The pituitary gland was also removed and frozen on dry ice. Brain and pituitary were transferred to a −80° C freezer for storage until later dissection. Additionally, thymus and adrenal glands were removed and weighed. Thymus and adrenal weights are reliable indicators of chronic activation of the HPA axis (Proulx and Gorski, 1965; Akana et al., 1985). Frozen brains were thawed on ice to permit dissection of the following brain regions: medial prefrontal cortex, dorsomedial hypothalamus (including the PVN), ventromedial hypothalamus (including the arcuate nucleus, ventral medial nucleus of the hypothalamus, and median eminence), amygdala, dorsal hippocampus, and ventral subiculum. Brains were placed into a stainless steel brain block cooled to −20°C and slices (1 or 2 mm) were cut using a straight edge razor blade. Tissues were hand dissected as shown in Figure 5. Coronal slices from which tissues were dissected were defined according to coordinates determined from Paxinos and Watson (1998). Briefly, mPFC was dissected from a 1 mm slice that extended from bregma +3.20 to +2.20 mm. The dorsomedial hypothalamus (containing the PVN) was dissected from a 1 mm slice that extended from bregma −0.80 mm to −1.80 mm. The ventromedial hypothalamus was dissected from the same slice and from a second 1 mm slice that extended from bregma −1.80 mm to −2.80 mm. The amygdala was dissected from the same slice and from a second 1 mm slice that extended from bregma −2.80 mm to −3.80 mm. The dorsal hippocampus was dissected −4.80 mm. The ventral subiculum was dissected from a 1 mm slice that extended from bregma −6.30 mm to −7.30 mm. Samples were homogenized by sonication on ice using a sonic dismembrator in a Tris homogenization buffer (50 mM Tris, pH 7.2) containing 10% (wt/vol) sucrose, 6mM MgCl2 and protease inhibitors (1mM EDTA, 1mM PMSF, 3mM benzamidine, 5 μg/ml leupeptin, 1 μg/ml pepstatin A, 1 μg/ml aprotinin, 5 μg/ml bestatin, 2 μg/ml E64) and were frozen at −80° C.
Figure 5.
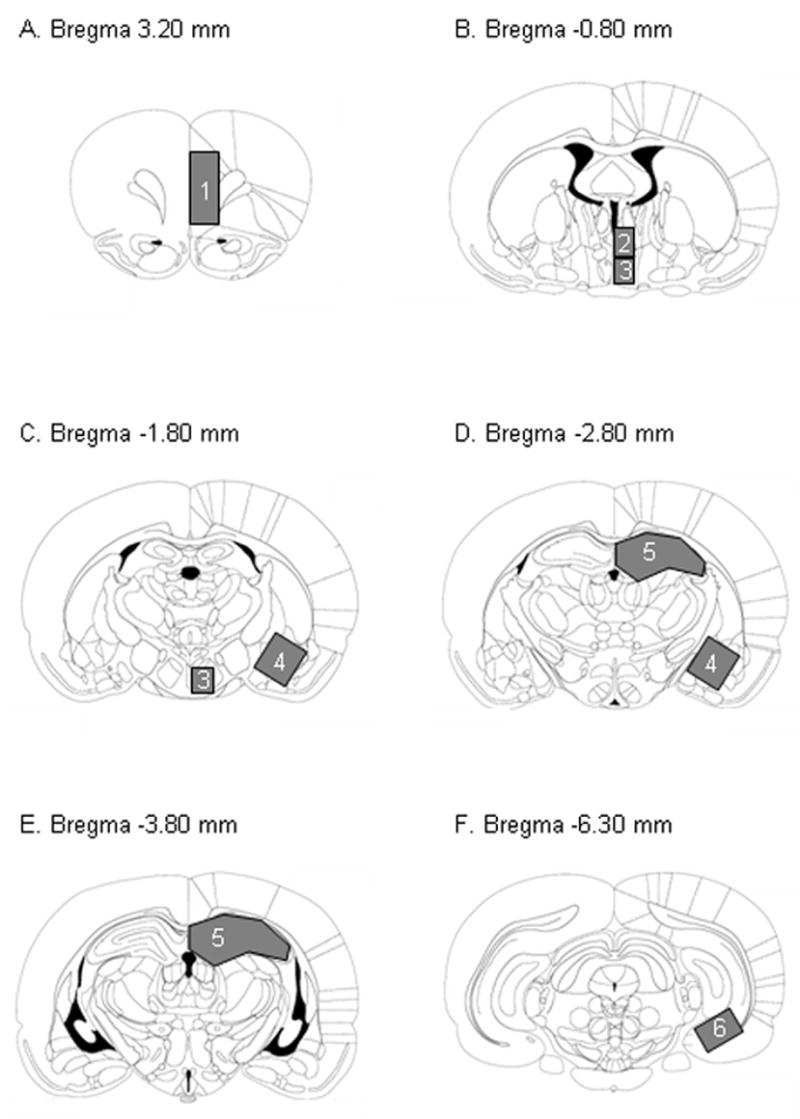
Schematic of brain tissue dissections for regional GR determination. Panels represent coronal sections reproduced from Paxinos and Watson (1998) with the coronal plane defined according to coordinates in relation to Bregma. Blocked areas represent dissected brain regions (see Experimental Procedure). Numbers correspond to the following regions: 1. medial prefrontal cortex; 2. dorsomedial hypothalamus; 3. ventromedial hypothalamus; 4. amygdala; 5. dorsal hippocampus; 6. ventral subiculum.
PAGE Western Blot Analysis
GR protein expression was measured in singulate in samples of whole cell tissue homogenate using polyacrylamide gel electrophoresis (Laemmli, 1970) followed by Western blot analysis with the polyclonal antibody, GR M-20, raised against a peptide corresponding to the N-terminus of the mouse GR (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Following protein determination using the BCA (bicinchoninic acid) assay (Wiechelman et al., 1988), samples were diluted in buffer (pH=6.8; 0.25 M Tris-HCl, 4 M urea, 10% glycerol, 1% SDS, 5% beta-mercaptoethanol) and loaded onto a 7.5% polyacrylamide gel (30 μg protein/lane). Gel electrophoresis was performed using a Bio-Rad Mini-protean 3 system at 200 V for 20 min followed by 35–40 min at 180 V. Samples were then transferred onto PVDF membranes (Immun-Blot™; Bio-Rad Laboratories) at 100 V for 50 min at 4°C using a Bio-Rad Mini-Transblot wet transfer system. Following washes and blocking in a TBS solution containing 10% non-fat dried milk and 0.5 μl/ml Tween 20 for 90 min, membranes were incubated with GR M-20 (1:500) for 2 hrs at room temperature and then with a secondary horseradish peroxidase-labeled antibody (donkey anti-rabbit; Amersham Biosciences; 1:5000) for 90 min also at room temperature. An enhanced chemi-luminescent detection system (Amersham) was used to detect immunoreactive bands. Optical density readings were determined using a Kodak ImageStation 4000MM system and Kodak Molecular Imaging software. Raw optical density data were converted to percentages of the mean of the optical density readings for SAL SA control samples on the same membrane. Each membrane contained at least three SAL control samples.
4.6: Experiment #2: Effects of Cocaine SA on Basal and Stressor-Induced CRH mRNA Levels in the PVN
Thirty-two rats were used for this experiment: 16 cocaine SA rats and 16 saline SA rats. As described for Experiment #2, these rats were provided access to cocaine (1.0 mg/kg/inf) or saline for SA under a FR4 schedule during 6-h sessions for 14 days. Twenty-one days after the final SA test session, rats were sacrificed under basal conditions (i.e., immediately after removal from the home-cages; n=8 each for the saline and cocaine SA groups) or 90 min after the termination of 30 min of restraint (n=8/group). As described above rats were decapitated and brain was rapidly excised and frozen in a −30° C isopentane solution and stored at −80º C. Brains were later sectioned on a cryostat at 16 μm, mounted onto polylysine coated slides and stored at −80º C until processing.
In Situ Hybridization
35S-labeled anti-sense probes for corticotropin releasing hormone (CRH) were generated from linearized cDNA encoding a 760bp segment spanning from exon 2 to the 3′ untranslated region. The specificity of this probe has been previously characterized (Herman et al., 1992). Linearized DNA template was transcribed to anti-sense riboprobe with T7 polymerase, followed by isolation of probe by high salt/ethanol precipitation. Prior to hybridization, slides with 16 μm cryosectioned coronal brain slices containing the PVN (Paxinos and Watson, 1996) were removed from −80°C storage and subjected to standard pre-hybridization treatment (post-fixation in 4% paraformaldehyde; glycine treatment; acetylation; dehydration/delipidation in ethanols/chloroform; air-drying). All solutions used were treated with diethylpyrocarbonate. Probe was diluted in commercial hybridization buffer (Amresco Solon, OH) with 100 mM DTT. Each slide was hybridized with 50μl hybridization medium containing 1 × 106 cpm probe, coverslipped, and incubated overnight at 55°C in chambers humidified with 50% formamide. Following incubation, coverslips were removed and slides were post-treated (rinses in 2X SSC; 200 μg/ml RNAse digestion of unbound probe; SSC rinses; 55°C hot bath in 0.2X SSC; dehydration in ethanols; air drying). Slides were exposed to Kodak BioMAX MR autoradiographic film for 1 week. Densitometric image analysis was performed using Scion Image 1.59 software. Data are presented as corrected gray level (gray level over sampled area minus gray level of a non-specifically labeled background region (i.e., the corpus callosum) in the same section).
4.6 Statistical Analyses
SA by cocaine and saline rats across the 14 days of testing was examined using two-way ANOVA, followed by analysis of simple main effects within each group using one-way ANOVA and post-hoc analysis using two-tailed Dunnett t-tests with all comparisons with day one of SA testing. The significance of differences in thymus, adrenal and body masses and regional GR levels between saline and cocaine self-administering rats was determined using two-tailed Student’s t-tests. The significance of differences in plasma CORT and/or CRH mRNA under basal conditions, following restraint, and following DEX administration was determined using two-way ANOVA followed, when appropriate, by further analysis within groups using one-way ANOVA and post-hoc testing using a two-tailed Dunnett t-test in the case of multiple comparisons or a two-tailed t-test in the case of single comparisons. CORT responses to restraint and DEX were also expressed as percent changes from baseline values. Since Kolmogerov-Smirnov (K-S) tests indicated that these percent change data were likely not normally distributed, Mann-Whitney U tests were used to determine the significance of differences in the percent baseline CORT response between cocaine and saline SA rats. Simple linear regression analysis was used to examine the significance of the relationship between basal CORT levels and the CORT response to restraint. All statistical analyses were performed using SPSS 14.0 software (Chicago, IL). For all analyses, significance was defined as P<0.05.
Acknowledgments
This work was supported by NIDA grant number DA15758 to JRM. The authors would like to thank Joseph Serge, David Francis, and Tanveer Sajan for their technical assistance and Dr. Pastor Couceyro for his guidance with the in situ hybridization experiment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Cador M. Dissociation of psychomotor sensitization from compulsive cocaine consumption. Neuropsychopharmacol. 2006;31:563–571. doi: 10.1038/sj.npp.1300834. [DOI] [PubMed] [Google Scholar]
- Akana SF, Cascio CS, Shinsako J, Dallman MF. Corticosterone: narrow range required for normal body and thymus weight and ACTH. Am J Physiol. 1985;249:R527–32. doi: 10.1152/ajpregu.1985.249.5.R527. [DOI] [PubMed] [Google Scholar]
- Barr AM, Hofmann CE, Weinberg J, Phillips AG. Exposure to repeated, intermittent d-amphetamine induces sensitization of HPA axis to a subsequent stressor. Neuropsychopharmacol. 2002;26:286–94. doi: 10.1016/S0893-133X(01)00308-6. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Gendron TM, Becketts KM, Henningfield JE, Gorelick DA, Rothman RB. Effects of intravenous cocaine on plasma cortisol and prolactin in human cocaine abusers. Biol Psychiatry. 1995;38:751–755. doi: 10.1016/0006-3223(95)00083-6. [DOI] [PubMed] [Google Scholar]
- Birnberg NC, Lissitzky JC, Hinman M, Herbert E. Glucocorticoids regulate proopiomelanocortin gene expression in vivo at the levels of transcription and secretion. Proc Natl Acad Sci USA. 1983;80:6982–6. doi: 10.1073/pnas.80.22.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowsky B, Kuhn CM. Chronic cocaine administration sensitizes behavioral but not neuroendocrine responses. Brain Res. 1991;543:301–306. doi: 10.1016/0006-8993(91)90041-s. [DOI] [PubMed] [Google Scholar]
- Broadbear JH, Winger G, Cicero TJ, Woods JH. Effects of response contingent and noncontingent cocaine injection on hypothalamic-pituitary-adrenal activity in rhesus monkeys. J Pharmacol Exp Ther. 1999;290:393–402. [PubMed] [Google Scholar]
- Buydens-Branchey L, Branchey M, Hudson J, Dorota Majewska M. Perturbations of plasma cortisol and DHEA-S following discontinuation of cocaine use in cocaine addicts. Psychoneuroendocrinol. 2002;27:83–97. doi: 10.1016/s0306-4530(01)00037-3. [DOI] [PubMed] [Google Scholar]
- Campbell UC, Carroll ME. Effects of ketoconazole on the acquisition of intravenous cocaine self-administration under different feeding conditions in rats. Psychopharmacol. 2001;154:311–318. doi: 10.1007/s002130000627. [DOI] [PubMed] [Google Scholar]
- Contoreggi C, Herning RI, Koeppl B, Simpson PM, Negro PJ, Jr, Fortner-Burton C, Hess J. Treatment-seeking inpatient cocaine abusers show hypothalamic dysregulation of both basal prolactin and cortisol secretion. Neuroendocrinol. 2003;78:154–162. doi: 10.1159/000072797. [DOI] [PubMed] [Google Scholar]
- Deroche V, Marinelli M, Le Moal M, Piazza PV. Glucocorticoids and behavioral effects of psychostimulants. II: Cocaine intravenous self-administration and reinstatement depend on glucocorticoid levels. J Pharmacol Exp Ther. 1997;281:1401–1407. [PubMed] [Google Scholar]
- Elman I, Lukas SE, Karlsgodt KH, Gasic GP, Breiter HC. Acute cortisol administration triggers craving in individuals with cocaine dependence. Psychopharmacol Bull. 2003;37:84–89. [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J Neurosci. 1998;18:5529–36. doi: 10.1523/JNEUROSCI.18-14-05529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Talih M, Malison R, Anderson GM, Kreek MJ, Sinha R. Frequency of recent cocaine and alcohol use affects drug craving and associated responses to stress and drug-related cues. Psychoneuroendocrinol. 2005;30:880–91. doi: 10.1016/j.psyneuen.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Galici R, Pechnick RN, Poland RE, France CP. Comparison of noncontingent versus contingent cocaine administration on plasma corticosterone levels in rats. Eur J Pharmacol. 2000;387:59–62. doi: 10.1016/s0014-2999(99)00780-3. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Guerin GF. Role for corticosterone in intravenous cocaine self-administration in rats. Neuroendocrinol. 1996;64:337–348. doi: 10.1159/000127137. [DOI] [PubMed] [Google Scholar]
- Goeders NE. Stress and cocaine addiction. J Pharmacol Exp Ther. 2002;301:785–9. doi: 10.1124/jpet.301.3.785. [DOI] [PubMed] [Google Scholar]
- Heesch CM, Negus BH, Bost JE, Keffer JH, Snyder RW, II, Eichhorn EJ. Effects of cocaine on anterior pituitary and gonadal hormones. J Pharmacol Exp Ther. 1996;278:1195–1200. [PubMed] [Google Scholar]
- Herman JP, Schafer MK, Thompson RC, Watson SJ. Rapid regulation of corticotropin-releasing hormone gene transcription in vivo. Mol Endocrinol. 1992;6:1061–1069. doi: 10.1210/mend.6.7.1324419. [DOI] [PubMed] [Google Scholar]
- Hsu DT, Chen FL, Takahashi LK, Kalin NH. Rapid stress-induced elevations in corticotropin-releasing hormone mRNA in rat central nucleus and hypothalamic paraventricular nucleus: an in situ hybridization analysis. Brain Res. 1998;788:305–310. doi: 10.1016/s0006-8993(98)00032-8. [DOI] [PubMed] [Google Scholar]
- Karssen AM, Meijer OC, Berry A, Sanjuan Pinol R, de Kloet ER. Low doses of dexamethasone can produce a hypocorticosteroid state in the brain. Endocrinology. 2005;146:5587–95. doi: 10.1210/en.2005-0501. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Fuchs RA, See RE. Contributions of prolonged contingent and noncontingent cocaine exposure to enhanced reinstatement of cocaine seeking in rats. Psychopharmacol. 2006;187:60–67. doi: 10.1007/s00213-006-0386-3. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee B, Tiefenbacher S, Platt DM, Spealman RD. Role of the hypothalamic-pituitary-adrenal axis in reinstatement of cocaine-seeking behavior in squirrel monkeys. Psychopharmacol. 2003;168:177–183. doi: 10.1007/s00213-003-1391-4. [DOI] [PubMed] [Google Scholar]
- Levy AD, Li Q, Alvarez Sanz MC, Rittenhouse PA, Kerr JE, Van de Kar LD. Neuroendocrine responses to cocaine do not exhibit sensitization following repeated cocaine exposure. Life Sci. 1992;51:887–97. doi: 10.1016/0024-3205(92)90396-7. [DOI] [PubMed] [Google Scholar]
- Levy AD, Rittenhouse PA, Li Q, Yracheta J, Kunimoto K, Van de Kar LD. Influence of repeated cocaine exposure on the endocrine and behavioral responses to stress in rats. Psychopharmacol. 1994;113:547–54. doi: 10.1007/BF02245238. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Saphier D, Goeders NE. Corticosterone facilitates the acquisition of cocaine self-administration in rats: Opposite effects of the Type II glucocorticoid receptor agonist dexamethasone. J Pharmacol Exp Ther. 1998;287:72–80. [PubMed] [Google Scholar]
- Mantsch JR, Schlussman SD, Ho A, Kreek MJ. Effects of cocaine self-administration on plasma corticosterone and prolactin in rats. J Pharmacol Exp Ther. 2000;294:239–247. [PubMed] [Google Scholar]
- Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ. Neuroendocrine alterations in a high-dose, extended-access rats self-administration model of escalating cocaine use. Psychoneuroendocrinol. 2003;28:836–862. doi: 10.1016/s0306-4530(02)00088-4. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Yuferov V, Mathieu-Kia A-M, Ho A, Kreek MJ. Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Psychopharmacol. 2004;175:26–36. doi: 10.1007/s00213-004-1778-x. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Katz ES. Elevation of glucocorticoids is necessary but not sufficient for the escalation of cocaine self-administration by chronic electric footshock stress in rats. Neuropsychopharmacol. 2007;32:367–376. doi: 10.1038/sj.npp.1301077. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Taves S, Khan T, Katz ES, Sajan T, Tang LC, Cullinan WE, Ziegler DR. Restraint-induced corticosterone secretion and hypothalamic CRH mRNA expression are augmented during acute withdrawal from chronic cocaine administration. Neurosci Lett. 2007;415:269–273. doi: 10.1016/j.neulet.2007.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli M, Piazza PV. Glucocorticoid hormones, individual differences, and behavioral and dopaminergic responses to psychostimulant drugs. In: Steckler T, Kalin NH, Reul JMHM, editors. Handbook of Stress and the Brain. Vol. 15. Elsevier; Amsterdam: 2005. pp. 89–111. [Google Scholar]
- Mendelson JH, Teoh SK, Lange U, Mello NK, Weiss R, Skupny A, Ellingboe J. Anterior pituitary, adrenal, and gonadal hormones during cocaine withdrawal. Am J Psychiatry. 1988;145:1094–1098. doi: 10.1176/ajp.145.9.1094. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Teoh SKMello NK, Ellingboe J, Rhoades E. Acute effects of cocaine on plasma adrenocorticotropic hormone, luteinizing hormone, and prolactin levels in cocaine-dependent men. J Pharmacol Exp Ther. 1992;263:505–509. [PubMed] [Google Scholar]
- Mendelson JH, Mello NK, Sholar MB, Siegel AJ, Mutschler N, Halpern J. Temporal concordance of cocaine effects on mood states and neuroendocrine hormones. Psychoneuroendocrinol. 2002;27:71–82. doi: 10.1016/s0306-4530(01)00036-1. [DOI] [PubMed] [Google Scholar]
- Moldow RL, Fischman AJ. Cocaine induced secretion of ACTH, beta-endorphin, and corticosterone. Peptides. 1987;8:819–822. doi: 10.1016/0196-9781(87)90065-9. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. Academic Press; San Diego: 1998. [Google Scholar]
- Proulx RP, Gorski RA. Thyroid and adrenal regulation in the steroid-sterilized rat. Endocrinology. 1965;77:406–8. doi: 10.1210/endo-77-2-406. [DOI] [PubMed] [Google Scholar]
- Rivier C, Vale W. Cocaine stimulates adrenocorticotropin (ACTH) secretion through a corticotropin-releasing factor (CRF)-mediated mechanism. Brain Res. 1987;422:403–406. doi: 10.1016/0006-8993(87)90953-x. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Biro E, Penke B, Telegdy G. The cocaine-induced elevation of plasma corticosterone is mediated by corticotrophin-releasing factor (CRF) in rats. Brain Res. 1992;589:154–156. doi: 10.1016/0006-8993(92)91176-f. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Mello NK, Mendelson JH, Erös-Sarnyai M, Mercer G. Effects of cocaine on pulsatile activity of the hypothalamic-pituitary-adrenal axis in male rhesus monkeys. Neuroendocrine and behavioral correlates. J Pharmacol Exp Ther. 1996;277:225–234. [PubMed] [Google Scholar]
- Sarnyai Z, Dhabhar FS, McEwen BS, Kreek MJ. Neuroendocrine-related effects of long-term, “binge” cocaine administration: Diminished individual differences in stress-induced corticosterone response. Neuroendocrinol. 1998;68:334–344. doi: 10.1159/000054382. [DOI] [PubMed] [Google Scholar]
- Schmidt ED, Tilders FJ, Janszen AW, Binnekade R, De Vries TJ, Schoffelmeer AN. Intermittent cocaine exposure causes delayed and long-lasting sensitization of cocaine-induced ACTH secretion in rats. Eur J Pharmacol. 1995;285:317–321. doi: 10.1016/0014-2999(95)00540-2. [DOI] [PubMed] [Google Scholar]
- Shalev U, Marinelli M, Baumann MH, Piazza PV, Shaham Y. The role of corticosterone in food deprivation-induced reinstatement of cocaine seeking in the rat. Psychopharmacol. 2003;168:170–176. doi: 10.1007/s00213-002-1200-5. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacol. 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63:324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Vescovi PP, Coiro V, Volpi R, Passeri M. Diurnal variations in plasma ACTH, cortisol, and beta-endorphin levels in cocaine addicts. Horm Res. 1992;37:221–224. doi: 10.1159/000182316. [DOI] [PubMed] [Google Scholar]
- Ward AS, Collins ED, Haney M, Foltin RW, Fischman MW. Blockade of coaine-induced increases in adrenocorticotrophic hormone and cortisol does not attenuate the subjective effects of smoked cocaine in humans. Behav Pharmacol. 1999;10:523–529. doi: 10.1097/00008877-199909000-00010. [DOI] [PubMed] [Google Scholar]
- Wiechelman KJ, Braun RD, Fitzpatrick JD. Investigation of the bicinchoninic acid protein assay: identification of the groups responsible for color formation. Anal Biochem. 1988;175:231–237. doi: 10.1016/0003-2697(88)90383-1. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Spangler R, LaForge KS, Maggos CE, Ho A, Kreek MJ. Corticotropin-releasing factor and type 1 corticotropin-releasing factor receptor messenger RNAs in rat brain and pituitary during “binge”-pattern cocaine administration and chronic withdrawal. J Pharmacol Exp Ther. 1996;279:351–358. [PubMed] [Google Scholar]
- Zhou Y, Spangler R, Schlussman SD, Ho A, Kreek MJ. Alterations in hypothalamic-pituitary-adrenal axis activity and in levels of proopiomelanocortin and corticotropin-releasing hormone-receptor 1 mRNAs in the pituitary and hypothalamus of the rat during chronic ‘binge’ cocaine and withdrawal. Brain Res. 2003;964:187–99. doi: 10.1016/s0006-8993(02)03929-x. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF-like immunoreactivity and plasma corticosterone during protracted withdrawal in dependent rats. Psychopharmacol. 2001;158:374–381. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]





