Abstract
Alien has characteristics of a corepressor for selected members of the nuclear hormone receptor (NHR) superfamily and also for transcription factors involved in cell cycle regulation and DNA repair. Alien mediates gene silencing and represses the transactivation of specific NHRs and other transcription factors to modulate hormone response and cell proliferation. Alien is a highly conserved protein and is expressed in a wide variety of tissues. Knockout of the gene encoding Alien in mice is embryonic lethal at a very early stage, indicating an important evolutionary role in multicellular organisms. From a mechanistic perspective, the corepressor function of Alien is in part mediated by histone deacetylase (HDAC) activity. In addition, Alien seems to modulate nucleosome assembly activity. This suggests that Alien is acting on chromatin not only through recruitment of histone-modifying activities, but also through enhancing nucleosome assembly.
Introduction
The name Alien was originally given to a gene in the Drosophila genome with an unknown function [Goubeaud et al., 1996] sharing high homologies to at that time partial gene sequence of TRIP15 isolated in a yeast 2-hybrid screen as a hormone-sensitive interacting partner for the thyroid hormone receptor (TR) [Lee et al., 1995]. Subsequently, Alien was characterized as a bona fide corepressor for TR, which enhanced receptor-mediated silencing [Burke and Baniahmad, 2000; Dressel et al., 1999], and was also found to function as a potent corepressor for the vitamin D receptor (VDR) [Polly et al., 2000].
Alien is found throughout the multicellular kingdom and is highly conserved between human, D. melanogaster and C. elegans, whereas no factor with significant homology has been found in S. cerevisiae. Alien is expressed in mice in the majority of tissues at levels detectable by in situ hybridization and at early embryonic stages. Notably, several Alien isoforms were detected in vivo for which the predominant forms were termed Alien α and Alien β [Tenbaum et al., 2003]. Alien β represents one of the eight subunits of the COP9 signalosome complex (CSN complex) and is hereafter termed CSN2.
The COP9 signalosome complex
Evolutionarily, the COP9 signalosome complex is highly conserved and its subunits possess remarkable homologies to the 19S lid of the 26S proteasome and are currently postulated to play a largely undetermined role in protein degradation [Richardson and Zundel, 2005]. The CSN was initially identified as a repressor of light-controlled development in Arabidopsis thaliana [Wei et al., 1994]. Functionally, the CSN has been found to play a central and necessary role in the degradation of multiple proteins that are known regulators of disease progression in diverse cancers. Although most of the proteins interact with the CSN5 subunit, it is unclear whether all these proteins are targets for degradation or if there are as yet undetermined functions of the CSN complex per se, or the individual CSN subunits. Furthermore, this complex possesses an associated yet unidentified kinase activity, which phosphorylates signaling molecules such as IκBa and c-Jun [Naumann et al., 1999; Seeger et al., 1998; Wei and Deng, 1998]. In addition, CSN mini-complexes have been isolated mainly localized in cytoplasm and were suggested to be involved in nuclear export of the cyclin-dependent kinase inhibitor p27/KIP1 [Callige et al., 2005; Tomoda et al., 2002]. Other reports found different subunits in both CSN-dependent and CSN-independent forms [Oron et al., 2002].
The COP9 signalosome also seems to be a multifunctional regulator essential for Drosophila development [Freilich et al., 1999; Harari-Steinberg et al., 2007; Lier and Paululat, 2002]. A CSN2/Alien knockout in Drosophila has not yet been described. Interestingly, phenotypic characterization of two other CSN subunit mutants in Drosophila indicates that they show both shared and unique in vivo phenotypes, which suggests specific roles for each subunit.
Notably, mutation of CSN4 led to phenotypes reminiscent of defects in ecdysone signaling [Oron et al., 2002], suggesting another link between a CSN subunit and ecdysone receptor-mediated signaling. Thus, CSN sub- and mini-complexes expand the complexity of the CSN regulatory network. Therefore, future work will shed light on the functional roles of individual CSN subunits, both within and independent of the CSN complex.
Knockout/mutational studies in a variety of organisms suggest that the CSN complex is involved in pleiotropic functions (including cell cycle progression, radiation sensitivity, genome stability, and cell survival) that largely overlap known Cullin-regulated phenotypes [Schwechheimer, 2004; Wei and Deng, 2003].
Furthermore, several components of the CSN-complex, including CSN2, have also been found to be directly associated with proto-oncogenes and tumor suppressors, and can regulate their function. Disruption of the CSN2 subunit in mice causes deficiencies in cell proliferation, accumulation of p53 and cyclin E, and embryonic death at a very early stage. In line with these observations, overexpression of CSN2 leads to an accelerated degradation of p53 [Huang et al., 2005]. Recently, the CSN has also been linked to chromatin and shown to be recruited to chromatin sites, ostensibly to modulate the histone code [O'Connell and Harper, 2007], thus opening a novel avenue of transcriptional regulation through heterochromatin, protein stability and degradation at the chromatin level.
Interaction of the corepressor Alien with nuclear hormone receptors
As mentioned, Alien was originally identified in a yeast 2-hybrid screening as a TR-interacting protein [Lee et al., 1995]. This interaction occurs in a ligand-sensitive manner; in the absence of thyroid hormone, Alien interacts with the thyroid hormone receptors TRα and TRβ, and this interaction is inhibited by treatment with thyroid hormone, thus suggesting a hormone-sensitive interaction of Alien with the TRs. Furthermore, Alien harbors an autonomous silencing function and enhances TR-mediated gene silencing [Dressel et al., 1999]. Similar findings were observed when analyzing the functional interaction of Alien with the VDR [Polly et al., 2000].
The VDR is a transcription factor, which after binding 1,25-dihydroxyvitamin D3 (VitD3), recruits coactivator proteins to specific DNA binding sites and promotes the transcriptional activation of its target genes [Carlberg, 1995]. Polly et al. showed that VDR interacts effectively with Alien and this interaction was found to be comparable with the Alien-TR interaction [Polly et al., 2000]. Interestingly, in vitro and in vivo experiments demonstrate that Alien interacts more effectively with VDR compared to the corepressor NCoR. Accordingly, higher concentrations of VitD3 are needed to dissociate the VDR-Alien complex, compared to the VDR-NCoR complexes [Polly et al., 2000]. Furthermore, Alien, but not NCoR, displays selectivity for different vitamin D response element motifs in order to mediate these repressive effects upon its target genes [Polly et al., 2000]. These results enhance the hypothesis that Alien and NCoR are using different interfaces for interaction with the VDR. Also, due to the observed selectivity of the Alien- or NCoR-mediated repression, it may be speculated that different signaling pathways are used depending on the vitamin D response elements.
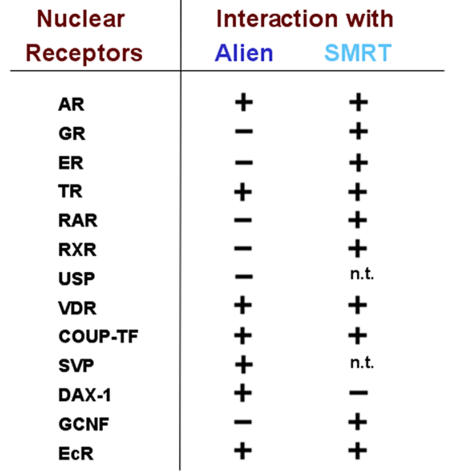
Notably, Alien seems to lack interaction with the heterodimer partners of TR, RXR and RAR [Dressel et al., 1999] and (Table 1). With regard to orphan receptors, Alien interacts with DAX-1 [Altincicek et al., 2000], whereas it fails to bind to the orphan receptor GCNF (germ cell nuclear factor) [Fuhrmann et al., 2001]. GCNF does, however, interact with the corepressors SMRT and NCoR, indicating a corepressor specificity for NHRs (Table 1). On the other hand, DAX-1 interacts with the corepressor Alien, but not with the corepressor SMRT [Altincicek et al., 2000]. DAX-1 is an unusual member of the NHR superfamily, since only the carboxy-terminus, but not the DNA-binding domain, is homologous to other members of the NHR superfamily [Muscatelli et al., 1994; Zanaria et al., 1994; Zazopoulos et al., 1997]. The interaction of DAX-1 with Alien is mediated by the DAX-1-silencing domain localized in the NHR-homologous regions. In line with this, the naturally-occurring mutants of the DAX-1 gene, that cause the X-linked disorder adrenal hypoplasia congenital and the associated hypogonadotropic hypogonadism [Habiby et al., 1996; Swain and Lovell-Badge, 1999], have lost silencing function and fail to interact with Alien. These findings suggest that corepressors possess receptor specificity and, on the other hand, NHRs exhibit corepressor-specific interactions. In addition, these findings suggest that dysregulated corepressor-NHR interaction leads to failure of normal development.
Table 1. Corepressor specificity for NHRs.
Interaction of the corepressors Alien or SMRT were compared and indicated by (+) for interaction and (-) for lack of interaction. The binding of these corepressors to the steroid receptors was compared in the presence of the corresponding antagonists. The table, a summary of interaction data from (Dressel et al., 1999; Altincicek et al., 2000; and Fuhrmann et al., 2001), indicates the receptor-corepressor specificity that might explain a possible biological role for the existence of several types of corepressors.
Based on the high degree of homology of Alien between Drosophila and mammals, insect-derived NHRs were tested for interaction with Alien. Interestingly, Alien binds to the ecdysone receptor, but not to its heterodimer partner ultraspiracle [Dressel et al., 1999]. Further, both COUP-TF1 and the Drosophila homolog seven-up revealed interaction with Alien, indicating some conserved similarities in the interaction pattern of Alien (Table 1). Whereas Fushi tarazu-F1 binds to Alien in vitro, other Drosophila NHRs, such as DHR3, DHR38, DHR78 and DHR96 did not reveal interaction with Alien [Dressel et al., 1999]. This indicates that Alien exhibits receptor-specific interaction with members of the NHR superfamily.
In contrast to most of the non-steroid hormone receptors, the steroid hormone receptors lack a classical silencing domain used for active gene repression. Nevertheless, the SMRT corepressor associates with steroid receptors, such as the AR, in the presence of both agonist and antagonist [Dotzlaw et al., 2002; Dotzlaw et al., 2003]. Interestingly, despite this, SMRT mediates corepression only in the presence of antagonists. Although Alien did not reveal an association with the glucocorticoid receptor or the estrogen receptor (unpublished observation), a ligand-specific interaction of Alien with the human androgen receptor (AR) was observed (Table 1). Alien interacts with AR with a strong preference in the presence of the partial androgen antagonist cyproterone acetate (CPA), but not in the presence of the nonsteroidal antiandrogens hydroxyflutamide and Casodex [Moehren et al., 2007]. This suggests that Alien also exhibits a ligand-induced interaction with NHRs. Notably, there are some similarities in the interaction of CPA-bound AR with the corepressors Alien and SMRT, since both corepressors bind to the amino-terminus of the receptor, with SUMOylation sites being involved in these interactions [Dotzlaw et al., 2002; Moehren et al., 2007].
Interestingly, the stable expression of Alien in LNCaP cells, a hormone-dependent prostate cancer cell line, led to potent inhibition of proliferation in the presence of CPA, but not in the presence of androgen agonists [Moehren et al., 2007]. These findings underline the importance of Alien for inhibition of prostate cancer cell growth by androgen antagonists.
Similar to the Alien-VDR interaction, the interaction of Alien with the CPA-bound AR also exhibits response element specificity. A modified 2-hybrid assay has shown a strong recruitment of both Alien and SMRT to the natural promoters of the probasin and the prostate specific antigen (PSA) genes (personal observation). Both PSA and probasin expression are indicators of androgen action and AR functionality, and therefore are used as important markers of prostate differentiation and prostate cancer growth [Cleutjens et al., 1997; Jenster, 1999; Zhang et al., 2000]. Also, employing the MMTV-enhancer-promoter (mouse mammary tumor virus-luciferase), the recruitment of both corepressors was observed. In contrast, the recruitment of SMRT to the perfect palindromic glucocorticoid response elements or the natural tyrosine amino transferase response sequences was not observed, whereas Alien seems to be recruited to the tyrosine amino transferase response elements in the presence of CPA. Thus, this indicates that CPA-bound AR exhibits, in addition to ligand-specificity, a corepressor selectivity, which is dictated by the response element. Taken together, these results enhance the theory that Alien represents a new class of corepressors that contributes to transcriptional regulation.
Alien interacts with transcription factors involved in cell cycle regulation and DNA repair
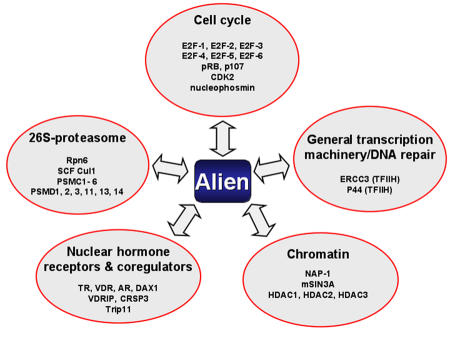
Protein-protein interaction techniques are a powerful method to detect protein complexes in a cell. An in vivo proteomic approach was assessed to identify Alien-interacting partners, combining detection of protein interactions of solely endogenously-expressed proteins with surface-enhanced laser desorption/ionization - mass spectrometry (SELDI-MS) and immunological techniques [Escher et al., 2007; Kob et al., 2007; Tenbaum et al., 2007]. Using this in vivo approach in addition to HDACs, several members of the E2F transcription factor family were identified as interacting partners of Alien (Figure 1 and [Escher et al., 2007]). E2F1 is known to be a transactivator and a positive regulator of cell cycle progression. Functionally, the interaction of Alien with E2F1 leads to repression of E2F1 transactivation, to reduced expression of endogenous E2F target gene, and to cell cycle inhibition [Tenbaum et al., 2007]. Notably, the repression of E2F1-mediated transactivation was independent of the presence of the retinoblastoma protein pRB. Whether Alien can replace pRB function during cell cycle progression is not yet known. Nevertheless, these findings indicate that Alien is not only a corepressor for members of the nuclear receptor family, but also for other transcription factors. Furthermore, Alien also interacts with those E2F family members that are known to be classic negative regulators, such as E2F5 and E2F6 [Escher et al., 2007]. Unclear is the functional role of Alien here, but the likelihood exists that negative gene regulation by these E2F members might be mediated by corepressors such as Alien.
Figure 1. Interaction profiling of Alien using in vivo proteomic analyses.
A list of factors, in addition to the COP9 signalosome (CSN), that were identified as factors interacting with Alien is depicted. Most factors were identified by affinity purification and subsequent SELDI combined with MS or other techniques such as the yeast 2-hybrid system. The interacting factors were grouped according to their known functions. This profiling indicates in addition to the function of the COP9 signalosome (CSN), a role for Alien in diverse regulatory processes including factors involved in gene repression at the chromatin level, protein degradation, DNA repair, cell cycle control and NHR biology.
Interestingly, CDK2 and other members of the pocket protein family were also identified as novel interacting partners of Alien in vivo (Figure 1). Both pRB and p107 are complexed with Alien [Escher et al., 2007]. Interaction of Alien with pRB was verified in vitro and in a yeast 2-hybrid system, and revealed that Alien interacts with the pocket domain itself, which is known to be a functional transcriptional silencing domain. In line with this, a mutant pRb, pRB706 mutated in the pocket domain that was isolated from a retinoblastoma tumor, lacks silencing function and also lacks interaction with Alien [Tenbaum et al., 2007], linking pRB-mediated silencing with interaction of Alien. In line with these results, it is conceivable that Alien not only exercises an inhibitory effect on the activity of nuclear hormone receptors, but also affects proteins involved in cell cycle regulation and transcription.
Recently, a link between the CSN and Drosophila pRB-related proteins was also identified in Drosophila [Ullah et al., 2007]. Similar to their mammalian counterparts, the Drosophila Rbf1 and Rbf2 retinoblastoma family members control cell cycle and developmentally-regulated gene expression. Mechanistically, the CSN co-occupies Rbf target gene promoters together with Rbf1 and Rbf2, suggesting an active role for the signalosome complex in transcriptional regulation.
Using the same in vivo proteomic approach (immunoprecipitation-SELDI-MS), several factors involved in cell cycle regulation and DNA repair were identified (Figure 1). One such factor was identified as B23/nucleophosmin. Recent studies of B23/nucleophosmin microarray profiling, accompanied by gene/protein pathway analyses, suggest that the predominant downstream effectors of B23/nucleophosmin are genes involved in the cell cycle, cell proliferation and cancer [Bergstralh et al., 2007]. Furthermore, it was recently revealed that B23/nucleophosmin has characteristics of a transcriptional corepressor [Liu et al., 2007]. In addition, the transcription factor complex TFIIH has several subunits, and two of them are associated with Alien: XPB, xeroderma pigmentosum group B protein and the p44 subunit [Kob et al., 2007]. TFIIH is one of the general transcription factors required for accurate transcription of protein-coding genes by RNA polymerase II. TFIIH has helicase and kinase activities, plays a role in promoter opening and promoter escape, and is also implicated in efficient activator-dependent transcription including nuclear hormone receptors [Drane et al., 2004; Lee et al., 2003; Liu et al., 2005; Nevado et al., 2004]. In addition, the TFIIH complex is involved in both transcription and DNA nucleotide excision repair, and impairs checkpoint triggering [Marini et al., 2006]. The functional role of Alien within these regulatory pathways is still unknown, but might counteract the reported activating function of nuclear receptors by TFIIH. Thus, taken together, the in vivo identification of Alien-interacting partners indicates that Alien is interacting within a network of proteins or protein complexes involved in hormonal control of transcriptional regulation, DNA repair, and the cell cycle.
Unclear, however, is whether Alien associates with these factors in several different protein complexes. Further, a detailed functional role of Alien in these protein networks remains to be elucidated. Moreover, an important issue is whether different Alien isoforms have different interaction patterns, and thus distinct functional roles. Furthermore, the role of CSN mini- or subcomplexes and the CSN-independent functions of each subunit need to be addressed. Here, a proteomic approach is required that separates each of the Alien complexes prior to the detection of interaction partners.
Expression of Alien
Alien seems to be expressed nearly ubiquitously. Expressed sequence tag databases suggest the presence of Alien mRNA in unfertilized eggs and at early developmental stages in mice. The important functions of Alien and its presence in early development may account for the severe phenotype of knockout mice. Nevertheless, data have been accumulated in recent years, including data from microarray expression analyses, concerning the regulation of Alien gene expression.
Multiple microarray analyses have identified Alien as a gene dysregulated in cancer and disease. Gene expression profiling of human substantia nigra pars compacta from Parkinson’s disease (PD) patients compared with healthy individuals was examined employing high-density microarrays. Among the 137 genes that have been identified as exhibiting alterations in their expression, Alien was one of the downregulated genes [Grunblatt et al., 2004]. In addition, a decline in the expression of the proteasome subunits PSMC4/TBP7 in the brains of PD patients was observed, which may contribute to the decreased levels of 26S proteasome complex, abnormal accumulation of ubiquitinated proteins and reduced rates of degradation of short-lived proteins such as cyclins, which in turn may induce cell defects [Coux et al., 1996; Voges et al., 1999].
The association between Alien upregulation and cancer was recently reported. Alien was identified as one of the genes that showed an increased expression in microarray assays focusing on cancer-associated hypomethylation in gastric cancer [Nishigaki et al., 2005]. Moreover, Alien was upregulated in cell lines with MLL-rearrangements, as well as in primary leukemias harboring 11q23/MLL rearrangements [Andersson et al., 2005].
An interesting observation was made while analyzing glucose and its potential to change gene expression profiles. Alien cDNA was identified by suppression-subtractive hybridization as being upregulated in mesangial cells cultured in high glucose. High extracellular glucose plays a pivotal role in the pathophysiology of diabetic nephropathy [Clarkson et al., 2002]. Thus, this suggests that Alien gene expression can also be regulated by glucose.
Interestingly, the expression of Alien seems to be under the control of NHR ligands. Recently, it was reported that treatment of MCF-7 cells with VitD3 results in upregulation of both the corepressor Alien and DRAP [Towsend et al., 2006]. Similarly, thyroid hormone induces Alien gene expression [Conrad et al., 2006; Tenbaum et al., 2003], which is discussed in more detail below.
DNA microarrays representing 30,000 human genes were analyzed for gene expression comparing the cornea, lens, iris, ciliary body, retina and optic nerve [Diehn et al., 2005]. Interestingly, a retina signature was identified containing enrichment in gene transcripts of Alien/TRIP15 and genes encoding thyroid releasing hormone (TRH), as well as numerous thyroid hormone receptor-related genes such as THRA, TRIP8 and TRAP100. Since previous work has demonstrated the importance of thyroid hormone in the developing rat retina [Sevilla-Romero et al., 2002], and the requirement of TRs for the development of green cone photoreceptor in rodents [Hurley and Chen, 2001], it indicates that TR-mediated gene regulation is associated with Alien expression.
Developmental regulation of Alien gene expression
Embryonic carcinoma (EC) P19 cells, which are derived from a mouse embryo, have been used extensively as a model system for in vitro neural differentiation [Bain et al., 1995; McBurney, 1993]. These multipotent cells can be maintained and propagated in an undifferentiated state. Interestingly, exposure of aggregated P19 cells to retinoic acid (RA) results in the differentiation of cells with many fundamental phenotypes of mammalian nervous system, being postmitotic, containing functional synapses and expressing a number of neurotransmitters. Interestingly, an induction of Alien mRNA expression at an early stage of neuronal differentiation was observed in the RA-treated P19 cells [Akiyama et al., 2003].
Further, the tissue-specific expression of Alien in mammals was analyzed. In adult rats, a high level of expression was observed in the heart, placenta, testis and spinal cord, a mediate level of expression in the brain, liver and ovary, and a low level of expression in thymus, spleen, adrenal gland and muscle [Akiyama et al., 2003]. Moreover, analyzing the developmental expression pattern, the expression of Alien mRNA in the rat brain was detected at embryonic day E14, increased thereafter, and reached the first peak at E18. The second peak was observed at postnatal day P5 and then gradually decreased towards the adult level of expression [Akiyama et al., 2003]. The second peak of Alien in the brain at P5 corresponds to the time of granulate neuron generation in the cerebellum. Analysis of the expression of Alien mRNA in the postnatal rat cerebellum demonstrates an increase of expression after birth, reaching a peak at postnatal day P9, and then a decrease reaching levels similar to those at P1. This indicates at least a biphasic regulation of Alien gene expression in brain during perinatal phases. Immunohistochemical analysis of the rat brain at P7 showed that Alien exists in the nuclei of Purkinje and Bergman glial cells, in Purkinje cell layer, and in the non-myelinated fibers [Akiyama et al., 2003]. Furthermore, Alien was detected in the granule cell nuclei.
Taken together, these results indicate that Alien is a predominantly nuclear protein that is regulated during development and plays a crucial role not only in neuronal differentiation and development, but also in maintaining neuronal functions.
Alien gene expression is controlled by thyroid hormone in brain
A main target organ of thyroid hormone in the developing embryo and at the neonatal stage is the brain. Hypothyroidism leads to severe mental retardation that is associated with lower myelination, and shorter axons and dendrites [Bernal, 2005; Bernal, 2007]. There is a developmental window within which thyroid hormone plays a pivotal role in neuronal differentiation, and this is around neonatal stage in rodents.
Analyzing the expression of Alien in brain, both Alien α and β (CSN2) were expressed at significantly lower levels in hypothyroid animals, both at the RNA and protein level [Tenbaum et al., 2003], Interestingly, injection of thyroxin in animals led to the induction of Alien gene expression. However, in hypothyroid animals, Alien levels were reduced only at perinatal days P0 and P5, but not at the later stages P10 or P15, indicating that thyroid hormone regulates the expression of Alien at the similar time period in brain for which TR is physiologically essential [Tenbaum et al., 2003]. Accordingly, following administration of thyroid hormone in chicken embryos and expression analysis in brain, Alien gene expression is drastically induced [Conrad et al., 2006].
These findings also strongly suggest that TR regulates the expression of its corepressor in brain. Consistent with this notion, low levels of T3, which is classically associated with TR being a gene silencer, represses the expression of Alien and thus reduces the level of silencing activity. On the other hand, at high levels of T3, which is associated with a transcriptional activating receptor, the levels of Alien corepressor are increased. Based on the likely assumption that there is a steady-state between repression and activation, as well as a highly dynamic coregulator recruitment, suggests that TR regulates the level of its own silencing function by regulating the expression of its corepressor.
Mechanisms of corepression by Alien
One mechanism by which Alien represses gene expression is through recruitment of HDAC-activity [Dressel et al., 1999]. Functionally, however, the HDAC-inhibitor, trichostatin A, reduces Alien-mediated gene silencing only partially, suggesting that Alien has both HDAC-dependent and HDAC-independent mechanisms of gene repression.
Interestingly, using the immunoprecipitation-SELDI-MS approach, the association of Alien with HDAC1 and HDCA3 was observed (unpublished observation), suggesting that HDAC1 and HDAC3 are complexed with the corepressor Alien. In addition to subunits of the 26S proteasome, HDAC2 was identified as being associated with Alien [Jung et al., 2005a; Jung et al., 2005b], indicating that Alien is associated with the class I HDACs to silence gene activity. In line with this, employing the yeast 2-hybrid system revealed that mSIN3A is a direct interacting partner for Alien [Moehren et al., 2004].
mSIN3A is known to be in a large protein complex together with HDAC1 and HDAC2. Noteworthy, not the paired amphipathic helices, which are emphasized to be important for protein-protein interaction, but rather the highly conserved region of mSIN3A, HCR, is mediating the interaction between mSIN3A and Alien. The interaction of Alien with Sin3A was confirmed by HeLa cell affinity purification-based profile of interacting factors for nuclear receptor coactivators [Jung et al., 2005a; Jung et al., 2005b]. Using chromatin immunoprecipitation (ChIP) experiments, analyzing at the chromatin level the VDR target gene CYP24, revealed that Alien and Sin3A are co-recruited to the VDREs in a timely, similar, and ordered manner [Moehren et al., 2004]. Accordingly, overexpressing the HCR alone, leads to de-repression and activation of the endogenous VDR target gene CYP24 [Moehren et al., 2004]. This indicates that VDR uses Alien, at least in part, as a corepressor on the CYP24 gene to silence CYP24 gene expression in the absence of ligand. It also suggests that the Alien-mediated gene silencing is mediated via interaction with the highly conserved region, HCR, of mSIN3a. Thus, these data suggest that Alien interacts with the mSin3A-HDAC protein complex.
Interestingly, VDR- and TR-interacting factors, TRIP230 and DRIP130, were also observed to interact with Alien in vivo [Kob et al., 2007]. It is noteworthy that these factors are in protein complexes associated with coactivator function, which implies that Alien might also play a repressive role on coactivators and on coactivator complexes. This also implies that Alien not only associates with the nuclear hormone receptors TR or VDR directly, but with protein complexes that associate with these nuclear receptors, as well.
A further exciting mechanism of Alien-mediated silencing might occur at the level of nucleosome assembly. Interestingly, NAP1, the nucleosome assembly protein 1, was initially identified as an interacting partner of Alien in a yeast 2-hybrid screen, which was further verified with in vivo and in vitro methods [Eckey et al., 2007] and (Figure 1). The transcriptional regulation is associated with rearrangements of chromatin structure that include histone modifications and changes in nucleosome structure [Ahmad and Henikoff, 2002; Boeger et al., 2005; Margueron et al., 2005; Sims et al., 2004]. Chromatin compaction and nucleosome arrangement themselves represent a barrier and a repressive state that have to be overcome for transcriptional activation. The assembly of nucleosomes into chromatin is essential for the compaction of DNA and inactivation of the DNA template to repress gene expression. NAP1 assembles nucleosomes independently of DNA-synthesis and was shown to enhance p300/CBP coactivator-mediated gene expression, thus suggesting a role for NAP1 in transcriptional regulation. Interestingly, tethering NAP1 to DNA leads to gene repression, indicating that NAP1 also mediates gene repression and plays a role in gene silencing through chromatin in vivo. Accordingly, the knockout of yeast NAP results in both down- and upregulation of many genes, confirming a role for NAP in both gene repression and activation.
Our recent findings suggest that Alien modulates NAP1 activity [Eckey et al., 2007]. Functional tests revealed that Alien increases the efficiency of nucleosome assembly with the notion that Alien represses transcription by reinforcing and sustaining a compact nucleosomal structure. Another feature of Alien is that it binds specifically to the histones H3 and H4, and this binding inhibits the accessibility of NAP1 to the histones H3/H4, indicating that Alien shifts the steady-state of nucleosome assembly towards prevention of histone displacement mediated by NAP1. The modulation of NAP1 activity by Alien was analyzed with in vitro systems using highly-purified, bacterially expressed proteins and the results excluded the involvement of HDAC activity. Therefore, here, the modulation of NAP1 activity by Alien points towards an HDAC-independent mechanism. NAP1 itself can be acetylated and acetylation might influence nucleosome assembly.
A further complexity is added by the fact that the association of Alien with HDAC activity in vivo might, in addition to the non-HDAC mechanism, influence nucleosome assembly through deacetylation. This, however, does not rule out other mechanisms of Alien-mediated gene silencing. Taken together, these findings strongly suggest that Alien uses at least two molecular mechanisms for transcriptional corepression.
Summary
Collectively, this review points out the most important characteristics and novel findings concerning the corepressor Alien. Alien seems to be a multifunctional protein, since it associates with factors involved in diverse regulatory functions in the cell. This includes factors involved in chromatin repression and transcriptional machinery, cell cycle regulation, interaction via the 26 proteasome for regulating protein stability and also modulating nuclear hormone receptor function. Further studies are necessary to clarify the exact mechanism of action of this multifunctional protein, which seems to be essential for life.
Abbreviations
- ChIP
chromatin immunoprecipitation
- CPA
cyproterone acetate
- CSN
COP9 signalosome
- HDAC
histone deacetylase
- NCoR
nuclear receptor corepressor
- NHR
nuclear hormone receptor
- PD
Parkinson’s disease
- pRB
retinoblastoma protein
- RA
retinoic acid
- SMRT
silencing mediator of RAR and TR
- VitD3
1,25-dihydroxyvitamin D3
References
- Ahmad K., Henikoff S. Epigenetic consequences of nucleosome dynamics. Cell. 2002;111:281–4. doi: 10.1016/s0092-8674(02)01081-4. [DOI] [PubMed] [Google Scholar]
- Akiyama H., Sugiyama A., Uzawa K., Fujisawa N., Tashiro Y., Tashiro F. Implication of Trip15/CSN2 in early stage of neuronal differentiation of P19 embryonal carcinoma cells. Brain Res Dev Brain Res. 2003;140:45–56. doi: 10.1016/s0165-3806(02)00574-6. [DOI] [PubMed] [Google Scholar]
- Altincicek B., Tenbaum S. P., Dressel U., Thormeyer D., Renkawitz R., Baniahmad A. Interaction of the corepressor Alien with DAX-1 is abrogated by mutations of DAX-1 involved in adrenal hypoplasia congenita. J Biol Chem. 2000;275:7662–7. doi: 10.1074/jbc.275.11.7662. [DOI] [PubMed] [Google Scholar]
- Andersson A., Olofsson T., Lindgren D., Nilsson B., Ritz C., Eden P., Lassen C., Rade J., Fontes M., Morse H., Heldrup J., Behrendtz M., Mitelman F., Hoglund M., Johansson B., Fioretos T. Molecular signatures in childhood acute leukemia and their correlations to expression patterns in normal hematopoietic subpopulations. Proc Natl Acad Sci U S A. 2005;102:19069–74. doi: 10.1073/pnas.0506637102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain G., Kitchens D., Yao M., Huettner J. E., Gottlieb D. I. Embryonic stem cells express neuronal properties in vitro. Dev Biol. 1995;168:342–57. doi: 10.1006/dbio.1995.1085. [DOI] [PubMed] [Google Scholar]
- Bergstralh D. T., Conti B. J., Moore C. B., Brickey W. J., Taxman D. J., Ting J. P. Global functional analysis of nucleophosmin in Taxol response, cancer, chromatin regulation, and ribosomal DNA transcription. Exp Cell Res. 2007;313:65–76. doi: 10.1016/j.yexcr.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal J. Pathophysiology of thyroid hormone deficiency during fetal development. J Pediatr Endocrinol Metab. 2005;18 Suppl 1:1253–6. doi: 10.1515/jpem.2005.18.s1.1253. [DOI] [PubMed] [Google Scholar]
- Bernal J. Thyroid hormone receptors in brain development and function. Nat Clin Pract Endocrinol Metab. 2007;3:249–59. doi: 10.1038/ncpendmet0424. [DOI] [PubMed] [Google Scholar]
- Boeger H., Bushnell D. A., Davis R., Griesenbeck J., Lorch Y., Strattan J. S., Westover K. D., Kornberg R. D. Structural basis of eukaryotic gene transcription. FEBS Lett. 2005;579:899–903. doi: 10.1016/j.febslet.2004.11.027. [DOI] [PubMed] [Google Scholar]
- Burke L. J., Baniahmad A. Co-repressors 2000. Faseb J. 2000;14:1876–88. doi: 10.1096/fj.99-0943rev. [DOI] [PubMed] [Google Scholar]
- Callige M., Kieffer I., Richard-Foy H. CSN5/Jab1 is involved in ligand-dependent degradation of estrogen receptor {α} by the proteasome. Mol Cell Biol. 2005;25:4349–58. doi: 10.1128/MCB.25.11.4349-4358.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlberg C. Mechanisms of nuclear signalling by vitamin D3. Interplay with retinoid and thyroid hormone signalling. Eur J Biochem. 1995;231:517–27. [PubMed] [Google Scholar]
- Clarkson M. R., Murphy M., Gupta S., Lambe T., Mackenzie H. S., Godson C., Martin F., Brady H. R. High glucose-altered gene expression in mesangial cells. Actin-regulatory protein gene expression is triggered by oxidative stress and cytoskeletal disassembly. J Biol Chem. 2002;277:9707–12. doi: 10.1074/jbc.M109172200. [DOI] [PubMed] [Google Scholar]
- Cleutjens K. B., van der Korput H. A., van Eekelen C. C., van Rooij H. C., Faber P. W., Trapman J. An androgen response element in a far upstream enhancer region is essential for high, androgen-regulated activity of the prostate-specific antigen promoter. Mol Endocrinol. 1997;11:148–61. doi: 10.1210/mend.11.2.9883. [DOI] [PubMed] [Google Scholar]
- Conrad A. H., Zhang Y., Walker A. R., Olberding L. A., Hanzlick A., Zimmer A. J., Morffi R., Conrad G. W. Thyroxine affects expression of KSPG-related genes, the carbonic anhydrase II gene, and KS sulfation in the embryonic chicken cornea. Invest Ophthalmol Vis Sci. 2006;47:120–32. doi: 10.1167/iovs.05-0806. [DOI] [PubMed] [Google Scholar]
- Coux O., Tanaka K., Goldberg A. L. Structure and functions of the 20S and 26S proteasomes. Annu Rev Biochem. 1996;65:801–47. doi: 10.1146/annurev.bi.65.070196.004101. [DOI] [PubMed] [Google Scholar]
- Diehn J. J., Diehn M., Marmor M. F., Brown P. O. Differential gene expression in anatomical compartments of the human eye. Genome Biol. 2005;6:R74. doi: 10.1186/gb-2005-6-9-r74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotzlaw H., Papaioannou M., Moehren U., Claessens F., Baniahmad A. Agonist-antagonist induced coactivator and corepressor interplay on the human androgen receptor. Mol Cell Endocrinol. 2003;213:79–85. doi: 10.1016/j.mce.2003.10.036. [DOI] [PubMed] [Google Scholar]
- Dotzlaw H., Moehren U., Mink S., Cato A. C., Iniguez Lluhi J. A., Baniahmad A. The amino terminus of the human AR is target for corepressor action and antihormone agonism. Mol Endocrinol. 2002;16:661–73. doi: 10.1210/mend.16.4.0798. [DOI] [PubMed] [Google Scholar]
- Drane P., Compe E., Catez P., Chymkowitch P., Egly J. M. Selective regulation of vitamin D receptor-responsive genes by TFIIH. Mol Cell. 2004;16:187–97. doi: 10.1016/j.molcel.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Dressel U., Thormeyer D., Altincicek B., Paululat A., Eggert M., Schneider S., Tenbaum S. P., Renkawitz R., Baniahmad A. Alien, a highly conserved protein with characteristics of a corepressor for members of the nuclear hormone receptor superfamily. Mol Cell Biol. 1999;19:3383–94. doi: 10.1128/mcb.19.5.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckey M., Hong W., Papaioannou M., Baniahmad A. The nucleosome assembly activity of NAP1 is enhanced by Alien. Mol Cell Biol. 2007;27:3557–68. doi: 10.1128/MCB.01106-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escher N., Kob R., Tenbaum S. P., Eisold M., Baniahmad A., von Eggeling F., Melle C. Various members of the E2F transcription factor family interact in vivo with the corepressor alien. J Proteome Res. 2007;6:1158–64. doi: 10.1021/pr060500c. [DOI] [PubMed] [Google Scholar]
- Freilich S., Oron E., Kapp Y., Nevo-Caspi Y., Orgad S., Segal D., Chamovitz D. A. The COP9 signalosome is essential for development of Drosophila melanogaster. Curr Biol. 1999;9:1187–90. doi: 10.1016/S0960-9822(00)80023-8. [DOI] [PubMed] [Google Scholar]
- Fuhrmann G., Chung A. C., Jackson K. J., Hummelke G., Baniahmad A., Sutter J., Sylvester I., Scholer H. R., Cooney A. J. Mouse germline restriction of Oct4 expression by germ cell nuclear factor. Dev Cell. 2001;1:377–87. doi: 10.1016/s1534-5807(01)00038-7. [DOI] [PubMed] [Google Scholar]
- Goubeaud A., Knirr S., Renkawitz-Pohl R., Paululat A. The Drosophila gene alien is expressed in the muscle attachment sites during embryogenesis and encodes a protein highly conserved between plants, Drosophila and vertebrates. Mech Dev. 1996;57:59–68. doi: 10.1016/0925-4773(96)00532-1. [DOI] [PubMed] [Google Scholar]
- Grunblatt E., Mandel S., Jacob-Hirsch J., Zeligson S., Amariglo N., Rechavi G., Li J., Ravid R., Roggendorf W., Riederer P., Youdim M. B. Gene expression profiling of parkinsonian substantia nigra pars compacta; alterations in ubiquitin-proteasome, heat shock protein, iron and oxidative stress regulated proteins, cell adhesion/cellular matrix and vesicle trafficking genes. J Neural Transm. 2004;111:1543–73. doi: 10.1007/s00702-004-0212-1. [DOI] [PubMed] [Google Scholar]
- Habiby R. L., Boepple P., Nachtigall L., Sluss P. M., Crowley W. F., Jr., Jameson J. L. Adrenal hypoplasia congenita with hypogonadotropic hypogonadism: evidence that DAX-1 mutations lead to combined hypothalmic and pituitary defects in gonadotropin production. J Clin Invest. 1996;98:1055–62. doi: 10.1172/JCI118866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harari-Steinberg O., Cantera R., Denti S., Bianchi E., Oron E., Segal D., Chamovitz D. A. COP9 signalosome subunit 5 (CSN5/Jab1) regulates the development of the Drosophila immune system: effects on Cactus, Dorsal and hematopoiesis. Genes Cells. 2007;12:183–95. doi: 10.1111/j.1365-2443.2007.01049.x. [DOI] [PubMed] [Google Scholar]
- Huang X., Hetfeld B. K., Seifert U., Kahne T., Kloetzel P. M., Naumann M., Bech-Otschir D., Dubiel W. Consequences of COP9 signalosome and 26S proteasome interaction. Febs J. 2005;272:3909–17. doi: 10.1111/j.1742-4658.2005.04807.x. [DOI] [PubMed] [Google Scholar]
- Hurley J. B., Chen J. Evaluation of the contributions of recoverin and GCAPs to rod photoreceptor light adaptation and recovery to the dark state. Prog Brain Res. 2001;131:395–405. doi: 10.1016/s0079-6123(01)31032-4. [DOI] [PubMed] [Google Scholar]
- Jenster G. The role of the androgen receptor in the development and progression of prostate cancer. Semin Oncol. 1999;26:407–21. [PubMed] [Google Scholar]
- Jung S. Y., Luo H., Malovannaya A., Kim T., Zhang J., Qin J., O'Malley B. W. NURSA Dataset: Proteomic analysis of steady-state nuclear hormone receptor coactivator complexes. Nuclear Receptor Signaling Atlas. 2005a. [DOI] [PubMed]
- Jung S. Y., Malovannaya A., Wei J., O'Malley B. W., Qin J. Proteomic analysis of steady-state nuclear hormone receptor coactivator complexes. Mol Endocrinol. 2005b;19:2451–65. doi: 10.1210/me.2004-0476. [DOI] [PubMed] [Google Scholar]
- Kob R., Baniahmad A., Escher N., von Eggeling F., Melle C. Detection and identification of transcription factors as interaction partners of alien in vivo. Cell Cycle. 2007;6:993–6. doi: 10.4161/cc.6.8.4108. [DOI] [PubMed] [Google Scholar]
- Lee D. K., Li M., Chang C. The second largest subunit of RNA polymerase II interacts with and enhances transactivation of androgen receptor. Biochem Biophys Res Commun. 2003;302:162–9. doi: 10.1016/s0006-291x(03)00126-8. [DOI] [PubMed] [Google Scholar]
- Lee J. W., Choi H. S., Gyuris J., Brent R., Moore D. D. Two classes of proteins dependent on either the presence or absence of thyroid hormone for interaction with the thyroid hormone receptor. Mol Endocrinol. 1995;9:243–54. doi: 10.1210/mend.9.2.7776974. [DOI] [PubMed] [Google Scholar]
- Lier S., Paululat A. The proteasome regulatory particle subunit Rpn6 is required for Drosophila development and interacts physically with signalosome subunit Alien/CSN2. Gene. 2002;298:109–19. doi: 10.1016/s0378-1119(02)00930-7. [DOI] [PubMed] [Google Scholar]
- Liu H., Tan B. C., Tseng K. H., Chuang C. P., Yeh C. W., Chen K. D., Lee S. C., Yung B. Y. Nucleophosmin acts as a novel AP2alpha-binding transcriptional corepressor during cell differentiation. EMBO Rep. 2007;8:394–400. doi: 10.1038/sj.embor.7400909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Ando S., Xia X., Yao R., Kim M., Fondell J., Yen P. M. p62, A TFIIH subunit, directly interacts with thyroid hormone receptor and enhances T3-mediated transcription. Mol Endocrinol. 2005;19:879–84. doi: 10.1210/me.2004-0385. [DOI] [PubMed] [Google Scholar]
- Margueron R., Trojer P., Reinberg D. The key to development: interpreting the histone code? Curr Opin Genet Dev. 2005;15:163–76. doi: 10.1016/j.gde.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Marini F., Nardo T., Giannattasio M., Minuzzo M., Stefanini M., Plevani P., Falconi M. M. DNA nucleotide excision repair-dependent signaling to checkpoint activation. Proc Natl Acad Sci U S A. 2006;103:17325–30. doi: 10.1073/pnas.0605446103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBurney M. W. P19 embryonal carcinoma cells. Int J Dev Biol. 1993;37:135–40. [PubMed] [Google Scholar]
- Moehren U., Papaioannou M., Reeb C. A., Hong W., Baniahmad A. Alien interacts with the human androgen receptor and inhibits prostate cancer cell growth. Mol Endocrinol. 2007;21:1039–48. doi: 10.1210/me.2006-0468. [DOI] [PubMed] [Google Scholar]
- Moehren U., Dressel U., Reeb C. A., Vaisanen S., Dunlop T. W., Carlberg C., Baniahmad A. The highly conserved region of the co-repressor Sin3A functionally interacts with the co-repressor Alien. Nucleic Acids Res. 2004;32:2995–3004. doi: 10.1093/nar/gkh621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatelli F., Strom T. M., Walker A. P., Zanaria E., Recan D., Meindl A., Bardoni B., Guioli S., Zehetner G., Rabl W. Mutations in the DAX-1 gene give rise to both X-linked adrenal hypoplasia congenita and hypogonadotropic hypogonadism. Nature. 1994;372:672–6. doi: 10.1038/372672a0. [DOI] [PubMed] [Google Scholar]
- Naumann M., Bech-Otschir D., Huang X., Ferrell K., Dubiel W. COP9 signalosome-directed c-Jun activation/stabilization is independent of JNK. J Biol Chem. 1999;274:35297–300. doi: 10.1074/jbc.274.50.35297. [DOI] [PubMed] [Google Scholar]
- Nevado J., Tenbaum S. P., Aranda A. hSrb7, an essential human Mediator component, acts as a coactivator for the thyroid hormone receptor. Mol Cell Endocrinol. 2004;222:41–51. doi: 10.1016/j.mce.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Nishigaki M., Aoyagi K., Danjoh I., Fukaya M., Yanagihara K., Sakamoto H., Yoshida T., Sasaki H. Discovery of aberrant expression of R-RAS by cancer-linked DNA hypomethylation in gastric cancer using microarrays. Cancer Res. 2005;65:2115–24. doi: 10.1158/0008-5472.CAN-04-3340. [DOI] [PubMed] [Google Scholar]
- O'Connell B. C., Harper J. W. Ubiquitin proteasome system (UPS): what can chromatin do for you? Curr Opin Cell Biol. 2007;19:206–14. doi: 10.1016/j.ceb.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Oron E., Mannervik M., Rencus S., Harari-Steinberg O., Neuman-Silberberg S., Segal D., Chamovitz D. A. COP9 signalosome subunits 4 and 5 regulate multiple pleiotropic pathways in Drosophila melanogaster. Development. 2002;129:4399–409. doi: 10.1242/dev.129.19.4399. [DOI] [PubMed] [Google Scholar]
- Polly P., Herdick M., Moehren U., Baniahmad A., Heinzel T., Carlberg C. VDR-Alien: a novel, DNA-selective vitamin D(3) receptor-corepressor partnership. Faseb J. 2000;14:1455–63. doi: 10.1096/fj.14.10.1455. [DOI] [PubMed] [Google Scholar]
- Richardson K. S., Zundel W. The emerging role of the COP9 signalosome in cancer. Mol Cancer Res. 2005;3:645–53. doi: 10.1158/1541-7786.MCR-05-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwechheimer C. The COP9 signalosome (CSN): an evolutionary conserved proteolysis regulator in eukaryotic development. Biochim Biophys Acta. 2004;1695:45–54. doi: 10.1016/j.bbamcr.2004.09.023. [DOI] [PubMed] [Google Scholar]
- Seeger M., Kraft R., Ferrell K., Bech-Otschir D., Dumdey R., Schade R., Gordon C., Naumann M., Dubiel W. A novel protein complex involved in signal transduction possessing similarities to 26S proteasome subunits. Faseb J. 1998;12:469–78. [PubMed] [Google Scholar]
- Sevilla-Romero E., Munoz A., Pinazo-Duran M. D. Low thyroid hormone levels impair the perinatal development of the rat retina. Ophthalmic Res. 2002;34:181–91. doi: 10.1159/000063885. [DOI] [PubMed] [Google Scholar]
- Sims R. J., 3rd, Mandal S. S., Reinberg D. Recent highlights of RNA-polymerase-II-mediated transcription. Curr Opin Cell Biol. 2004;16:263–71. doi: 10.1016/j.ceb.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Swain A., Lovell-Badge R. Mammalian sex determination: a molecular drama. Genes Dev. 1999;13:755–67. doi: 10.1101/gad.13.7.755. [DOI] [PubMed] [Google Scholar]
- Tenbaum S. P., Papaioannou M., Reeb C. A., Goeman F., Escher N., Kob R., von Eggeling F., Melle C., Baniahmad A. Alien inhibits E2F1 gene expression and cell proliferation. Biochim Biophys Acta. 2007:(In press). doi: 10.1016/j.bbamcr.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Tenbaum S. P., Juenemann S., Schlitt T., Bernal J., Renkawitz R., Munoz A., Baniahmad A. Alien/CSN2 gene expression is regulated by thyroid hormone in rat brain. Dev Biol. 2003;254:149–60. doi: 10.1016/s0012-1606(02)00023-4. [DOI] [PubMed] [Google Scholar]
- Tomoda K., Kubota Y., Arata Y., Mori S., Maeda M., Tanaka T., Yoshida M., Yoneda-Kato N., Kato J. Y. The cytoplasmic shuttling and subsequent degradation of p27Kip1 mediated by Jab1/CSN5 and the COP9 signalosome complex. J Biol Chem. 2002;277:2302–10. doi: 10.1074/jbc.M104431200. [DOI] [PubMed] [Google Scholar]
- Towsend K., Trevino V., Falciani F., Stewart P. M., Hewison M., Campbell M. J. Identification of VDR-responsive gene signatures in breast cancer cells. Oncology. 2006;71:111–23. doi: 10.1159/000100989. [DOI] [PubMed] [Google Scholar]
- Ullah Z., Buckley M. S., Arnosti D. N., Henry R. W. Retinoblastoma protein regulation by the COP9 signalosome. Mol Biol Cell. 2007;18:1179–86. doi: 10.1091/mbc.E06-09-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voges D., Zwickl P., Baumeister W. The 26S proteasome: a molecular machine designed for controlled proteolysis. Annu Rev Biochem. 1999;68:1015–68. doi: 10.1146/annurev.biochem.68.1.1015. [DOI] [PubMed] [Google Scholar]
- Wei N., Chamovitz D. A., Deng X. W. Arabidopsis COP9 is a component of a novel signaling complex mediating light control of development. Cell. 1994;78:117–24. doi: 10.1016/0092-8674(94)90578-9. [DOI] [PubMed] [Google Scholar]
- Wei N., Deng X. W. Characterization and purification of the mammalian COP9 complex, a conserved nuclear regulator initially identified as a repressor of photomorphogenesis in higher plants. Photochem Photobiol. 1998;68:237–41. doi: 10.1562/0031-8655(1998)068<0237:capotm>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- Wei N., Deng X. W. The COP9 signalosome. Annu Rev Cell Dev Biol. 2003;19:261–86. doi: 10.1146/annurev.cellbio.19.111301.112449. [DOI] [PubMed] [Google Scholar]
- Zanaria E., Muscatelli F., Bardoni B., Strom T. M., Guioli S., Guo W., Lalli E., Moser C., Walker A. P., McCabe E. R. An unusual member of the nuclear hormone receptor superfamily responsible for X-linked adrenal hypoplasia congenita. Nature. 1994;372:635–41. doi: 10.1038/372635a0. [DOI] [PubMed] [Google Scholar]
- Zazopoulos E., Lalli E., Stocco D. M., Sassone-Corsi P. DNA binding and transcriptional repression by DAX-1 blocks steroidogenesis. Nature. 1997;390:311–5. doi: 10.1038/36899. [DOI] [PubMed] [Google Scholar]
- Zhang J., Thomas T. Z., Kasper S., Matusik R. J. A small composite probasin promoter confers high levels of prostate-specific gene expression through regulation by androgens and glucocorticoids in vitro and in vivo. Endocrinology. 2000;141:4698–710. doi: 10.1210/endo.141.12.7837. [DOI] [PubMed] [Google Scholar]