Abstract
Members of the NR3B group of the nuclear receptor superfamily, known as the estrogen-related receptors (ERRs), were the first orphan receptors to be identified two decades ago. Despite the fact that a natural ligand has yet to be associated with the ERRs, considerable knowledge about their mode of action and biological functions has emerged through extensive biochemical, genetic and functional genomics studies. This review describes our current understanding of how the ERRs work as transcription factors and as such, how they control diverse developmental and physiological programs.
Introduction
The nuclear receptor (NR) superfamily was originally defined as a group of structurally-related transcription factors controlling gene expression in response to binding to small lipophilic ligands best represented by the steroid and thyroid hormones, as well as vitamin D and the active derivatives of vitamin A [Evans, 1988]. However, it was soon realized that the number of nuclear receptors exceeded the number of known, classic lipophilic hormones, and receptors that could not be matched with a natural ligand were labeled as orphan nuclear receptors [Giguere, 1999; Giguere et al., 1988]. The emerging challenge was to perform “reverse endocrinology”, starting with a gene encoding a putative receptor and ending up with a corresponding natural ligand and/or the recognition of the developmental and physiological processes modulated by these receptor-like proteins [Kliewer et al., 1999]. Here, we will review the current knowledge on the NR3B subgroup of nuclear receptors, commonly known as estrogen-related receptors (ERRs), the first orphan nuclear receptors identified, and still in search of a natural ligand.
The NR3B family
Multiple ERR isoforms
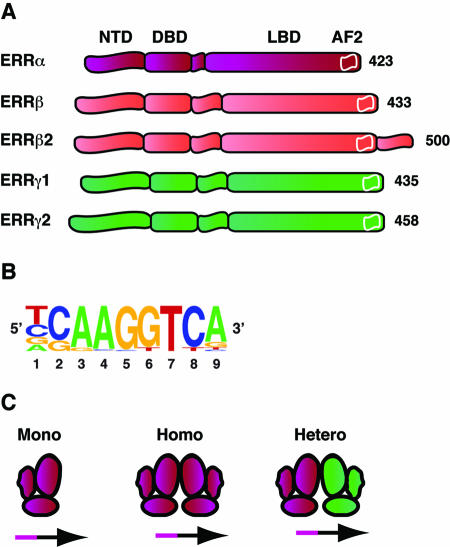
The NR3B subgroup includes three nuclear receptors referred to as ERRα (NR3B1, ERR1, ESRRA), ERRβ (NR3B2, ERR2, ESRRB) and ERRγ (NR3B3, ERR3, ESRRG), respectively. Members of the NR3B subgroup belong to the larger NR3 class of nuclear receptors that includes the classic steroid hormone receptors for estrogens, androgens, progesterone, aldosterone and cortisol. The first member of the subgroup (ERRα) was originally identified owing to the significant nucleotide and primary amino acid sequence similarities that it shared with the estrogen receptor α (NR2B1) gene and protein, while ERRβ was identified using the ERRα cDNA as a probe [Giguere et al., 1988]. Ironically, while ERRα was the first orphan nuclear receptor identified, ERRγ was the final addition to the superfamily [Eudy et al., 1998; Heard et al., 2000; Hong et al., 1999]. Although ERR homologs also exist in invertebrates such as Drosophila [Sullivan and Thummel, 2003] and amphioxus (Branchiostoma floridae) [Bardet et al., 2005], suggesting an ancient origin for the ERRs, it is not yet possible to identify the ancestral member of the NR3 group [Bardet et al., 2006]. The genomic organization of the three ERR loci also shares a structural characteristic unique among receptor isoforms within a subgroup of the superfamily. The exon encoding the amino terminal domain of the receptor also encodes the first zinc finger of the DNA binding domain, a genetic link between the two domains that could explain the unusual level of amino acid sequence identity present in the amino terminal domains of the three ERR isoforms. A single polypeptide of 423 amino acid residues encodes human ERRα, while several splice variants of ERRβ and γ have been identified in human (Figure 1A). The ERRβ2 variant contains an extended carboxy-terminal domain [Chen et al., 1999], ERRβ2Δ10 lacks exon 10 and encodes a different carboxy-terminal region [Zhou et al., 2006], the ERRγ2 splice variant possesses an additional 23 amino acids within its amino-terminal domain [Heard et al., 2000; Susens et al., 2000], while a new ERRγ splice variant lacking 39 amino acid residues of the second zinc finger of the DNA-binding domain has been found to be expressed in adipocytes and the prostate [Kojo et al., 2006]. The existence and relative abundance of most of these isoforms need to be confirmed by endogenous protein detection assays, while further studies are required to elucidate their putative physiological roles.
Figure 1. Schematic representation of ERR structures and DNA binding mode.
A) Schematic structure of the various ERR isoforms. Like most nuclear receptors, the ERRs possess three core domains, a regulatory amino-terminal domain (NTD), a DNA binding domain (DBD) and a ligand binding domain (LBD), in which the activation function 2 (AF-2, white box) is embedded at its carboxy terminal end. The AF-2 is required for ERR interaction with the coactivator PGC-1 and corepressor RIP140. B) Consensus ERR response element, as defined by motif-finding algorithms of ERR target gene promoters (Dufour et al., 2007). C) ERRs can bind to the ERRE as monomers, homodimers and heterodimers.
Expression of the ERR isoforms
In general, the ERRs display a similar tissue distribution in both mice and humans. The ERR isoforms are ubiquitously expressed. However, ERRα is generally more abundantly expressed than ERRγ, which in turn is more abundant than ERRβ. The three isoforms are expressed at elevated levels in tissues subjected to high energy demand, such as the heart and kidneys. ERRα is also expressed at high levels in the intestine, brown adipose tissue and skeletal muscles, while ERRγ mRNA can be found in abundance in the brain stem and the spinal cord. ERRβ can be found at relatively high levels in a subset of extra-embryonic ectoderm in the developing placenta and undifferentiated trophoblast stem cell lines, as well as in the adult eyes, inner ear, heart and kidneys [Bookout et al., 2006; Chen and Nathans, 2007; Giguere et al., 1988; Luo et al., 1997; Pettersson et al., 1996; Tremblay et al., 2001b]. The graphical views of the tissue-specific mRNA expression patterns for the three mouse ERR isoforms are available at [Bookout et al., 2005].
Interestingly, it was shown that the expression of all three ERR isoforms displays distinct diurnal rhythmicity in tissues such as the liver, white adipose, skeletal muscle, uterus and bone [Horard et al., 2004; Yang et al., 2006], suggesting that the ERRs may serve as a molecular link between the circadian oscillator and energy metabolism (see below). In addition, physiological stress signals such as exposure to cold, exercise or fasting also induce ERRα expression in brown fat, skeletal muscles and liver, respectively [Cartoni et al., 2005; Ichida et al., 2002; Schreiber et al., 2003]. It has also been shown that ERRα expression in bone marrow-derived macrophages is activated by lipopolysaccharide, interferon γ (IFN-γ) and interleukin 4 [Barish et al., 2005; Sonoda et al., 2007]. In addition, a 2-fold increase of ERRα mRNA expression has been demonstrated during differentiation of human bone marrow-derived mesodermal progenitor cells into osteoblasts [Qi et al., 2003].
Little is known about how the expression of the three ERR genes is controlled. The main regulator of ERRα expression has been shown to be the ERRs themselves [Laganiere et al., 2004; Liu et al., 2005; Mootha et al., 2004]. The ESRRA promoter contains a polymorphic 23 base pair sequence (ESRRA23) that is present in 1-4 copies in human. Each ESRRA23 element contains one perfect ERRE, as well as an additional nuclear receptor half-site. Transient transfection experiments have demonstrated that induction of the ESRRA promoter by PGC-1α is dependent on the presence of the ESRRA23 element, and that the strength of the activation correlates with its dosage [Laganiere et al., 2004].
Transcriptional activity of the ERRs
Interactions with coregulatory proteins
The three ERRs are constitutively active transcription factors. Their transactivation properties are independent of any exogenously added natural ligand and their relative potency as transcriptional activators varies in cell context- and promoter-dependent manners. The three ERR isoforms bind to a number of coregulator proteins, which they also share with other NRs. Their transcriptional activity is increased by members of the steroid receptor coactivator (SRC) family (SRC-1, TIF-2/SRC-2, AIB1/ACTR/SRC-3) [Hong et al., 1999; Xie et al., 1999; Zhang and Teng, 2000], the peroxisome proliferator-activated receptor γ coactivator-1 (PGC-1) α and β [Huss et al., 2002; Kamei et al., 2003; Laganiere et al., 2004; Schreiber et al., 2003; Sonoda et al., 2007], as well as the proline-rich nuclear receptor coregulatory protein (PNRC), PNRC2 and transducin-like enhancer of split 1 [Hentschke and Borgmeyer, 2003; Zhou and Chen, 2001; Zhou et al., 2000]. The transcriptional activity of the ERRs is also modulated by the nuclear receptor corepressor RIP140/Nrip1 [Augereau et al., 2006; Castet et al., 2006; Debevec et al., 2007; Sanyal et al., 2004]. The ERRs can interact with the orphan nuclear receptor small heterodimer partner (SHP; NR0B2), which represses their transcriptional activity [Sanyal et al., 2002]. SHP lacks a conventional DNA binding domain and interacts with several other members of the nuclear receptor superfamily to inhibit their receptor transcriptional activity. It is interesting to note that the mutations in SHP that have been associated with moderate obesity in humans prevent the inhibition of ERRγ activity [Sanyal et al., 2002].
ERR activity can be modulated by synthetic ligands
Despite the absence of response to natural estrogens, it has been reported that the transcriptional activity of all ERR isoforms is inhibited by the synthetic estrogen analog diethylstilbestrol (DES) [Coward et al., 2001; Tremblay et al., 2001b]. The constitutive transcriptional activity of ERRβ and ERRγ, but not ERRα, can also be repressed by the selective estrogen receptor modulator (SERM) 4-hydroxytamoxifen (OHT), which behaves like a selective inverse agonist, causing the dissociation of coactivator protein [Coward et al., 2001; Tremblay et al., 2001a]. Recently, bisphenol A, a ubiquitous environmental contaminant with estrogenic activity, was shown to bind to ERRγ and antagonize the repression of ERRγ activity induced by OHT [Takayanagi et al., 2006]. Also, toxaphene and chlordane, two organochlorine pesticides with estrogen-like activity, have been identified as weak antagonists for ERRα [Yang and Chen, 1999]. However, mutation of phenylalanine 329, an amino acid crucial for the constitutive activity of the receptor, to an alanine residue, allowed toxaphene to act as an ERRα agonist instead [Chen et al., 2001]. In addition, the isoflavones genistein, daidzein, and biochanin A and the flavone 6,3',4'-trihydroxyflavone were identified as agonists of the ERRs by mammalian two-hybrid experiments under comparable conditions to those for the activation of ERα and ERβ [Suetsugi et al., 2003]. It should be noted that binding of the organochlorine pesticide molecules to ERRα could not be directly demonstrated [Tremblay et al., 2001b] and that structure-based predictions showed that flavone and isoflavone cannot be accommodated within the ERR binding pocket [Greschik et al., 2002]. Although these compounds constitute useful tools to study the regulation of the ERRs, they share the pitfall of being non-specific to the ERRs, as they modulate the activity of other nuclear receptors such as the ERs. However, several synthetic compounds have recently been characterized as ERR-specific ligands. XCT790 is a highly specific inverse agonist for ERRα that disrupts the interaction between ERRα and PGC-1α and has no effect on either the ERs or the other ERR isoforms [Busch et al., 2004; Willy et al., 2004], while GSK5182, a tamoxifen analog, showed improved inverse agonist selectivity for ERRγ [Chao et al., 2006]. On the other hand, the structurally-related phenolic acyl hydrazones GSK4716 and DY131 were reported to effectively and selectively activate ERRβ and ERRγ [Yu and Forman, 2005; Zuercher et al., 2005].
The possible existence of a natural ligand for the ERRs remains an unresolved question. The initial crystal structure of unliganded ERRγ showed that the ligand-binding pocket is very small, approximately 280 Å [Greschik et al., 2002]. Likewise, the unoccupied volume of the ligand-binding pocket of the unliganded ERRα bound to a PGC-1α coactivator peptide was reported to be ~100 Å [Kallen et al., 2004], suggesting that a natural agonist would have to be composed of at most four to five non-hydrogen atoms. However, the crystal structures of the GSK4716 agonist-bound ERRγ and of the cyclohexylmethyl-(1-p-tolyl-1H-indol-3-ylmethyl)-amine inverse agonist-bound ERRα revealed that these ligands can force the rearrangement of amino acid residues in the respective ligand binding domains that allows access of the ligands to a larger ligand-binding pocket. The results of these experiments thus suggest that the plasticity of the ERR ligand binding pockets could allow for larger compounds to act as natural ligands [Kallen et al., 2007; Wang et al., 2006]. The crystal structures of ERRα and ERRγ ligand-binding domains also provided strong evidence for ligand-independent transactivation by these receptors. In both cases, the apo-receptors are in a permanent active configuration and thus ready to interact with coregulatory proteins [Greschik et al., 2002; Kallen et al., 2007; Kallen et al., 2004; Wang et al., 2006]. Because binding of the agonist GSK4617 does not affect the orientation of the AF-2 helix, the mechanism by which GSK4617 activates ERRγ is currently unknown. In contrast, binding of DES and 4-OHT to ERRγ induces the displacement of the AF-2 helix to a position that interferes with the recruitment of coactivators [Greschik et al., 2004; Wang et al., 2006].
ERR target genes
Identification of the binding site
The physiological roles played by nuclear receptors can often be ascribed by investigating their target genes. Unbiased binding site selection and characterization of the first ERR-responsive genes defined the ERR response element (ERRE) as the consensus nucleotide sequence TCAAGGTCA [Johnston et al., 1997; Sladek et al., 1997a; Vega and Kelly, 1997]. Bioinformatic analysis of a large set of ERR target promoters identified using a combination of chromatin immunoprecipitation (ChIP) and genomic DNA arrays (ChIP-on-chip) confirmed that the TCAAGGTCA motif serves as the main ERR binding site in vivo (Figure 1B) [Dufour et al., 2007]. Although the consensus ERRE contains a single nuclear receptor core binding half-site, the ERRs can bind DNA either as monomers, homodimers or heterodimers (Figure 1C) [Barry et al., 2006; Dufour et al., 2007; Gearhart et al., 2003; Huppunen and Aarnisalo, 2004; Johnston et al., 1997; Sladek et al., 1997a; Vanacker et al., 1999a]. ERRα and ERRβ have also been shown to bind the estrogen response element (ERE) as homodimers [Vanacker et al., 1999b; Yang et al., 1996; Zhang and Teng, 2000], and two studies even suggested that ERα and ERRα could heterodimerize in vitro [Johnston et al., 1997; Yang et al., 1996]. However, convincing evidence that the ERRs can commonly bind to EREs and/or interact in a physiologically significant manner with the ERs in vivo is still lacking. A study of the TFF1 (pS2) gene showed that while the promoter contains both an ERE and an ERRE, regulation by the ERRs is lost only when the ERRE is ablated, suggesting that the ERRE is the ERRs’ legitimate binding site, at least on this promoter [Lu et al., 2001].
ERR target promoters
Biochemical purification of HeLa cell nuclear extract proteins that bind to the Simian Virus 40 (SV40) late promoter first identified ERRα as a repressor of the transcription of the SV40 late genes [Wiley et al., 1993; Zuo and Mertz, 1995]. In contrast, the ERRs have been shown to mainly activate the expression of cellular genes. Until recently, most ERR target genes had been identified through the discovery of a putative ERRE in the promoters of genes of interest. These included genes encoding the medium-chain acyl coenzyme A dehydrogenase (MCAD or Acadm) [Sladek et al., 1997a; Vega and Kelly, 1997], osteopontin (OPN) [Bonnelye et al., 1997; Vanacker et al., 1998b], the thyroid receptor α (TRα) [Vanacker et al., 1998a], aromatase [Yang et al., 1998], lactoferrin [Yang et al., 1996], the orphan nuclear receptor SHP [Sanyal et al., 2002], endothelial nitric oxide synthase (eNOS) [Sumi and Ignarro, 2003], PPARα [Huss et al., 2004], pyruvate dehydrogenase kinase 4 (PDK4) [Araki and Motojima, 2006; Wende et al., 2005; Zhang et al., 2006a], monoamine oxidase B (MAO-B) [Willy et al., 2004; Zhang et al., 2006b], ERRα itself [Laganiere et al., 2004; Liu et al., 2005; Mootha et al., 2004], apolipoprotein A4 (ApoA4) [Carrier et al., 2004], phosphoenolpyruvate carboxykinase (PEPCK) [Herzog et al., 2006], surfactant protein A (SP-A) [Liu et al., 2006], RIP-140/Nrip1 [Nichol et al., 2006], mitofusin 2 [Soriano et al., 2006], Polo-like kinase 2 (Plk2) [Park et al., 2007] and uncoupling protein 1 (UCP-1) [Debevec et al., 2007]. A second, more global approach to identifying ERR-responsive genes was to exploit the observation that ERRα transcriptional activity is highly stimulated in the presence of PGC-1α. Various cell lines were first infected with an adenovirus expressing either wild-type PGC-1α or a PGC-1α variant engineered to specifically interact with ERRα, and differential gene expression profiling was then carried out using DNA microarrays [Gaillard et al., 2006; Mootha et al., 2004; Rangwala et al., 2007; Schreiber et al., 2004]. A small subset of bona fide ERRα targets were further identified using a combination of computational biology, small inhibitory RNAs (siRNAs) against ERRα, a specific antagonist, as well as DNA binding assays and cotransfection of reporter genes. These targets included several genes involved in oxidative phosphorylation (OXPHOS) and mitochondrial biogenesis, such as ATP synthase b (ATPsynb), cytochrome c (CYCS), COX4, GABPA, adenine nucleotide translocator 1 (ANT1) and carnitine palmytoyltransferase 1A (CPT1A), thus suggesting that ERRα could serve as a conduit for PGC-1α action in mitochondrial biogenesis and the OXPHOS pathway.
A powerful approach to identify direct target genes of nuclear receptors is to determine their occupancy on a genome-wide scale by using the ChIP-on-chip technology [Carroll et al., 2005; Carroll et al., 2006; Laganiere et al., 2005; Odom et al., 2004]. This technique was recently used to appraise the role of ERRα and ERRγ in the adult heart [Dufour et al., 2007], of ERRγ in the newborn heart [Alaynick et al., 2007] and of ERRα in bone marrow-derived macrophages [Sonoda et al., 2007]. Together, these studies identified more than 500 promoters that considerably expanded the repertoire of direct ERR target genes encoding cytosolic and mitochondrial proteins involved in the control of energy metabolism. In addition, these studies showed that the ERRs control tissue-specific functions, such as fuel sensing and contractile work in the heart and bacterial clearance in macrophages. Further analyses of the ChIP-on-chip data demonstrated that ERRα and ERRγ target the same promoter as non-obligatory heterodimers and cooperate with other transcription factors, namely CREB and STAT3, to control of the expression of metabolic genes [Dufour et al., 2007].
Regulatory networks
Review of the data gathered on ERR coregulatory proteins and target genes described above also reveals an important emerging concept of ERRs functioning in a regulatory network. First, induction of PGC-1α expression by physiological stimuli leads to the upregulation of ERRα, which in turn stimulates its own expression, thereby generating a positive feedback loop [Laganiere et al., 2004; Mootha et al., 2004]. Second, ERRα can also stimulate the expression of the corepressor RIP140 and SHP, thereby providing an inhibitory feedback mechanism to control the expression of its target genes [Nichol et al., 2006; Sanyal et al., 2002]. Third, since the three ERR isoforms can regulate the same target genes as homo- or heterodimers, the expression of these genes can be differentially regulated depending on the levels of individual ERR isoform in each tissue. The existence of such networks has been shown in vivo where, in the ERRα null heart, the expression of RIP140 is downregulated, while that of PGC-1α is upregulated at a time when the expression of ERRγ is also elevated [Dufour et al., 2007]. The compensatory mechanism was particularly evident in HL-1 cardiomyocytes, where ERRγ was shown to play an important role in maintaining the expression of ERRα target genes. A similar compensatory mechanism was observed in the neonatal heart lacking ERRγ [Alaynick et al., 2007].
Phenotypic analyses of ERR null mice
ERRα null mice: metabolic defects have tissue-specific consequences
Initial phenotypic analysis of the ERRα null mice showed them to be viable and fertile with no gross anatomical alterations, with the exception of reduced body weight and peripheral fat deposits [Luo et al., 2003]. The ERRα null mice also showed altered expression of genes involved in lipid metabolism and OXPHOS in several tissues, including white adipose tissue, muscle and small intestine [Carrier et al., 2004; Huss et al., 2004; Luo et al., 2003]. Although the changes observed in the expression of metabolic genes in these tissues should, in theory, lead the mice to burn less fat and spend less energy, the mice are paradoxically lean and resistant to high fat diet-induced obesity [Luo et al., 2003]. However, it was observed that, in the white adipose tissue of ERRα null mice, genes involved in fat catabolism were upregulated, while genes involved in triglyceride synthesis were downregulated. These data suggest that the ERRs can potentially function as both repressor and activator of gene expression in a tissue- and gene-specific manner. Together, these observations imply a more complex and tissue-specific role for ERRα in the control of energy metabolism in the whole animal. Indeed, subsequent investigation of the ERRα null mice showed that enterocytes from the null mice displayed a lower capacity for β-oxidation, and that ERRα-deficient pups exhibited a significant lipid malabsorption and fat transport [Carrier et al., 2004]. In addition, ERRα null mice display a reduced mitochondrial mass in brown adipose tissue and impaired thermogenic capacity in response to cold temperatures, leading to hypothermia and slower recovery to a normal body temperature [Villena et al., 2007]. ERRα is thus essential for the organism in situations of high energy demand and defects in ERRα function could contribute to pathological states caused by mitochondrial dysfunction.
Given the observation that ERRα orchestrates a comprehensive transcriptional program in the heart [Dufour et al., 2007] and the known link between metabolic disturbance and pathologic cardiac hypertrophy, ERRα null mice were recently tested for their response to stressors known to cause heart failure. Hearts of ERRα null mice subjected to pressure overload displayed chamber dilatation, reduced left ventricular shortening, depletion of the phosphocreatine pool and reduced ATP synthesis [Huss et al., 2007], indicating that ERRα is essential for the bioenergetic and functional adaptation to cardiac hemodynamic stressors.
Finally, recent studies of ERRα null macrophages showed that ERRα is required for the induction of mitochondrial reactive oxygen species (ROS) production and efficient clearance of pathogens in response to IFN-γ [Sonoda et al., 2007]. Consequently, the mitochondrial metabolic defect renders the ERRα null mice susceptible to infection by Listeria monocytogenes. The activation of ERRα by IFN-γ was also found to be dependent on the presence of the coactivator PGC-1β.
ERRβ: control of cell fate
Expression of ERRβ during early embryogenesis defines a subset of extra-embryonic ectoderm predestined to form the dome of the chorion [Luo et al., 1997; Pettersson et al., 1996]. Phenotypic analysis of the ERRβ null mice indeed showed abnormal chorion development associated with an overabundance of trophoblast giant cells and a severe deficiency of diploid trophoblasts [Luo et al., 1997]. Consequently, the ERRβ null mice die in utero at 10.5 days post-coitum due to impaired placental formation. Strikingly, treatment of trophoblast stem cells with DES, a synthetic estrogen that promotes coactivator release from ERRβ and inhibits its transcriptional activity, led to their differentiation toward the polyploid giant cell lineage [Tremblay et al., 2001b]. In addition, DES-treated pregnant mice exhibited abnormal early placenta development associated with an overabundance of trophoblast giant cells and an absence of diploid trophoblast. These studies thus provided evidence for steroid-like control of trophoblast development.
Although essential for placental formation, tetraploid rescue experiments, as well as tissue-specific ablation of Esrrb, demonstrated that the ERRβ null embryos can develop normally and generate fertile adult animals of both sexes [Chen and Nathans, 2007; Luo et al., 1997; Mitsunaga et al., 2004]. However, the ERRβ null mice of both sexes were found to have a reduced number of primordial germ cells in their gonads [Mitsunaga et al., 2004]. In addition, ERRβ null mice display abnormal development of the endolymph-producing cells of the inner ear, suggesting that potential ERRβ ligands could be used to treat some disorders of hearing and balance [Chen and Nathans, 2007]. Taken together, these phenotypes advocate a more precise role for ERRβ than the other two ERR isoforms in the control of the fate of specific cell types during development.
ERRγ is required for the transition to oxidative metabolism in the postnatal heart
ERRγ is expressed at high levels in the fetal and postnatal heart. ERRγ null mice display a reduction in ventricular mass and die shortly after birth [Alaynick et al., 2007]. Loss of ERRγ blocks the transition from a predominant dependence on carbohydrates as substrates during the fetal period to greater dependence on oxidative metabolism in postnatal life. The impairment in this switch resulted in lactatemia, electrocardiogram abnormalities, high mitochondrial genome number and altered electron transport chain biochemical activities [Alaynick et al., 2007]. Since pathologic conditions such as heart failure and cardiac hypertrophy can re-induce carbohydrate utilization, these findings suggest that like ERRα, ERRγ could be targeted for the management and/or treatment of cardiomyopathies.
ERRs and human diseases
Cancer
Evidence is accumulating towards a potential implication of the ERRs in the etiology of various types of cancer. The expression of all three ERR isoforms has been monitored in cancer cell lines or primary tumors derived from breast [Ariazi et al., 2002; Lu et al., 2001; Suzuki et al., 2004; Zhou et al., 2006], ovary [Sun et al., 2005], prostate [Cheung et al., 2005; Fujimura et al., 2007], endometrium [Gao et al., 2006; Sun et al., 2006; Watanabe et al., 2006] and colon [Cavallini et al., 2005]. In particular, ERRα has been identified as a bad prognosis indicator owing to its correlation with unfavorable biomarkers such as ERα negativity and elevated ErbB2 expression. On the other hand, ERRγ was associated with more favorable biomarkers such as hormone responsiveness in estrogen/progesterone receptor-positive tumors and expression of ErbB4, and its presence is therefore considered as a better prognosis indicator. Increased ERRα levels have also been associated with a higher risk of recurrence and poor clinical outcome in human breast carcinoma [Ariazi et al., 2002; Suzuki et al., 2004]. At the molecular level, it has been demonstrated that ERRα is phosphorylated in response to EGF signaling in MCF-7 breast cancer cells, a posttranslational modification that was linked to selective gene activation by ERRα [Barry and Giguere, 2005]. Likewise, ERRα phosphorylation status and transcriptional activity were shown to be higher in a breast cancer cell line expressing high levels of ErbB2, while inhibition of ErbB2 signaling abrogated ERRα-induced transcription in these cells [Ariazi et al., 2007]. Lastly, coordinated upregulation of ERRα and its coactivators, PGC-1α and PGC-1β, as well as genes involved in glycolysis, tricarboxylic acid cycle and the OXPHOS pathway was observed in a human breast cancer model for brain metastasis [Chen et al., 2007]. These findings suggest that ERRα may direct a transcriptional switch that supports expression of oxidative metabolic pathways in metastatic cancer cells in the brain.
Osteoporosis, bone maintenance and cartilage formation
Given the structural and functional similarities between the ERRs and ERs, and the important role that estrogens play in bone formation and maintenance, a potential role for ERRα in bone physiology is under investigation [Bonnelye and Aubin, 2005; Giguere, 2002]. ERRα is expressed in bone cells [Bonnelye and Aubin, 2002; Bonnelye et al., 2002; Bonnelye et al., 2001; Bonnelye et al., 1997; Sladek et al., 1997a] and directly regulates osteoblast-associated genes such as OPN [Vanacker et al., 1998b], lactoferrin [Yang et al., 1996], TRα [Vanacker et al., 1998a], aromatase [Yang et al., 1998] and eNOS [Sumi and Ignarro, 2003]. In addition, ERRα expression has been shown to be regulated by estradiol, both in cultured rat calvaria cells and in vivo [Bonnelye et al., 2002]. However, it is currently unknown whether ERRα regulates estrogen-sensitive genes or interferes with estrogen signaling through indirect mechanisms. A statistically significant association between bone mass density (BMD) of white premenopausal women and ESRRA23 was also observed in a genetic association study [Laflamme et al., 2005]. Women with more copies of ESRRA23 showed a higher BMD. The study concluded that lower copy number of ESRRA23 is associated with lower BMD and likely increased risk of bone fracture.
Expression of ERRα has also been reported in fetal and adult rat chondrocytes in growth plate and articular cartilage and in the rat chondrogenic cell line C5.18 [Bonnelye et al., 2007]. Overexpression of ERRα in C5.18 cells induces the expression of the transcription factor SOX9, an important gene in cartilage formation. On the other hand, reduction of ERRα expression by a siRNA led to inhibition of cartilage formation and maturation of proliferating chondrocytes into hypertrophic chondrocytes in vitro.
Obesity
As noted above, ERRα is expressed in tissues with a high capacity for β-oxidation, is a main conduit for PGC-1α and PGC-1β activity, and the ERRα null mice have reduced body weight and are resistant to high fat diet-induced obesity. In addition, ESRRA is located on chromosome 11q13 [Shi et al., 1997; Sladek et al., 1997b], a region previously linked to body mass index (BMI) and fat content [Perusse et al., 2005]. Association studies between ESRRA variants located either in the promoter (ESRRA23, [Laganiere et al., 2004]) or in the coding region (Pro116Pro, [Larsen et al., 2007]) with obesity and type 2 diabetes have so far given mixed results. While Kamei et al. [Kamei et al., 2005] found that the 2.3 genotype of ESRRA23 was associated with a higher BMI in Japanese individuals, Larsen et al. [Larsen et al., 2007] found no association of the ESRRA23 or Pro116Pro variants with obesity or type 2 diabetes in Danish whites.
Closing remarks
While the ERRs remain orphan nuclear receptors as the search for a natural ligand continues, it has become clear that the three ERR isoforms are essential transcriptional regulators of development and physiology (Table 1). Control of energy metabolism, through their interaction with members of the PGC-1 family of coactivator proteins, as well as with the corepressor RIP140, appears to be at the core of ERR biological activities (Figure 2). Given that the ERRs are proven druggable targets, it is hoped that the development of isoform-specific ERR agonists and antagonists will lead to new therapeutic approaches to manage and/or treat a variety of diseases that could include cardiomyopathies, osteoporosis, infection by pathogens, diabetes, obesity and cancer.
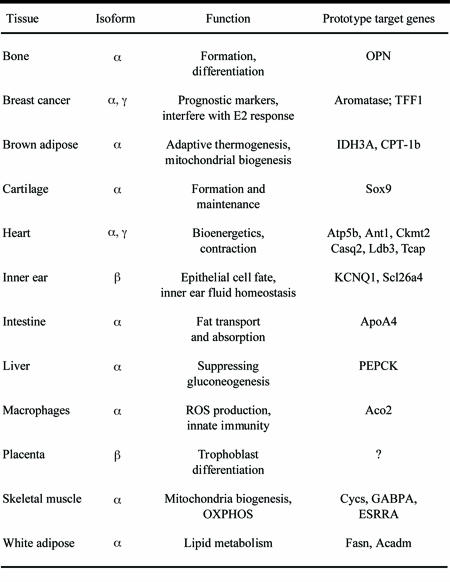
Table 1. List of known ERR functions and associated target genes.
The table shows a list of physiological and developmental functions regulated by the three ERR isoforms in diverse tissues. Prototypic ERR target genes for each tissue are also listed. References to ERR functions and target genes can be found in the main text.
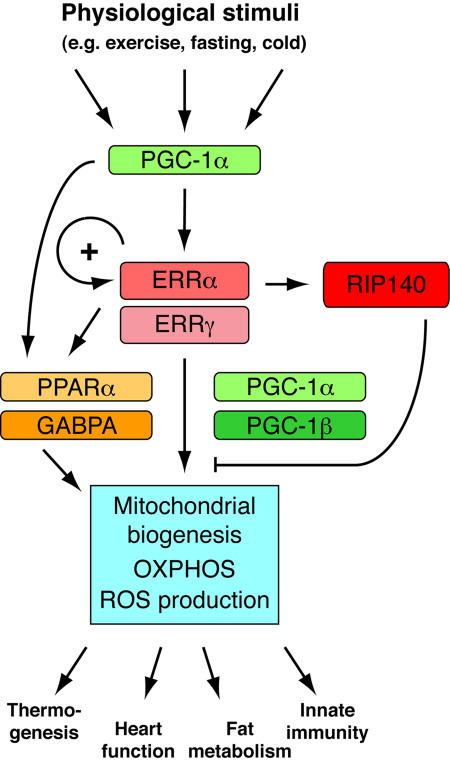
Figure 2. Central role played by ERRα and ERRγ in the control of energy metabolism.
Induction of the expression of the coactivator PGC-1α upon diverse physiological stimuli leads to an augmentation in ERR transcriptional activity, as well as an increase in ERRα levels through an autoregulatory mechanism. The ERRs then upregulate the expression of other transcription factors, such as PPARα and GABPA, thus amplifying the original signal. These factors, working in concert with PGC1α and PGC-β, stimulate the expression of a vast genetic program controlling mitochondrial biogenesis, OXPHOS and ROS production. ERRα also stimulates the expression of RIP140, which provides an inhibitory feedback mechanism. Mitochondrial output is then used in a tissue-specific manner to modulate diverse biological responses.
Abbreviations
- 4-OHT
4-hydroxytamoxifen
- AF-2
activation function 2
- BMI
body mass index
- CREB
cyclic AMP response element binding protein
- DES
diethylstilbestrol
- eNOS
endothelial nitric oxide synthase
- ER
estrogen receptor
- ERR
estrogen-related receptor
- IFN-γ
interferon γ
- NR
nuclear receptor
- OPN
osteopontin
- OXPHOS
oxidative phosphorylation
- PDK4
pyruvate dehydrogenase kinase 4
- PGC-1
PPARγ coactivator 1
- PPAR
peroxisome proliferator activated receptor
- RIP140
receptor interacting protein of 140 kDa
- ROS
reactive oxygen species
- SRC
steroid receptor coactivator
- STAT3
signal transducer and activator of transcription 3
References
- Alaynick W. A., Kondo R. P., Xie W., He W., Dufour C. R., Downes M., Jonker J. W., Giles W., Naviaux R. K., Giguere V., Evans R. M. ERRgamma directs and maintains the transition to oxidative metabolism in the postnatal heart. Cell Metab. 2007;6:13–24. doi: 10.1016/j.cmet.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Araki M., Motojima K. Identification of ERRalpha as a specific partner of PGC-1alpha for the activation of PDK4 gene expression in muscle. Febs J. 2006;273:1669–80. doi: 10.1111/j.1742-4658.2006.05183.x. [DOI] [PubMed] [Google Scholar]
- Ariazi E. A., Clark G. M., Mertz J. E. Estrogen-related receptor α and estrogen-related receptor γ associate with unfavorable and favorable biomarkers, respectively, in human breast cancer. Cancer Res. 2002;62:6510–8. [PubMed] [Google Scholar]
- Ariazi E. A., Kraus R. J., Farrell M. L., Jordan V. C., Mertz J. E. Estrogen-related receptor alpha1 transcriptional activities are regulated in part via the ErbB2/HER2 signaling pathway. Mol Cancer Res. 2007;5:71–85. doi: 10.1158/1541-7786.MCR-06-0227. [DOI] [PubMed] [Google Scholar]
- Augereau P., Badia E., Carascossa S., Castet A., Fritsch S., Harmand P. O., Jalaguier S., Cavailles V. The nuclear receptor transcriptional coregulator RIP140. Nucl Recept Signal. 2006;4:e024. doi: 10.1621/nrs.04024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardet P. L., Schubert M., Horard B., Holland L. Z., Laudet V., Holland N. D., Vanacker J. M. Expression of estrogen-receptor related receptors in amphioxus and zebrafish: implications for the evolution of posterior brain segmentation at the invertebrate-to-vertebrate transition. Evol Dev. 2005;7:223–33. doi: 10.1111/j.1525-142X.2005.05025.x. [DOI] [PubMed] [Google Scholar]
- Bardet P. L., Laudet V., Vanacker J. M. Studying non-mammalian models? Not a fool's ERRand! Trends Endocrinol Metab. 2006;17:166–71. doi: 10.1016/j.tem.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barish G. D., Downes M., Alaynick W. A., Yu R. T., Ocampo C. B., Bookout A. L., Mangelsdorf D. J., Evans R. M. A Nuclear Receptor Atlas: macrophage activation. Mol Endocrinol. 2005;19:2466–77. doi: 10.1210/me.2004-0529. [DOI] [PubMed] [Google Scholar]
- Barry J. B., Laganiere J., Giguere V. A single nucleotide in an estrogen-related receptor α site can dictate mode of binding and peroxisome proliferator-activated receptor γ coactivator 1alpha activation of target promoters. Mol Endocrinol. 2006;20:302–10. doi: 10.1210/me.2005-0313. [DOI] [PubMed] [Google Scholar]
- Barry J. B., Giguere V. Epidermal growth factor-induced signaling in breast cancer cells results in selective target gene activation by orphan nuclear receptor estrogen-related receptor α. Cancer Res. 2005;65:6120–9. doi: 10.1158/0008-5472.CAN-05-0922. [DOI] [PubMed] [Google Scholar]
- Bonnelye E., Aubin J. E. Differential expression of estrogen receptor-related receptor α and estrogen receptors α and β in osteoblasts in vivo and in vitro. J Bone Miner Res. 2002a;17:1392–400. doi: 10.1359/jbmr.2002.17.8.1392. [DOI] [PubMed] [Google Scholar]
- Bonnelye E., Kung V., Laplace C., Galson D. L., Aubin J. E. Estrogen receptor-related receptor α impinges on the estrogen axis in bone: potential function in osteoporosis. Endocrinology. 2002b;143:3658–70. doi: 10.1210/en.2002-220095. [DOI] [PubMed] [Google Scholar]
- Bonnelye E., Aubin J. E. Estrogen receptor-related receptor α: a mediator of estrogen response in bone. J Clin Endocrinol Metab. 2005;90:3115–21. doi: 10.1210/jc.2004-2168. [DOI] [PubMed] [Google Scholar]
- Bonnelye E., Vanacker J. M., Dittmar T., Begue A., Desbiens X., Denhardt D. T., Aubin J. E., Laudet V., Fournier B. The ERR-1 orphan receptor is a transcriptional activator expressed during bone development. Mol Endocrinol. 1997;11:905–16. doi: 10.1210/mend.11.7.9948. [DOI] [PubMed] [Google Scholar]
- Bonnelye E., Merdad L., Kung V., Aubin J. E. The orphan nuclear estrogen receptor-related receptor α (ERRalpha) is expressed throughout osteoblast differentiation and regulates bone formation in vitro. J Cell Biol. 2001;153:971–84. doi: 10.1083/jcb.153.5.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnelye E., Zirngibl R. A., Jurdic P., Aubin J. E. The orphan nuclear estrogen receptor-related receptor-α regulates cartilage formation in vitro: implication of Sox9. Endocrinology. 2007;148:1195–205. doi: 10.1210/en.2006-0962. [DOI] [PubMed] [Google Scholar]
- Bookout A. L., Jeong Y., Downes M., Yu R. T., Evans R. M., Mangelsdorf D. J. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell. 2006;126:789–99. doi: 10.1016/j.cell.2006.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookout A.L., Jeong Y., Downes M., Yu R. , Evans R.M., Mangelsdorf D.J. NURSA Dataset: Tissue-specific expression patterns of nuclear receptors. 2005.
- Busch B. B., Stevens W. C., Jr., Martin R., Ordentlich P., Zhou S., Sapp D. W., Horlick R. A., Mohan R. Identification of a selective inverse agonist for the orphan nuclear receptor estrogen-related receptor α. J Med Chem. 2004;47:5593–6. doi: 10.1021/jm049334f. [DOI] [PubMed] [Google Scholar]
- Carrier J. C., Deblois G., Champigny C., Levy E., Giguere V. Estrogen-related receptor α (ERRalpha) is a transcriptional regulator of apolipoprotein A-IV and controls lipid handling in the intestine. J Biol Chem. 2004;279:52052–8. doi: 10.1074/jbc.M410337200. [DOI] [PubMed] [Google Scholar]
- Carroll J. S., Liu X. S., Brodsky A. S., Li W., Meyer C. A., Szary A. J., Eeckhoute J., Shao W., Hestermann E. V., Geistlinger T. R., Fox E. A., Silver P. A., Brown M. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell. 2005;122:33–43. doi: 10.1016/j.cell.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Carroll J. S., Meyer C. A., Song J., Li W., Geistlinger T. R., Eeckhoute J., Brodsky A. S., Keeton E. K., Fertuck K. C., Hall G. F., Wang Q., Bekiranov S., Sementchenko V., Fox E. A., Silver P. A., Gingeras T. R., Liu X. S., Brown M. Genome-wide analysis of estrogen receptor binding sites. Nat Genet. 2006;38:1289–97. doi: 10.1038/ng1901. [DOI] [PubMed] [Google Scholar]
- Cartoni R., Leger B., Hock M. B., Praz M., Crettenand A., Pich S., Ziltener J. L., Luthi F., Deriaz O., Zorzano A., Gobelet C., Kralli A., Russell A. P. Mitofusins 1/2 and ERRalpha expression are increased in human skeletal muscle after physical exercise. J Physiol. 2005;567:349–58. doi: 10.1113/jphysiol.2005.092031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castet A., Herledan A., Bonnet S., Jalaguier S., Vanacker J. M., Cavailles V. Receptor-interacting protein 140 differentially regulates estrogen receptor-related receptor transactivation depending on target genes. Mol Endocrinol. 2006;20:1035–47. doi: 10.1210/me.2005-0227. [DOI] [PubMed] [Google Scholar]
- Cavallini A., Notarnicola M., Giannini R., Montemurro S., Lorusso D., Visconti A., Minervini F., Caruso M. G. Oestrogen receptor-related receptor α (ERRalpha) and oestrogen receptors (ERalpha and ERbeta) exhibit different gene expression in human colorectal tumour progression. Eur J Cancer. 2005;41:1487–94. doi: 10.1016/j.ejca.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Chao E. Y., Collins J. L., Gaillard S., Miller A. B., Wang L., Orband-Miller L. A., Nolte R. T., McDonnell D. P., Willson T. M., Zuercher W. J. Structure-guided synthesis of tamoxifen analogs with improved selectivity for the orphan ERRgamma. Bioorg Med Chem Lett. 2006;16:821–4. doi: 10.1016/j.bmcl.2005.11.030. [DOI] [PubMed] [Google Scholar]
- Chen E. I., Hewel J., Krueger J. S., Tiraby C., Weber M. R., Kralli A., Becker K., Yates J. R., 3rd, Felding-Habermann B. Adaptation of energy metabolism in breast cancer brain metastases. Cancer Res. 2007b;67:1472–86. doi: 10.1158/0008-5472.CAN-06-3137. [DOI] [PubMed] [Google Scholar]
- Chen J., Nathans J. Estrogen-Related Receptor β/NR3B2 Controls Epithelial Cell Fate and Endolymph Production by the Stria Vascularis. Dev Cell. 2007a;13:325–37. doi: 10.1016/j.devcel.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Chen F., Zhang Q., McDonald T., Davidoff M. J., Bailey W., Bai C., Liu Q., Caskey C. T. Identification of two hERR2-related novel nuclear receptors utilizing bioinformatics and inverse PCR. Gene. 1999;228:101–9. doi: 10.1016/s0378-1119(98)00619-2. [DOI] [PubMed] [Google Scholar]
- Chen S., Zhou D., Yang C., Sherman M. Molecular basis for the constitutive activity of estrogen-related receptor α-1. J Biol Chem. 2001;276:28465–70. doi: 10.1074/jbc.M102638200. [DOI] [PubMed] [Google Scholar]
- Cheung C. P., Yu S., Wong K. B., Chan L. W., Lai F. M., Wang X., Suetsugi M., Chen S., Chan F. L. Expression and functional study of estrogen receptor-related receptors in human prostatic cells and tissues. J Clin Endocrinol Metab. 2005;90:1830–44. doi: 10.1210/jc.2004-1421. [DOI] [PubMed] [Google Scholar]
- Coward P., Lee D., Hull M. V., Lehmann J. M. 4-Hydroxytamoxifen binds to and deactivates the estrogen-related receptor γ. Proc Natl Acad Sci U S A. 2001;98:8880–4. doi: 10.1073/pnas.151244398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debevec D., Christian M., Morganstein D., Seth A., Herzog B., Parker M., White R. Receptor interacting protein 140 regulates expression of uncoupling protein 1 in adipocytes through specific peroxisome proliferator activated receptor isoforms and estrogen-related receptor α. Mol Endocrinol. 2007;21:1581–92. doi: 10.1210/me.2007-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour C. R., Wilson B. J., Huss J. M., Kelly D. P., Alaynick W. A., Downes M., Evans R. M., Blanchette M., Giguere V. Genome-wide orchestration of cardiac functions by the orphan nuclear receptors ERRalpha and γ. Cell Metab. 2007;5:345–56. doi: 10.1016/j.cmet.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Eudy J. D., Yao S., Weston M. D., Ma-Edmonds M., Talmadge C. B., Cheng J. J., Kimberling W. J., Sumegi J. Isolation of a gene encoding a novel member of the nuclear receptor superfamily from the critical region of Usher syndrome type IIa at 1q41. Genomics. 1998;50:382–4. doi: 10.1006/geno.1998.5345. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–95. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura T., Takahashi S., Urano T., Kumagai J., Ogushi T., Horie-Inoue K., Ouchi Y., Kitamura T., Muramatsu M., Inoue S. Increased expression of estrogen-related receptor α (ERRalpha) is a negative prognostic predictor in human prostate cancer. Int J Cancer. 2007;120:2325–30. doi: 10.1002/ijc.22363. [DOI] [PubMed] [Google Scholar]
- Gaillard S., Grasfeder L. L., Haeffele C. L., Lobenhofer E. K., Chu T. M., Wolfinger R., Kazmin D., Koves T. R., Muoio D. M., Chang C. Y., McDonnell D. P. Receptor-selective coactivators as tools to define the biology of specific receptor-coactivator pairs. Mol Cell. 2006;24:797–803. doi: 10.1016/j.molcel.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Gao M., Sun P., Wang J., Zhao D., Wei L. Expression of estrogen receptor-related receptor isoforms and clinical significance in endometrial adenocarcinoma. Int J Gynecol Cancer. 2006;16:827–33. doi: 10.1111/j.1525-1438.2006.00527.x. [DOI] [PubMed] [Google Scholar]
- Gearhart M. D., Holmbeck S. M., Evans R. M., Dyson H. J., Wright P. E. Monomeric complex of human orphan estrogen related receptor-2 with DNA: a pseudo-dimer interface mediates extended half-site recognition. J Mol Biol. 2003;327:819–32. doi: 10.1016/s0022-2836(03)00183-9. [DOI] [PubMed] [Google Scholar]
- Giguere V., Yang N., Segui P., Evans R. M. Identification of a new class of steroid hormone receptors. Nature. 1988;331:91–4. doi: 10.1038/331091a0. [DOI] [PubMed] [Google Scholar]
- Giguere V. Orphan nuclear receptors: from gene to function. Endocr Rev. 1999;20:689–725. doi: 10.1210/edrv.20.5.0378. [DOI] [PubMed] [Google Scholar]
- Giguere V. To ERR in the estrogen pathway. Trends Endocrinol Metab. 2002;13:220–5. doi: 10.1016/s1043-2760(02)00592-1. [DOI] [PubMed] [Google Scholar]
- Greschik H., Wurtz J. M., Sanglier S., Bourguet W., van Dorsselaer A., Moras D., Renaud J. P. Structural and functional evidence for ligand-independent transcriptional activation by the estrogen-related receptor 3. Mol Cell. 2002;9:303–13. doi: 10.1016/s1097-2765(02)00444-6. [DOI] [PubMed] [Google Scholar]
- Greschik H., Flaig R., Renaud J. P., Moras D. Structural basis for the deactivation of the estrogen-related receptor γ by diethylstilbestrol or 4-hydroxytamoxifen and determinants of selectivity. J Biol Chem. 2004;279:33639–46. doi: 10.1074/jbc.M402195200. [DOI] [PubMed] [Google Scholar]
- Heard D. J., Norby P. L., Holloway J., Vissing H. Human ERRgamma, a third member of the estrogen receptor-related receptor (ERR) subfamily of orphan nuclear receptors: tissue-specific isoforms are expressed during development and in the adult. Mol Endocrinol. 2000;14:382–92. doi: 10.1210/mend.14.3.0431. [DOI] [PubMed] [Google Scholar]
- Hentschke M., Borgmeyer U. Identification of PNRC2 and TLE1 as activation function-1 cofactors of the orphan nuclear receptor ERRgamma. Biochem Biophys Res Commun. 2003;312:975–82. doi: 10.1016/j.bbrc.2003.11.025. [DOI] [PubMed] [Google Scholar]
- Herzog B., Cardenas J., Hall R. K., Villena J. A., Budge P. J., Giguere V., Granner D. K., Kralli A. Estrogen-related receptor α is a repressor of phosphoenolpyruvate carboxykinase gene transcription. J Biol Chem. 2006;281:99–106. doi: 10.1074/jbc.M509276200. [DOI] [PubMed] [Google Scholar]
- Hong H., Yang L., Stallcup M. R. Hormone-independent transcriptional activation and coactivator binding by novel orphan nuclear receptor ERR3. J Biol Chem. 1999;274:22618–26. doi: 10.1074/jbc.274.32.22618. [DOI] [PubMed] [Google Scholar]
- Horard B., Rayet B., Triqueneaux G., Laudet V., Delaunay F., Vanacker J. M. Expression of the orphan nuclear receptor ERRalpha is under circadian regulation in estrogen-responsive tissues. J Mol Endocrinol. 2004;33:87–97. doi: 10.1677/jme.0.0330087. [DOI] [PubMed] [Google Scholar]
- Huppunen J., Aarnisalo P. Dimerization modulates the activity of the orphan nuclear receptor ERRgamma. Biochem Biophys Res Commun. 2004;314:964–70. doi: 10.1016/j.bbrc.2003.12.194. [DOI] [PubMed] [Google Scholar]
- Huss J. M., Torra I. P., Staels B., Giguere V., Kelly D. P. Estrogen-related receptor α directs peroxisome proliferator-activated receptor α signaling in the transcriptional control of energy metabolism in cardiac and skeletal muscle. Mol Cell Biol. 2004;24:9079–91. doi: 10.1128/MCB.24.20.9079-9091.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huss J. M., Kopp R. P., Kelly D. P. Peroxisome proliferator-activated receptor coactivator-1alpha (PGC-1alpha) coactivates the cardiac-enriched nuclear receptors estrogen-related receptor-α and -γ. Identification of novel leucine-rich interaction motif within PGC-1alpha. J Biol Chem. 2002;277:40265–74. doi: 10.1074/jbc.M206324200. [DOI] [PubMed] [Google Scholar]
- Huss J. M., Imahashi K., Dufour C. R., Weinheimer C. J., Courtois M., Kovacs A., Giguere V., Murphy E., Kelly D. P. The nuclear receptor ERRalpha is required for the bioenergetic and functional adaptation to cardiac pressure overload. Cell Metab. 2007;6:25–37. doi: 10.1016/j.cmet.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Ichida M., Nemoto S., Finkel T. Identification of a specific molecular repressor of the peroxisome proliferator-activated receptor γ Coactivator-1 α (PGC-1alpha) J Biol Chem. 2002;277:50991–5. doi: 10.1074/jbc.M210262200. [DOI] [PubMed] [Google Scholar]
- Johnston S. D., Liu X., Zuo F., Eisenbraun T. L., Wiley S. R., Kraus R. J., Mertz J. E. Estrogen-related receptor α 1 functionally binds as a monomer to extended half-site sequences including ones contained within estrogen-response elements. Mol Endocrinol. 1997;11:342–52. doi: 10.1210/mend.11.3.9897. [DOI] [PubMed] [Google Scholar]
- Kallen J., Lattmann R., Beerli R., Blechschmidt A., Blommers M. J., Geiser M., Ottl J., Schlaeppi J. M., Strauss A., Fournier B. Crystal structure of human estrogen-related receptor α in complex with a synthetic inverse agonist reveals its novel molecular mechanism. J Biol Chem. 2007;282:23231–9. doi: 10.1074/jbc.M703337200. [DOI] [PubMed] [Google Scholar]
- Kallen J., Schlaeppi J. M., Bitsch F., Filipuzzi I., Schilb A., Riou V., Graham A., Strauss A., Geiser M., Fournier B. Evidence for ligand-independent transcriptional activation of the human estrogen-related receptor α (ERRalpha): crystal structure of ERRalpha ligand binding domain in complex with peroxisome proliferator-activated receptor coactivator-1alpha. J Biol Chem. 2004;279:49330–7. doi: 10.1074/jbc.M407999200. [DOI] [PubMed] [Google Scholar]
- Kamei Y., Ohizumi H., Fujitani Y., Nemoto T., Tanaka T., Takahashi N., Kawada T., Miyoshi M., Ezaki O., Kakizuka A. PPARgamma coactivator 1beta/ERR ligand 1 is an ERR protein ligand, whose expression induces a high-energy expenditure and antagonizes obesity. Proc Natl Acad Sci U S A. 2003;100:12378–83. doi: 10.1073/pnas.2135217100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei Y., Lwin H., Saito K., Yokoyama T., Yoshiike N., Ezaki O., Tanaka H. The 2.3 genotype of ESRRA23 of the ERR α gene is associated with a higher BMI than the 2.2 genotype. Obes Res. 2005;13:1843–4. doi: 10.1038/oby.2005.225. [DOI] [PubMed] [Google Scholar]
- Kliewer S. A., Lehmann J. M., Willson T. M. Orphan nuclear receptors: shifting endocrinology into reverse. Science. 1999;284:757–60. doi: 10.1126/science.284.5415.757. [DOI] [PubMed] [Google Scholar]
- Kojo H., Tajima K., Fukagawa M., Isogai T., Nishimura S. A novel estrogen receptor-related protein γ splice variant lacking a DNA binding domain exon modulates transcriptional activity of a moderate range of nuclear receptors. J Steroid Biochem Mol Biol. 2006;98:181–92. doi: 10.1016/j.jsbmb.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Laflamme N., Giroux S., Loredo-Osti J. C., Elfassihi L., Dodin S., Blanchet C., Morgan K., Giguere V., Rousseau F. A frequent regulatory variant of the estrogen-related receptor α gene associated with BMD in French-Canadian premenopausal women. J Bone Miner Res. 2005;20:938–44. doi: 10.1359/JBMR.050203. [DOI] [PubMed] [Google Scholar]
- Laganiere J., Tremblay G. B., Dufour C. R., Giroux S., Rousseau F., Giguere V. A polymorphic autoregulatory hormone response element in the human estrogen-related receptor α (ERRalpha) promoter dictates peroxisome proliferator-activated receptor γ coactivator-1alpha control of ERRalpha expression. J Biol Chem. 2004;279:18504–10. doi: 10.1074/jbc.M313543200. [DOI] [PubMed] [Google Scholar]
- Laganiere J., Deblois G., Giguere V. Functional genomics identifies a mechanism for estrogen activation of the retinoic acid receptor alpha1 gene in breast cancer cells. Mol Endocrinol. 2005;19:1584–92. doi: 10.1210/me.2005-0040. [DOI] [PubMed] [Google Scholar]
- Larsen L. H., Rose C. S., Sparso T., Overgaard J., Torekov S. S., Grarup N., Jensen D. P., Albrechtsen A., Andersen G., Ek J., Glumer C., Borch-Johnsen K., Jorgensen T., Hansen T., Pedersen O. Genetic analysis of the estrogen-related receptor α and studies of association with obesity and type 2 diabetes. Int J Obes (Lond) 2007;31:365–70. doi: 10.1038/sj.ijo.0803408. [DOI] [PubMed] [Google Scholar]
- Liu D., Hinshelwood M. M., Giguere V., Mendelson C. R. Estrogen related receptor-α enhances surfactant protein-A gene expression in fetal lung type II cells. Endocrinology. 2006;147:5187–95. doi: 10.1210/en.2006-0664. [DOI] [PubMed] [Google Scholar]
- Liu D., Zhang Z., Teng C. T. Estrogen-related receptor-γ and peroxisome proliferator-activated receptor-γ coactivator-1alpha regulate estrogen-related receptor-α gene expression via a conserved multi-hormone response element. J Mol Endocrinol. 2005;34:473–87. doi: 10.1677/jme.1.01586. [DOI] [PubMed] [Google Scholar]
- Luo J., Sladek R., Bader J. A., Matthyssen A., Rossant J., Giguere V. Placental abnormalities in mouse embryos lacking the orphan nuclear receptor ERR-β. Nature. 1997;388:778–82. doi: 10.1038/42022. [DOI] [PubMed] [Google Scholar]
- Luo J., Sladek R., Carrier J., Bader J. A., Richard D., Giguere V. Reduced fat mass in mice lacking orphan nuclear receptor estrogen-related receptor α. Mol Cell Biol. 2003;23:7947–56. doi: 10.1128/MCB.23.22.7947-7956.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D., Kiriyama Y., Lee K. Y., Giguere V. Transcriptional regulation of the estrogen-inducible pS2 breast cancer marker gene by the ERR family of orphan nuclear receptors. Cancer Res. 2001;61:6755–61. [PubMed] [Google Scholar]
- Mitsunaga K., Araki K., Mizusaki H., Morohashi K., Haruna K., Nakagata N., Giguere V., Yamamura K., Abe K. Loss of PGC-specific expression of the orphan nuclear receptor ERR-β results in reduction of germ cell number in mouse embryos. Mech Dev. 2004;121:237–46. doi: 10.1016/j.mod.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Mootha V. K., Handschin C., Arlow D., Xie X., St Pierre J., Sihag S., Yang W., Altshuler D., Puigserver P., Patterson N., Willy P. J., Schulman I. G., Heyman R. A., Lander E. S., Spiegelman B. M. Erralpha and Gabpa/b specify PGC-1alpha-dependent oxidative phosphorylation gene expression that is altered in diabetic muscle. Proc Natl Acad Sci U S A. 2004;101:6570–5. doi: 10.1073/pnas.0401401101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol D., Christian M., Steel J. H., White R., Parker M. G. RIP140 expression is stimulated by estrogen-related receptor α during adipogenesis. J Biol Chem. 2006;281:32140–7. doi: 10.1074/jbc.M604803200. [DOI] [PubMed] [Google Scholar]
- Odom D. T., Zizlsperger N., Gordon D. B., Bell G. W., Rinaldi N. J., Murray H. L., Volkert T. L., Schreiber J., Rolfe P. A., Gifford D. K., Fraenkel E., Bell G. I., Young R. A. Control of pancreas and liver gene expression by HNF transcription factors. Science. 2004;303:1378–81. doi: 10.1126/science.1089769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y. Y., Kim S. H., Kim Y. J., Kim S. Y., Lee T. H., Lee I. K., Park S. B., Choi H. S. Polo-like kinase 2 gene expression is regulated by the orphan nuclear receptor estrogen receptor-related receptor γ (ERRgamma) Biochem Biophys Res Commun. 2007;362:107–13. doi: 10.1016/j.bbrc.2007.07.170. [DOI] [PubMed] [Google Scholar]
- Perusse L., Rankinen T., Zuberi A., Chagnon Y. C., Weisnagel S. J., Argyropoulos G., Walts B., Snyder E. E., Bouchard C. The human obesity gene map: the 2004 update. Obes Res. 2005;13:381–490. doi: 10.1038/oby.2005.50. [DOI] [PubMed] [Google Scholar]
- Pettersson K., Svensson K., Mattsson R., Carlsson B., Ohlsson R., Berkenstam A. Expression of a novel member of estrogen response element-binding nuclear receptors is restricted to the early stages of chorion formation during mouse embryogenesis. Mech Dev. 1996;54:211–23. doi: 10.1016/0925-4773(95)00479-3. [DOI] [PubMed] [Google Scholar]
- Qi H., Aguiar D. J., Williams S. M., La Pean A., Pan W., Verfaillie C. M. Identification of genes responsible for osteoblast differentiation from human mesodermal progenitor cells. Proc Natl Acad Sci U S A. 2003;100:3305–10. doi: 10.1073/pnas.0532693100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangwala S. M., Li X., Lindsley L., Wang X., Shaughnessy S., Daniels T. G., Szustakowski J., Nirmala N. R., Wu Z., Stevenson S. C. Estrogen-related receptor α is essential for the expression of antioxidant protection genes and mitochondrial function. Biochem Biophys Res Commun. 2007;357:231–6. doi: 10.1016/j.bbrc.2007.03.126. [DOI] [PubMed] [Google Scholar]
- Sanyal S., Matthews J., Bouton D., Kim H. J., Choi H. S., Treuter E., Gustafsson J. A. Deoxyribonucleic acid response element-dependent regulation of transcription by orphan nuclear receptor estrogen receptor-related receptor γ. Mol Endocrinol. 2004;18:312–25. doi: 10.1210/me.2003-0165. [DOI] [PubMed] [Google Scholar]
- Sanyal S., Kim J. Y., Kim H. J., Takeda J., Lee Y. K., Moore D. D., Choi H. S. Differential regulation of the orphan nuclear receptor small heterodimer partner (SHP) gene promoter by orphan nuclear receptor ERR isoforms. J Biol Chem. 2002;277:1739–48. doi: 10.1074/jbc.M106140200. [DOI] [PubMed] [Google Scholar]
- Schreiber S. N., Emter R., Hock M. B., Knutti D., Cardenas J., Podvinec M., Oakeley E. J., Kralli A. The estrogen-related receptor α (ERRalpha) functions in PPARgamma coactivator 1alpha (PGC-1alpha)-induced mitochondrial biogenesis. Proc Natl Acad Sci U S A. 2004;101:6472–7. doi: 10.1073/pnas.0308686101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S. N., Knutti D., Brogli K., Uhlmann T., Kralli A. The transcriptional coactivator PGC-1 regulates the expression and activity of the orphan nuclear receptor estrogen-related receptor α (ERRalpha) J Biol Chem. 2003;278:9013–8. doi: 10.1074/jbc.M212923200. [DOI] [PubMed] [Google Scholar]
- Shi H., Shigeta H., Yang N., Fu K., O'Brian G., Teng C. T. Human estrogen receptor-like 1 (ESRL1) gene: genomic organization, chromosomal localization, and promoter characterization. Genomics. 1997;44:52–60. doi: 10.1006/geno.1997.4850. [DOI] [PubMed] [Google Scholar]
- Sladek R., Beatty B., Squire J., Copeland N. G., Gilbert D. J., Jenkins N. A., Giguere V. Chromosomal mapping of the human and murine orphan receptors ERRalpha (ESRRA) and ERRbeta (ESRRB) and identification of a novel human ERRalpha-related pseudogene. Genomics. 1997b;45:320–6. doi: 10.1006/geno.1997.4939. [DOI] [PubMed] [Google Scholar]
- Sladek R., Bader J. A., Giguere V. The orphan nuclear receptor estrogen-related receptor α is a transcriptional regulator of the human medium-chain acyl coenzyme A dehydrogenase gene. Mol Cell Biol. 1997a;17:5400–9. doi: 10.1128/mcb.17.9.5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda J., Laganiere J., Mehl I. R., Barish G. D., Chong L. W., Li X., Scheffler I. E., Mock D. C., Bataille A. R., Robert F., Lee C. H., Giguere V., Evans R. M. Nuclear receptor ERR α and coactivator PGC-1 β are effectors of IFN-γ-induced host defense. Genes Dev. 2007;21:1909–20. doi: 10.1101/gad.1553007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano F. X., Liesa M., Bach D., Chan D. C., Palacin M., Zorzano A. Evidence for a mitochondrial regulatory pathway defined by peroxisome proliferator-activated receptor-γ coactivator-1 α, estrogen-related receptor-α, and mitofusin 2. Diabetes. 2006;55:1783–91. doi: 10.2337/db05-0509. [DOI] [PubMed] [Google Scholar]
- Suetsugi M., Su L., Karlsberg K., Yuan Y. C., Chen S. Flavone and isoflavone phytoestrogens are agonists of estrogen-related receptors. Mol Cancer Res. 2003;1:981–91. [PubMed] [Google Scholar]
- Sullivan A. A., Thummel C. S. Temporal profiles of nuclear receptor gene expression reveal coordinate transcriptional responses during Drosophila development. Mol Endocrinol. 2003;17:2125–37. doi: 10.1210/me.2002-0430. [DOI] [PubMed] [Google Scholar]
- Sumi D., Ignarro L. J. Estrogen-related receptor α 1 up-regulates endothelial nitric oxide synthase expression. Proc Natl Acad Sci U S A. 2003;100:14451–6. doi: 10.1073/pnas.2235590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun P. M., Gao M., Wei L. H., Mustea A., Wang J. L., Konsgen D., Lichtenegger W., Sehouli J. An estrogen receptor α-dependent regulation of estrogen receptor-related receptor α in the proliferation of endometrial carcinoma cells. Int J Gynecol Cancer. 2006;16 Suppl 2:564–8. doi: 10.1111/j.1525-1438.2006.00697.x. [DOI] [PubMed] [Google Scholar]
- Sun P., Sehouli J., Denkert C., Mustea A., Konsgen D., Koch I., Wei L., Lichtenegger W. Expression of estrogen receptor-related receptors, a subfamily of orphan nuclear receptors, as new tumor biomarkers in ovarian cancer cells. J Mol Med. 2005;83:457–67. doi: 10.1007/s00109-005-0639-3. [DOI] [PubMed] [Google Scholar]
- Susens U., Hermans-Borgmeyer I., Borgmeyer U. Alternative splicing and expression of the mouse estrogen receptor-related receptor γ. Biochem Biophys Res Commun. 2000;267:532–5. doi: 10.1006/bbrc.1999.1976. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Miki Y., Moriya T., Shimada N., Ishida T., Hirakawa H., Ohuchi N., Sasano H. Estrogen-related receptor α in human breast carcinoma as a potent prognostic factor. Cancer Res. 2004;64:4670–6. doi: 10.1158/0008-5472.CAN-04-0250. [DOI] [PubMed] [Google Scholar]
- Takayanagi S., Tokunaga T., Liu X., Okada H., Matsushima A., Shimohigashi Y. Endocrine disruptor bisphenol A strongly binds to human estrogen-related receptor γ (ERRgamma) with high constitutive activity. Toxicol Lett. 2006;167:95–105. doi: 10.1016/j.toxlet.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Tremblay G. B., Bergeron D., Giguere V. 4-Hydroxytamoxifen is an isoform-specific inhibitor of orphan estrogen-receptor-related (ERR) nuclear receptors β and γ. Endocrinology. 2001a;142:4572–5. doi: 10.1210/endo.142.10.8528. [DOI] [PubMed] [Google Scholar]
- Tremblay G. B., Kunath T., Bergeron D., Lapointe L., Champigny C., Bader J. A., Rossant J., Giguere V. Diethylstilbestrol regulates trophoblast stem cell differentiation as a ligand of orphan nuclear receptor ERR β. Genes Dev. 2001b;15:833–8. doi: 10.1101/gad.873401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanacker J. M., Delmarre C., Guo X., Laudet V. Activation of the osteopontin promoter by the orphan nuclear receptor estrogen receptor related α. Cell Growth Differ. 1998b;9:1007–14. [PubMed] [Google Scholar]
- Vanacker J. M., Bonnelye E., Delmarre C., Laudet V. Activation of the thyroid hormone receptor α gene promoter by the orphan nuclear receptor ERR α. Oncogene. 1998a;17:2429–35. doi: 10.1038/sj.onc.1202167. [DOI] [PubMed] [Google Scholar]
- Vanacker J. M., Bonnelye E., Chopin-Delannoy S., Delmarre C., Cavailles V., Laudet V. Transcriptional activities of the orphan nuclear receptor ERR α (estrogen receptor-related receptor-α) Mol Endocrinol. 1999a;13:764–73. doi: 10.1210/mend.13.5.0281. [DOI] [PubMed] [Google Scholar]
- Vanacker J. M., Pettersson K., Gustafsson J. A., Laudet V. Transcriptional targets shared by estrogen receptor- related receptors (ERRs) and estrogen receptor (ER) α, but not by ERbeta. Embo J. 1999b;18:4270–9. doi: 10.1093/emboj/18.15.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega R. B., Kelly D. P. A role for estrogen-related receptor α in the control of mitochondrial fatty acid β-oxidation during brown adipocyte differentiation. J Biol Chem. 1997;272:31693–9. doi: 10.1074/jbc.272.50.31693. [DOI] [PubMed] [Google Scholar]
- Villena J. A., Hock M. B., Chang W. Y., Barcas J. E., Giguere V., Kralli A. Orphan nuclear receptor estrogen-related receptor α is essential for adaptive thermogenesis. Proc Natl Acad Sci U S A. 2007;104:1418–23. doi: 10.1073/pnas.0607696104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Zuercher W. J., Consler T. G., Lambert M. H., Miller A. B., Orband-Miller L. A., McKee D. D., Willson T. M., Nolte R. T. X-ray crystal structures of the estrogen-related receptor-γ ligand binding domain in three functional states reveal the molecular basis of small molecule regulation. J Biol Chem. 2006;281:37773–81. doi: 10.1074/jbc.M608410200. [DOI] [PubMed] [Google Scholar]
- Watanabe A., Kinoshita Y., Hosokawa K., Mori T., Yamaguchi T., Honjo H. Function of estrogen-related receptor α in human endometrial cancer. J Clin Endocrinol Metab. 2006;91:1573–7. doi: 10.1210/jc.2005-1990. [DOI] [PubMed] [Google Scholar]
- Wende A. R., Huss J. M., Schaeffer P. J., Giguere V., Kelly D. P. PGC-1alpha coactivates PDK4 gene expression via the orphan nuclear receptor ERRalpha: a mechanism for transcriptional control of muscle glucose metabolism. Mol Cell Biol. 2005;25:10684–94. doi: 10.1128/MCB.25.24.10684-10694.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley S. R., Kraus R. J., Zuo F., Murray E. E., Loritz K., Mertz J. E. SV40 early-to-late switch involves titration of cellular transcriptional repressors. Genes Dev. 1993;7:2206–19. doi: 10.1101/gad.7.11.2206. [DOI] [PubMed] [Google Scholar]
- Willy P. J., Murray I. R., Qian J., Busch B. B., Stevens W. C., Jr., Martin R., Mohan R., Zhou S., Ordentlich P., Wei P., Sapp D. W., Horlick R. A., Heyman R. A., Schulman I. G. Regulation of PPARgamma coactivator 1alpha (PGC-1alpha) signaling by an estrogen-related receptor α (ERRalpha) ligand. Proc Natl Acad Sci U S A. 2004;101:8912–7. doi: 10.1073/pnas.0401420101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W., Hong H., Yang N. N., Lin R. J., Simon C. M., Stallcup M. R., Evans R. M. Constitutive activation of transcription and binding of coactivator by estrogen-related receptors 1 and 2. Mol Endocrinol. 1999;13:2151–62. doi: 10.1210/mend.13.12.0381. [DOI] [PubMed] [Google Scholar]
- Yang N., Shigeta H., Shi H., Teng C. T. Estrogen-related receptor, hERR1, modulates estrogen receptor-mediated response of human lactoferrin gene promoter. J Biol Chem. 1996;271:5795–804. doi: 10.1074/jbc.271.10.5795. [DOI] [PubMed] [Google Scholar]
- Yang C., Zhou D., Chen S. Modulation of aromatase expression in the breast tissue by ERR α-1 orphan receptor. Cancer Res. 1998;58:5695–700. [PubMed] [Google Scholar]
- Yang X., Downes M., Yu R. T., Bookout A. L., He W., Straume M., Mangelsdorf D. J., Evans R. M. Nuclear receptor expression links the circadian clock to metabolism. Cell. 2006;126:801–10. doi: 10.1016/j.cell.2006.06.050. [DOI] [PubMed] [Google Scholar]
- Yang C., Chen S. Two organochlorine pesticides, toxaphene and chlordane, are antagonists for estrogen-related receptor α-1 orphan receptor. Cancer Res. 1999;59:4519–24. [PubMed] [Google Scholar]
- Yu D. D., Forman B. M. Identification of an agonist ligand for estrogen-related receptors ERRbeta/γ. Bioorg Med Chem Lett. 2005;15:1311–3. doi: 10.1016/j.bmcl.2005.01.025. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Teng C. T. Estrogen receptor-related receptor α 1 interacts with coactivator and constitutively activates the estrogen response elements of the human lactoferrin gene. J Biol Chem. 2000;275:20837–46. doi: 10.1074/jbc.M001880200. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Ma K., Sadana P., Chowdhury F., Gaillard S., Wang F., McDonnell D. P., Unterman T. G., Elam M. B., Park E. A. Estrogen-related receptors stimulate pyruvate dehydrogenase kinase isoform 4 gene expression. J Biol Chem. 2006a;281:39897–906. doi: 10.1074/jbc.M608657200. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Chen K., Shih J. C., Teng C. T. Estrogen-related receptors-stimulated monoamine oxidase B promoter activity is down-regulated by estrogen receptors. Mol Endocrinol. 2006b;20:1547–61. doi: 10.1210/me.2005-0252. [DOI] [PubMed] [Google Scholar]
- Zhou W., Liu Z., Wu J., Liu J. H., Hyder S. M., Antoniou E., Lubahn D. B. Identification and characterization of two novel splicing isoforms of human estrogen-related receptor β. J Clin Endocrinol Metab. 2006;91:569–79. doi: 10.1210/jc.2004-1957. [DOI] [PubMed] [Google Scholar]
- Zhou D., Quach K. M., Yang C., Lee S. Y., Pohajdak B., Chen S. PNRC: a proline-rich nuclear receptor coregulatory protein that modulates transcriptional activation of multiple nuclear receptors including orphan receptors SF1 (steroidogenic factor 1) and ERRalpha1 (estrogen related receptor α-1) Mol Endocrinol. 2000;14:986–98. doi: 10.1210/mend.14.7.0480. [DOI] [PubMed] [Google Scholar]
- Zhou D., Chen S. PNRC2 is a 16 kDa coactivator that interacts with nuclear receptors through an SH3-binding motif. Nucleic Acids Res. 2001;29:3939–48. doi: 10.1093/nar/29.19.3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuercher W. J., Gaillard S., Orband-Miller L. A., Chao E. Y., Shearer B. G., Jones D. G., Miller A. B., Collins J. L., McDonnell D. P., Willson T. M. Identification and structure-activity relationship of phenolic acyl hydrazones as selective agonists for the estrogen-related orphan nuclear receptors ERRbeta and ERRgamma. J Med Chem. 2005;48:3107–9. doi: 10.1021/jm050161j. [DOI] [PubMed] [Google Scholar]
- Zuo F., Mertz J. E. Simian virus 40 late gene expression is regulated by members of the steroid/thyroid hormone receptor superfamily. Proc Natl Acad Sci U S A. 1995;92:8586–90. doi: 10.1073/pnas.92.19.8586. [DOI] [PMC free article] [PubMed] [Google Scholar]