Abstract
Although the importance of the progesterone receptor (PR) to female reproductive and mammary gland biology is firmly established, the coregulators selectively co-opted by PR in these systems have not been clearly delineated. A selective gene-knockout approach applied to the mouse, which abrogates gene function only in cell types that express PR, recently disclosed steroid receptor coactivator 2 (SRC-2, also known as TIF-2 or GRIP-1) to be an indispensable coregulator for uterine and mammary gland responses that require progesterone. Uterine cells positive for PR (but devoid of SRC-2) were found to be incapable of facilitating embryo implantation, a necessary first step toward the establishment of the materno-fetal interface. Importantly, such an implantation defect is not exhibited by knockouts for SRC-1 or SRC-3, underscoring the unique coregulator importance of SRC-2 in peri-implantation biology. Moreover, despite normal levels of PR, SRC-1 and SRC-3, progesterone-dependent branching morphogenesis and alveologenesis fails to occur in the murine mammary gland in the absence of SRC-2, thereby establishing a critical coregulator role for SRC-2 in signaling cascades that mediate progesterone-induced mammary epithelial proliferation. Finally, the recent detection of SRC-2 in the human endometrium and breast suggests that this coregulator may represent a new clinical target for the future management of female reproductive health and/or breast cancer.
Family ties: SRC-2 is a member of the steroid receptor coactivator/p160 family
Pioneering in vitro studies by the O’Malley group revealed that the transactivational potency of agonist bound progesterone receptor (PR) can be significantly enhanced by increasing the cellular level of members of the steroid receptor coactivator (SRC/p160) family of coregulators, reviewed in [McKenna and O'Malley, 2002]. The SRC/p160 family consists of three members: SRC-1 (ERAP140/ERAP160/NcoA-1); SRC-2 (TIF-2/GRIP-1/NcoA-2); and SRC-3 (p/CIP/RAC3/AIB1/TRAM-1/ACTR/NcoA-3); reviewed in [Lonard and O'Malley, 2005]. To enhance nuclear receptor (NR)-mediated transactivation, each SRC family member has been shown to directly contact - through discreet LXXLL motifs within their NR interaction domain (Figure 1) - the highly conserved activation 2 domain located in the C-terminal region of NRs. Furthermore, two activation domains (AD1 and AD2) positioned in the C-terminal region of each SRC are responsible for recruiting secondary coregulators (or co-coregulators). For example, AD1 is known to interact with histone acetyltransferases (HATs) p300 and the related cyclic AMP-response element binding protein (CREB)-binding protein (CBP), whereas AD2 is known to recruit arginine methyltransferases such as coactivator-associated arginine methyltransferase 1 (CARM1), reviewed in [Lonard and O'Malley, 2006]. The histone-modifying activities of these secondary coregulators (in addition to the weak intrinsic HAT activity of SRC members) facilitate local chromatin remodeling that allows the general transcriptional machinery open access to promoter regions of NR target genes. Apart from histones, these co-coregulators have been shown to posttranslationally modify other target proteins (i.e., other coregulators and transcription factors) within the transcriptional complex. The N-terminally positioned basic helix loop helix-Per/ARNT/Sim (bHLH-PAS) domain is the most conserved structural motif among SRC members (Figure 1) and is also responsible for co-opting additional coregulators and transcription factors. For SRC-2, these coregulators include coiled-coil coactivator (CoCoA) [Kim et al., 2003], flightless-I (Fli-I) [Lee and Stallcup, 2006], GRIP1-associated coactivator 63 (GAC63) [Chen et al., 2005], as well as the transcription factors myocyte-enhancer factor 2C (MEF-2C) [Chen et al., 2000b] and TEF4 [Belandia and Parker, 2000]. In the case of other regulatory proteins, the bHLH-PAS motif has been shown to be involved in both DNA and ligand binding [Gu et al., 2000; Huang et al., 1993], indicating that this structural domain feature may be involved in SRC regulatory functions beyond those currently known.
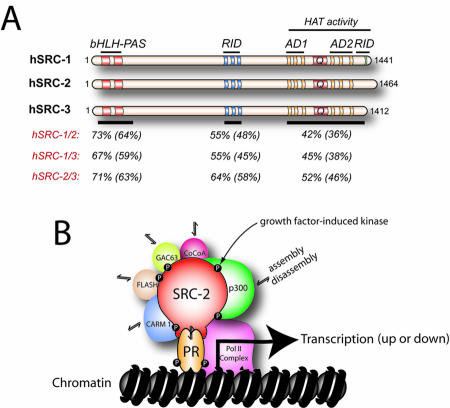
Figure 1. The SRC/p160 family of coregulators.
Panel A shows the domain structure of human (h) SRC-1, -2, and -3 proteins. The basic helix loop helix, Per/ARNT/Sim, receptor interaction and activation domains are indicated by bHLH, PAS, RID, and AD, respectively. The amino acid region associated with histone acetyl transferase (HAT) activity in SRC-1 and -3 is also denoted; Q indicates the glutamine-rich region. Also shown is the similarity and (identity) of amino acid sequences within key functional domains of SRC members. Overall amino acid similarity and (identity) between SRC members is: hSRC1/2, 54% (46%); hSRC1/3, 50% (43%); and hSRC2/3, 55% (48%). Amino acid sequence alignments were conducted using LALNVIEW software (Duret et al., 1996). Panel B shows an schematic model in which the pairing of SRC-2 with PR at the genome comprises part of a dynamic multiprotein transcriptional complex in which such co-coregulators as p300 (Chen et al., 2000a), CARM-1 (Chen et al., 1999), FLASH (Kino et al., 2004), GAC63 (Chen et al., 2005), and CoCoA (Kim and Stallcup, 2004) differentially assemble and disassemble depending on a particular input signal, such as a distinct phosphorylation event mediated by a growth factor or cell survival signal-induced kinase.
Superimposed on the myriad of protein-protein interactions that enable SRC-2 to relay (and control) signaling inputs dispatched from ligand-bound NR to the general transcriptional complex, a multiplicity of interacting signaling inputs (i.e., phosphorylation events triggered by extracellular growth and cell survival factors [Duong et al., 2006; Frigo et al., 2006]) are also being transduced by SRC-2 within the multicomponent transcriptional machinery. Although SRC-2 has been primarily considered a coactivator, a subset of investigations provide strong support for a corepressor role for SRC-2 within certain cellular contexts [Gupta et al., 2007; He and Simons, 2007; Rogatsky et al., 2002; Wang et al., 2007]; these studies highlight the versatility and complexity of this multifunctional coregulator.
While in vitro experiments disclosed the existence of the SRC family, subsequent experimental mouse genetics would uncover important overlapping and non-overlapping roles for the three SRCs in progestin-initiated signaling events in vivo. Further underscoring their multifunctional properties, mouse studies have also uncovered critical roles for each SRC in signaling processes that reside outside the physiologic area of progestin control.
A subgroup of progestin-dependent physiological processes require SRC members: insights from mouse studies
The SRC-1 knockout (KO) mouse was shown to display an attenuated decidual response in the uterus [Xu et al., 1998], suggesting that this coregulator (in concert with others) is required for complete manifestation of this morphological response, which requires initial progesterone stimulation. Although the SRC-3KO exhibits a normal decidual response, a partial block in hormone-induced mammary ductal side-branching and alveologenesis is observed in this animal [Xu et al., 2000]. Collectively, these observations support the proposal that SRC-1 and -3 are required for a subgroup of PR-mediated transcriptional responses in the uterus and mammary gland, respectively. Investigations on the PR activity indicator (PRAI) model provide further support for this supposition [Han et al., 2006; Han et al., 2005]. Mouse studies have also underscored important roles for SRC-1 and SRC-3 in areas of normal physiology and disease which are outside the realm of progesterone control, these include: cell growth [Wang et al., 2000; Xu et al., 2000], metabolism [Louet et al., 2006; Wang et al., 2006], thyroid hormone-based physiology [Ying et al., 2005], bone homeostasis [Modder et al., 2004], prostate biology [Zhou et al., 2005] and B-cell lymphoma developmental progression [Coste et al., 2006].
In contrast to KOs for SRC-1 and -3, the global KO for SRC-2 (termed: Transcriptional Intermediary Factor 2 KO (or TIF2-/-)) displays striking reproductive abnormalities in both sexes [Gehin et al., 2002]. In the female, abrogation of SRC-2 function triggers placental hypoplasia, which results in a severe hypofertility defect. Subsequent studies found that TIF2-/- pups (both sexes) are significantly underrepresented in litters from TIF2+/- crosses (TIF2-/- females resulting from such crosses are infertile (personal observations)). Similar to KOs for SRC-1 and -3, global ablation of SRC-2 function results in physiological defects not directly linked to reproductive biology, such as a decrease in early postnatal survival [Mark et al., 2004], a breakdown in energy homeostasis [Jeong et al., 2006], as well as elaboration of insidious adrenocortical insufficiency [Patchev et al., 2007]).
Cell lineage-specific abrogation of SRC-2 function in the mouse reveals a critical coregulator role for SRC-2 in a subset of physiological processes that require progesterone action
The placental defect exhibited by the global KO for SRC-2, in conjunction with the recent observation that a subset of murine cell lineages express both PR and SRC-2 [Mukherjee et al., 2006b], suggested that SRC-2 (like SRC-1 and -3) may occupy a critical coregulator role in a subgroup of physiological processes that require PR function. To test this hypothesis, a PRCre/+SRC-2flox/flox bigenic mouse was created [Mukherjee et al., 2006b] by crossing a PRCre/+ knockin mouse [Soyal et al., 2005] with a SRC-2flox/flox mouse in which exon 11 of the SRC-2 gene was floxed to enable cre-mediated excision [Gehin et al., 2002]; exon 11 encodes the receptor-interacting domain (RID). Therefore, the PRCre/+SRC-2flox/flox bigenic is designed to abrogate SRC-2 function specifically in cell lineages that are PR positive [Mukherjee et al., 2006b]. The utility of this genetic strategy is that SRC-2’s role in PR-regulated transcriptional programs can be directly investigated at the whole-animal level without interference from other, unrelated phenotypes resulting from SRC-2’s absence (a key advantage over the global KO for SRC-2).
SRC-2 is required for uterine receptivity
To date, female and male PRCre/+SRC-2flox/flox mice show normal postnatal development; however, the PRCre/+SRC-2flox/flox female is sterile [Mukherjee et al., 2006b]. Unlike the TIF2-/- mouse, male PRCre/+SRC-2flox/flox mice exhibit normal fertility and neither sex displays phenotypes (outside progestin control) previously described for the TIF2-/- model [Gehin et al., 2002].
The absence of implantation sites along the uterine horn of the PRCre/+SRC-2flox/flox mouse (5.5 days post-coitum) is the primary underlying cause for the infertility defect displayed by the PRCre/+SRC-2flox/flox female (Figure 2A). This result suggests a pivotal role played by SRC-2 in the early cellular changes in the uterus that are required for embryo implantation. Although the SRC-2flox/flox uterus (a positive control) exhibits a full decidual response to an artificial deciduogenic stimulus (Figure 2B and C), the PRCre/+SRC-2flox/flox uterus displays only a partial decidual response (Figure 2B and C). These findings support the proposal that a subgroup of PR-mediated transcriptional events are dependent on SRC-2 to launch a complete decidual reaction. The incomplete decidual response phenotype shared by the SRC-1KO [Xu et al., 1998] and PRCre/+SRC-2flox/flox mouse suggests that both SRC coregulators may be required together in PR-mediated signaling cascades that result in a fully decidualized uterus. To test this hypothesis, the SRC-1KO mutation was introduced into the PRCre/+SRC-2flox/flox germline to generate a PRCre/+SRC-2flox/flox SRC-1KO trigenic model. Figure 2B and C shows that the trigenic uterus fails to mount a decidual response, thereby furnishing essential in vivo support for a cooperative involvement for SRC-1 and SRC-2 in the progesterone-dependent decidual reaction. Note that the PRCre/+SRC-2flox/flox uterine phenotype is not explained by changes in the normal levels of uterine SRC-1 and/or SRC-3 protein (Figure 2D).
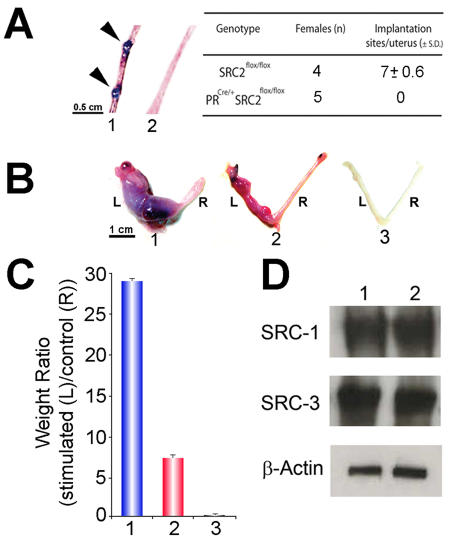
Figure 2. Abrogation of uterine SRC-2 results in a block in embryo implantation and a partial decidual response.
In panel A, arrows show the location of implantation sites in the uterus (1) of a SRC-2flox/flox (or wild-type (WT)) mouse (5.5 days post coitum (d.p.c.)). However, implantation sites were not detected in uteri from similarly treated PRCre/+SRC-2flox/flox (2) mice. The average number of implantation sites per genotype per total number of mice examined is tabulated. In panel B, the gross morphological response of the left (L) uterine horn to a deciduogenic stimulus for SRC-2flox/flox (1), PRCre/+SRC-2flox/flox (2), and PRCre/+SRC-2flox/flox SRC-1KO trigenic (3) mice is shown. The right (R) uterine horn represents the unstimulated control. Although the PRCre/+SRC-2flox/flox uterus (2) exhibits a limited decidual response, note the absence of a decidual response in the PRCre/+SRC-2flox/flox SRC-1KO trigenic uterus (3). Panel C graphically presents the average weight ratios (± standard deviation (SD)) of stimulated (L) to control (R) horn for SRC-2flox/flox (1), PRCre/+SRC-2flox/flox (2), and PRCre/+SRC-2flox/flox SRC-1KO trigenic (3) uteri. Western analysis in panel D reveals uterine tissue from untreated adult virgin SRC-2flox/flox (1) and PRCre/+SRC-2flox/flox (2) mice show equivalent levels of uterine SRC-1 and SRC-3 (loading control is β-actin). Modified from (Mukherjee et al., 2006b) (Copyright (2006) American Society for Microbiology).
In contrast to the uterus, ovarian and pituitary function is not compromised in the PRCre/+SRC-2flox/flox mouse, suggesting that PR enlists other coregulators in these systems (normal ovarian and pituitary functionality is severely diminished in the PRKO [Lydon et al., 1995]). Furthermore, SRC-2 is not required for progesterone-inhibition of PR expression or suppression of estrogen-induced luminal epithelial proliferation in the uterus. Collectively, these observations suggest that the selective enlistment of SRC-2 by PR in female reproductive tissues may provide one explanation as to why different reproductive tissues display different responses to the same progesterone signal.
Having disclosed a central role for uterine SRC-2 in murine peri-implantation biology, future questions to be addressed include: (1) Is uterine SRC-2 expression in the epithelial, stromal, or both cellular compartments required for the development of the receptive uterus? (2) Is SRC-2 expressed in the embryonic-derived trophectoderm? If so, is trophectoderm-derived SRC-2 required for embryo implantation? (3) Does uterine SRC-2 possess coregulator functions necessary for later stages of pregnancy? For example, in the regulation of the onset of parturition; and (4) Does SRC-2 have a role in the etiopathogenesis of such endometrial disorders as uterine hyperplasia and/or endometriosis?
Postnatal mammary morphogenesis requires SRC-2 function
The detection of SRC-2 protein in mammary epithelial cells that are PR positive [Mukherjee et al., 2006b] suggested that mammary SRC-2 may occupy a crucial role in PR-mediated proliferative programs which result in ductal side-branching and alveolar morphogenesis in the mammary gland of the adult. This assumption was supported by the observation that the PRCre/+SRC-2flox/flox mammary gland fails to exhibit the typical morphological changes that occur with combined estrogen and progestin exposure (Figure 3A-D). Like the PRKO [Lydon et al., 1999], the underlying cause of the PRCre/+SRC-2flox/flox mammary phenotype is a failure of the mammary epithelium to proliferate in response to hormone (Figure 3E). These results support an essential role for SRC-2 in progesterone-induced signaling programs which are required for mammary morphogenesis in the adult. Of note, the PRCre/+SRC-2flox/flox mammary defect was not compensated for by SRC-3 (Figure 3E). Although SRC-3 has been shown to be involved in steroid-induced mammary morphogenesis [Xu et al., 2000], as well as tumorigenesis [Kuang et al., 2005; Kuang et al., 2004; Torres-Arzayus et al., 2004], our data to date suggest that SRC-2 and -3 are operationally distinct in the murine mammary epithelial cell. Irrespective of the functional interrelationships between SRC-2 and other members of the SRC family in this tissue, our studies reveal SRC-2 to be an important coactivator for progestin-initiated signaling in the mammary epithelium. An important question for the future will be to determine whether SRC-2 (like SRC-3/AIB-1 [Anzick et al., 1997]) can act as a mammary oncogene.
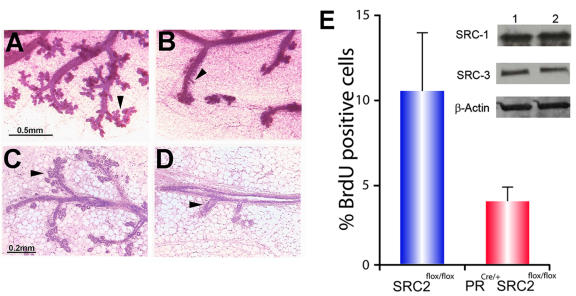
Figure 3. Absence of mammary SRC-2 function blocks progestin-induced ductal side-branching and alveologenesis.
Panels A and B show whole-mounts of mammary glands from SRC-2flox/flox and PRCre/+SRC-2flox/flox mice (following three weeks of estrogen plus progesterone (EP) exposure), respectively. Unlike the SRC-2flox/flox mammary gland (positive control), note the marked reduction in branching morphogenesis (black arrow) in the PRCre/+SRC-2flox/flox gland. Panels C and D represent hematoxylin and eosin (H&E) stained sections of tissue shown in panels A and B, respectively. Compared to the SRC-2flox/flox gland (panel C), note the conspicuous reduction in the epithelial compartment in the PRCre/+SRC-2flox/flox gland (panel D (arrowhead)). The graph in panel E displays the average percentage of mammary epithelial cells (± standard deviation (S.D.)) positive for BrdU incorporation in the hormone-treated SRC-2flox/flox and PRCre/+SRC-2flox/flox glands. Inset shows an SRC-2flox/flox (1) and PRCre/+SRC-2flox/flox (2) immunoblot for mammary SRC-1 and -3. In comparison to SRC-2flox/flox, changes in SRC-1 and -3 protein levels are not observed in the PRCre/+SRC-2flox/flox mammary gland (β-actin acts as a loading control). Scale bars in panels A and C apply to B and D, respectively. Modified from (Mukherjee et al., 2006b) (Copyright (2006) American Society for Microbiology).
Relevance to the human…
Although state-of-the-art genetics demonstrated a critical role for SRC-2 in a subset of progesterone responses in the uterus and mammary gland of the mouse, whether these findings translate to the human is now an important research focus. As previously shown [Hofman et al., 2002], the transactivational potency of the human PR ortholog is significantly enhanced with increasing levels of human SRC-2 (Figure 4A). These observations provide strong support for a coregulator involvement for SRC-2 in progestin-dependent physiological processes in the human. As further support for this supposition, immunohistochemistry demonstrates that SRC-2 protein is expressed in a subset of steroid-responsive target tissues in the human (Figure 4B-F). In the case of human prostate, (Figure 4B), SRC-2 expression is regionally restricted to the epithelial compartment, a known cellular target-site for androgen receptor-mediated signaling and neoplastic transformation [Berrevoets et al., 2004; Culig et al., 2002; Ye et al., 2005]. In the human endometrium, immunohistochemical studies clearly demonstrate that SRC-2 and PR are expressed in identical cell types within the stromal and epithelial compartments (Figure 4C-D); similar findings have been described for the mouse [Mukherjee et al., 2006b].
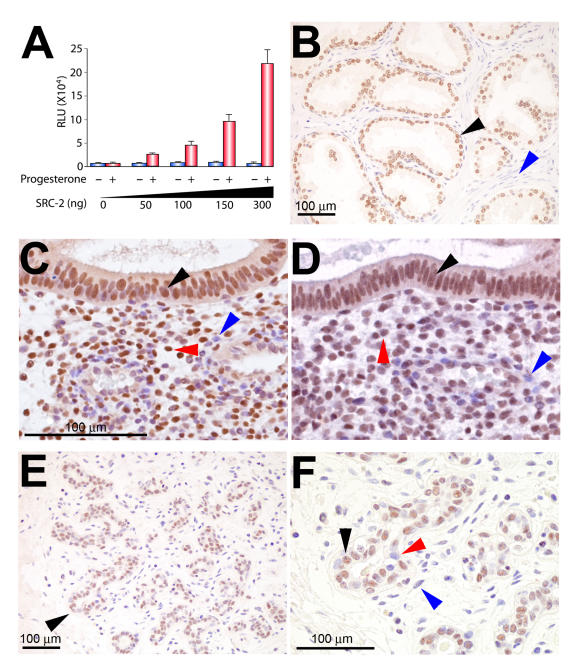
Figure 4. Steroid hormone responsive tissues express SRC-2 in the human.
Panel A shows the increase in ligand-dependent transactivational potency of human PR-B is dependent on increased levels of human SRC-2 (red bars ± S.D.); in the absence of ligand, this increase is not observed (blue bars). For these experiments, human PR-B; SRC-2 (both cloned into pCR3.1) and the luciferase reporter pGRE.E1b.LUC were transiently cotransfected into HeLa cells in the presence or absence of 10-7M R5020, as described previously (Lonard et al., 2004); results are expressed in relative light units (RLU). Panel B shows SRC-2 is expressed in the majority of epithelial cells of the human prostate (black arrow), an established cellular target for androgen receptor action (Culig et al., 2002); note: the stromal compartment registers negative for SRC-2 expression (blue arrow). Panels C and D show transverse sections of the luminal and stromal compartment (with surrounding stroma) of the human endometrium stained for PR and SRC-2 expression, respectively. Note that PR and SRC-2 are detected in nuclei of the same cell types in both cellular compartments (black and red arrows, respectively). The blue arrow in panels C and D highlights a stromal cell negative for PR and SRC-2 expression, respectively; scale bar in panel C applies to panel D. Endometrial biopsies were obtained by endometrial pipelle from healthy women with normal cycles (aged between 18-35 years) during the mid-secretory (luteal) phase of the menstrual cycle (days 20-24, which is based on the ideal 28 day cycle, in which day 1 represents the first day of menstrual flow and day 14 the day of ovulation); cycle phase was determined relative to the timing of the urinary luteinizing hormone (LH) surge. Immunohistochemical detection of human SRC-2 and PR was undertaken using established methods previously reported by our group (Lee et al., 2005; Mukherjee et al., 2006b). Panel E shows a representative example of a normal type 1 terminal ductal lobular unit (TDLU) of the human breast in which SRC-2 expression is restricted to the epithelial compartment (black arrow). Panel F is a higher magnification of the region indicated by the black arrow in panel E. Note that SRC-2 expression is confined to a subset of epithelial cells of the TDLU (black arrow indicates an epithelial cell scoring positive for SRC-2 expression, whereas the red arrow highlights an epithelial cell which is negative for SRC-2 expression; blue arrow denotes a stromal cell which is negative for SRC-2 expression). Interestingly, the spatial expression pattern of mammary SRC-2 resembles that previously reported for ER-α and PR in the human breast (Clarke et al., 1997). With institutional review board approval, human tissue samples were obtained from Baylor College of Medicine affiliated hospitals. Modified from (Mukherjee et al., 2006a) (Copyright (2006) Elsevier, B.V.).
Similar to the murine mammary gland [Mukherjee et al., 2006b], immunohistochemical investigations have shown that a subgroup of epithelial cells within the normal human breast express SRC-2 (Figure 4E and F). Interestingly, the punctate spatial expression pattern for SRC-2 in the human breast parallels a similar spatial expression pattern reported for PR in the rodent and human breast [Clarke et al., 1997]. However, whether (like the mouse [Mukherjee et al., 2006b]) SRC-2 and PR localize to identical cells in the human breast has yet to be demonstrated. Further progress in this area is important, as separation of PR positive mammary epithelial cells from PR negative cells that undergo cell division in response to progesterone is now recognized as an evolutionarily conserved feature that underpins a proposed paracrine mechanism action for PR in the normal breast, reviewed in [Fernandez-Valdivia et al., 2005].
Conclusions and perspective
Despite over 200 known coactivators reported [Lonard and O'Malley, 2006], it is significant that PR action is singularly dependent on the coregulator functions of SRC-2 for a subgroup of progesterone-induced physiological responses that are necessary for the maintenance of female fertility and postnatal mammary morphogenesis in the mouse.
Overtly distinct from SRC-1 and SRC-3, coregulator properties of which subserve only a selection of progesterone-initiated transcriptional responses either in the uterus or mammary gland, SRC-2 exerts potent coregulator activities in both progesterone target tissues. From a clinical standpoint, the indispensable role of SRC-2 in murine peri-implantation biology demands further study, since recurrent implantation failure is now recognized as a key underlying factor that precludes the establishment of a successful pregnancy [Norwitz et al., 2001]). Importantly, abnormal increases in endometrial SRC-2 levels have also been associated with infertility in women with polycystic ovarian syndrome (PCOS) and with a subset of endometrial cancers [Gregory et al., 2002; Pathirage et al., 2006]. Although preliminary, these latter observations suggest a possible connection between perturbation in SRC-2 protein levels and the etiopathogenesis of these uterine disorders.
In the case of the murine mammary gland, previous studies demonstrated that PR action is necessary for parity-induced mammary proliferation, which represents a prerequisite developmental step prior to terminal differentiation of this tissue; importantly, the progesterone signal can also influence breast cancer susceptibility, reviewed in [Fernandez-Valdivia et al., 2005]. The finding that SRC-2 ablation results in a mammary phenotype similar to the PRKO mammary defect has prompted three key questions: (1) Can upregulation of SRC-2 expression promote neoplastic transformation in the murine mammary gland? (2) If so, does SRC-2 have an involvement in hormone-responsive breast cancers in the human? and (3) Does the established oncogenic effects of SRC-3 require the presence of SRC-2?
Obviously, addressing these questions will extend our current understanding of progesterone’s role in breast cancer promotion and/or progression, and thus may enable the formulation of more powerful diagnostic, prognostic and/or therapeutic approaches in the future clinical containment of this cancer.
Acknowledgments
The technical expertise of Jie Li, Yan Ying, and Jie Han is gratefully acknowledged. We also thank Drs. Pierre Chambon and Martine Gehin, Institut Clinique de la Souris, (ICS-IGBMC), BP10142, 67404 ILLKIRCH Cedex France, for kindly providing the floxed TIF-2 mouse. These studies were supported by NIH and private grants HD-42311 (F.J.D.) and CA-77530 and Susan G. Komen Breast Cancer Research Program (J.P.L.)
Abbreviations
- AD
activation domain
- AIB-1
amplified in breast cancer-1
- CARM-1
coactivator associated arginine methyltransferase-1
- CoCoA
coiled-coil coactivator
- GAC63
GRIP-1 associated coactivator 63
- GRIP-1
glucocorticoid receptor interacting protein-1
- HAT
histone acetyltransferase
- MEF-2C
myocyte enhancer factor 2C
- NCOA
nuclear receptor coactivator
- NR
nuclear receptor
- PCOS
polycystic ovarian syndrome
- PRAI
progesterone receptor activity indicator
- PRKO
progesterone receptor knockout
- SRC
steroid receptor coactivator
- TIF-2
transcriptional intermediary factor-2
References
- Anzick S. L., Kononen J., Walker R. L., Azorsa D. O., Tanner M. M., Guan X. Y., Sauter G., Kallioniemi O. P., Trent J. M., Meltzer P. S. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997;277:965–8. doi: 10.1126/science.277.5328.965. [DOI] [PubMed] [Google Scholar]
- Belandia B., Parker M. G. Functional interaction between the p160 coactivator proteins and the transcriptional enhancer factor family of transcription factors. J Biol Chem. 2000;275:30801–5. doi: 10.1074/jbc.C000484200. [DOI] [PubMed] [Google Scholar]
- Berrevoets C. A., Umar A., Trapman J., Brinkmann A. O. Differential modulation of androgen receptor transcriptional activity by the nuclear receptor co-repressor (N-CoR) Biochem J. 2004;379:731–8. doi: 10.1042/BJ20031456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. H., Kim J. H., Stallcup M. R. GAC63, a GRIP1-dependent nuclear receptor coactivator. Mol Cell Biol. 2005;25:5965–72. doi: 10.1128/MCB.25.14.5965-5972.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. L., Dowhan D. H., Hosking B. M., Muscat G. E. The steroid receptor coactivator, GRIP-1, is necessary for MEF-2C-dependent gene expression and skeletal muscle differentiation. Genes Dev. 2000b;14:1209–28. [PMC free article] [PubMed] [Google Scholar]
- Clarke R. B., Howell A., Potten C. S., Anderson E. Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res. 1997;57:4987–91. [PubMed] [Google Scholar]
- Coste A., Antal M. C., Chan S., Kastner P., Mark M., O'Malley B. W., Auwerx J. Absence of the steroid receptor coactivator-3 induces B-cell lymphoma. Embo J. 2006;25:2453–64. doi: 10.1038/sj.emboj.7601106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culig Z., Klocker H., Bartsch G., Hobisch A. Androgen receptors in prostate cancer. Endocr Relat Cancer. 2002;9:155–70. doi: 10.1677/erc.0.0090155. [DOI] [PubMed] [Google Scholar]
- Duong B. N., Elliott S., Frigo D. E., Melnik L. I., Vanhoy L., Tomchuck S., Lebeau H. P., David O., Beckman B. S., Alam J., Bratton M. R., McLachlan J. A., Burow M. E. AKT Regulation of Estrogen Receptor {β} Transcriptional Activity in Breast Cancer. Cancer Res. 2006;66:8373–81. doi: 10.1158/0008-5472.CAN-05-3845. [DOI] [PubMed] [Google Scholar]
- Fernandez-Valdivia R., Mukherjee A., Mulac-Jericevic B., Conneely O. M., DeMayo F. J., Amato P., Lydon J. P. Revealing progesterone's role in uterine and mammary gland biology: insights from the mouse. Semin Reprod Med. 2005;23:22–37. doi: 10.1055/s-2005-864031. [DOI] [PubMed] [Google Scholar]
- Frigo D. E., Basu A., Nierth-Simpson E. N., Weldon C. B., Dugan C. M., Elliott S., Collins-Burow B. M., Salvo V. A., Zhu Y., Melnik L. I., Lopez G. N., Kushner P. J., Curiel T. J., Rowan B. G., McLachlan J. A., Burow M. E. p38 mitogen-activated protein kinase stimulates estrogen-mediated transcription and proliferation through the phosphorylation and potentiation of the p160 coactivator glucocorticoid receptor-interacting protein 1. Mol Endocrinol. 2006;20:971–83. doi: 10.1210/me.2004-0075. [DOI] [PubMed] [Google Scholar]
- Gehin M., Mark M., Dennefeld C., Dierich A., Gronemeyer H., Chambon P. The function of TIF2/GRIP1 in mouse reproduction is distinct from those of SRC-1 and p/CIP. Mol Cell Biol. 2002;22:5923–37. doi: 10.1128/MCB.22.16.5923-5937.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory C. W., Wilson E. M., Apparao K. B., Lininger R. A., Meyer W. R., Kowalik A., Fritz M. A., Lessey B. A. Steroid receptor coactivator expression throughout the menstrual cycle in normal and abnormal endometrium. J Clin Endocrinol Metab. 2002;87:2960–6. doi: 10.1210/jcem.87.6.8572. [DOI] [PubMed] [Google Scholar]
- Gupta P., Park S. W., Farooqui M., Wei L. N. Orphan nuclear receptor TR2, a mediator of preadipocyte proliferation, is differentially regulated by RA through exchange of coactivator PCAF with corepressor RIP140 on a platform molecule GRIP1. Nucleic Acids Res. 2007;35:2269–82. doi: 10.1093/nar/gkl1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y. Z., Hogenesch J. B., Bradfield C. A. The PAS superfamily: sensors of environmental and developmental signals. Annu Rev Pharmacol Toxicol. 2000;40:519–61. doi: 10.1146/annurev.pharmtox.40.1.519. [DOI] [PubMed] [Google Scholar]
- Han S. J., Jeong J., Demayo F. J., Xu J., Tsai S. Y., Tsai M. J., O'Malley B. W. Dynamic cell type specificity of SRC-1 coactivator in modulating uterine progesterone receptor function in mice. Mol Cell Biol. 2005;25:8150–65. doi: 10.1128/MCB.25.18.8150-8165.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S. J., DeMayo F. J., Xu J., Tsai S. Y., Tsai M. J., O'Malley B. W. Steroid receptor coactivator (SRC)-1 and SRC-3 differentially modulate tissue-specific activation functions of the progesterone receptor. Mol Endocrinol. 2006;20:45–55. doi: 10.1210/me.2005-0310. [DOI] [PubMed] [Google Scholar]
- He Y., Simons S. S., Jr. STAMP, a novel predicted factor assisting TIF2 actions in glucocorticoid receptor-mediated induction and repression. Mol Cell Biol. 2007;27:1467–85. doi: 10.1128/MCB.01360-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofman K., Swinnen J. V., Verhoeven G., Heyns W. Coactivation of an endogenous progesterone receptor by TIF2 in COS-7 cells. Biochem Biophys Res Commun. 2002;295:469–74. doi: 10.1016/s0006-291x(02)00698-8. [DOI] [PubMed] [Google Scholar]
- Huang Z. J., Edery I., Rosbash M. PAS is a dimerization domain common to Drosophila period and several transcription factors. Nature. 1993;364:259–62. doi: 10.1038/364259a0. [DOI] [PubMed] [Google Scholar]
- Jeong J. W., Kwak I., Lee K. Y., White L. D., Wang X. P., Brunicardi F. C., O'Malley B. W., DeMayo F. J. The genomic analysis of the impact of steroid receptor coactivators ablation on hepatic metabolism. Mol Endocrinol. 2006;20:1138–52. doi: 10.1210/me.2005-0407. [DOI] [PubMed] [Google Scholar]
- Kim J. H., Li H., Stallcup M. R. CoCoA, a nuclear receptor coactivator which acts through an N-terminal activation domain of p160 coactivators. Mol Cell. 2003;12:1537–49. doi: 10.1016/s1097-2765(03)00450-7. [DOI] [PubMed] [Google Scholar]
- Kuang S. Q., Liao L., Zhang H., Lee A. V., O'Malley B. W., Xu J. AIB1/SRC-3 deficiency affects insulin-like growth factor I signaling pathway and suppresses v-Ha-ras-induced breast cancer initiation and progression in mice. Cancer Res. 2004;64:1875–85. doi: 10.1158/0008-5472.can-03-3745. [DOI] [PubMed] [Google Scholar]
- Kuang S. Q., Liao L., Wang S., Medina D., O'Malley B. W., Xu J. Mice lacking the amplified in breast cancer 1/steroid receptor coactivator-3 are resistant to chemical carcinogen-induced mammary tumorigenesis. Cancer Res. 2005;65:7993–8002. doi: 10.1158/0008-5472.CAN-05-1179. [DOI] [PubMed] [Google Scholar]
- Lee Y. H., Stallcup M. R. Interplay of Fli-I and FLAP1 for regulation of β-catenin dependent transcription. Nucleic Acids Res. 2006;34:5052–9. doi: 10.1093/nar/gkl652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonard D. M., O'Malley B. W. Expanding functional diversity of the coactivators. Trends Biochem Sci. 2005;30:126–32. doi: 10.1016/j.tibs.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Lonard D. M., O'Malley B. W. The expanding cosmos of nuclear receptor coactivators. Cell. 2006;125:411–4. doi: 10.1016/j.cell.2006.04.021. [DOI] [PubMed] [Google Scholar]
- Louet J. F., Coste A., Amazit L., Tannour-Louet M., Wu R. C., Tsai S. Y., Tsai M. J., Auwerx J., O'Malley B. W. Oncogenic steroid receptor coactivator-3 is a key regulator of the white adipogenic program. Proc Natl Acad Sci U S A. 2006;103:17868–73. doi: 10.1073/pnas.0608711103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydon J. P., DeMayo F. J., Funk C. R., Mani S. K., Hughes A. R., Montgomery C. A., Jr., Shyamala G., Conneely O. M., O'Malley B. W. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–78. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- Lydon J. P., Ge G., Kittrell F. S., Medina D., O'Malley B. W. Murine mammary gland carcinogenesis is critically dependent on progesterone receptor function. Cancer Res. 1999;59:4276–84. [PubMed] [Google Scholar]
- Mark M., Yoshida-Komiya H., Gehin M., Liao L., Tsai M. J., O'Malley B. W., Chambon P., Xu J. Partially redundant functions of SRC-1 and TIF2 in postnatal survival and male reproduction. Proc Natl Acad Sci U S A. 2004;101:4453–8. doi: 10.1073/pnas.0400234101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna N. J., O'Malley B. W. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell. 2002;108:465–74. doi: 10.1016/s0092-8674(02)00641-4. [DOI] [PubMed] [Google Scholar]
- Modder U. I., Sanyal A., Kearns A. E., Sibonga J. D., Nishihara E., Xu J., O'Malley B. W., Ritman E. L., Riggs B. L., Spelsberg T. C., Khosla S. Effects of loss of steroid receptor coactivator-1 on the skeletal response to estrogen in mice. Endocrinology. 2004;145:913–21. doi: 10.1210/en.2003-1089. [DOI] [PubMed] [Google Scholar]
- Mukherjee A., Soyal S. M., Fernandez-Valdivia R., Gehin M., Chambon P., Demayo F. J., Lydon J. P., O'Malley B W. Steroid receptor coactivator 2 is critical for progesterone-dependent uterine function and mammary morphogenesis in the mouse. Mol Cell Biol. 2006b;26:6571–83. doi: 10.1128/MCB.00654-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norwitz E. R., Schust D. J., Fisher S. J. Implantation and the survival of early pregnancy. N Engl J Med. 2001;345:1400–8. doi: 10.1056/NEJMra000763. [DOI] [PubMed] [Google Scholar]
- Patchev A. V., Fischer D., Wolf S. S., Herkenham M., Gotz F., Gehin M., Chambon P., Patchev V. K., Almeida O. F. Insidious adrenocortical insufficiency underlies neuroendocrine dysregulation in TIF-2 deficient mice. Faseb J. 2007;21:231–8. doi: 10.1096/fj.06-6952com. [DOI] [PubMed] [Google Scholar]
- Pathirage N., Di Nezza L. A., Salmonsen L. A., Jobling T., Simpson E. R., Clyne C. D. Expression of aromatase, estrogen receptors, and their coactivators in patients with endometrial cancer. Fertil Steril. 2006;86:469–72. doi: 10.1016/j.fertnstert.2005.12.057. [DOI] [PubMed] [Google Scholar]
- Rogatsky I., Luecke H. F., Leitman D. C., Yamamoto K. R. Alternate surfaces of transcriptional coregulator GRIP1 function in different glucocorticoid receptor activation and repression contexts. Proc Natl Acad Sci U S A. 2002;99:16701–6. doi: 10.1073/pnas.262671599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyal S. M., Mukherjee A., Lee K. Y., Li J., Li H., DeMayo F. J., Lydon J. P. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis. 2005;41:58–66. doi: 10.1002/gene.20098. [DOI] [PubMed] [Google Scholar]
- Torres-Arzayus M. I., Font de Mora J., Yuan J., Vazquez F., Bronson R., Rue M., Sellers W. R., Brown M. High tumor incidence and activation of the PI3K/AKT pathway in transgenic mice define AIB1 as an oncogene. Cancer Cell. 2004;6:263–74. doi: 10.1016/j.ccr.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Wang D., Wang Q., Awasthi S., Simons S. S., Jr. Amino-terminal domain of TIF2 is involved in competing for corepressor binding to glucocorticoid and progesterone receptors. Biochemistry. 2007;46:8036–49. doi: 10.1021/bi7004575. [DOI] [PubMed] [Google Scholar]
- Wang Z., Qi C., Krones A., Woodring P., Zhu X., Reddy J. K., Evans R. M., Rosenfeld M. G., Hunter T. Critical roles of the p160 transcriptional coactivators p/CIP and SRC-1 in energy balance. Cell Metab. 2006;3:111–22. doi: 10.1016/j.cmet.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Wang Z., Rose D. W., Hermanson O., Liu F., Herman T., Wu W., Szeto D., Gleiberman A., Krones A., Pratt K., Rosenfeld R., Glass C. K., Rosenfeld M. G. Regulation of somatic growth by the p160 coactivator p/CIP. Proc Natl Acad Sci U S A. 2000;97:13549–54.. doi: 10.1073/pnas.260463097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Qiu Y., DeMayo F. J., Tsai S. Y., Tsai M. J., O'Malley B. W. Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science. 1998;279:1922–5. doi: 10.1126/science.279.5358.1922. [DOI] [PubMed] [Google Scholar]
- Xu J., Liao L., Ning G., Yoshida-Komiya H., Deng C., O'Malley B. W. The steroid receptor coactivator SRC-3 (p/CIP/RAC3/AIB1/ACTR/TRAM-1) is required for normal growth, puberty, female reproductive function, and mammary gland development. Proc Natl Acad Sci U S A. 2000;97:6379–84. doi: 10.1073/pnas.120166297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X., Han S. J., Tsai S. Y., DeMayo F. J., Xu J., Tsai M. J., O'Malley B. W. Roles of steroid receptor coactivator (SRC)-1 and transcriptional intermediary factor (TIF) 2 in androgen receptor activity in mice. Proc Natl Acad Sci U S A. 2005;102:9487–92. doi: 10.1073/pnas.0503577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying H., Furuya F., Willingham M. C., Xu J., O'Malley B. W., Cheng S. Y. Dual functions of the steroid hormone receptor coactivator 3 in modulating resistance to thyroid hormone. Mol Cell Biol. 2005;25:7687–95. doi: 10.1128/MCB.25.17.7687-7695.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H. J., Yan J., Luo W., Ayala G., Lin S. H., Erdem H., Ittmann M., Tsai S. Y., Tsai M. J. SRC-3 is required for prostate cancer cell proliferation and survival. Cancer Res. 2005;65:7976–83. doi: 10.1158/0008-5472.CAN-04-4076. [DOI] [PubMed] [Google Scholar]