Abstract
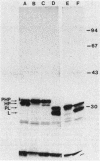
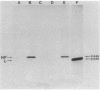
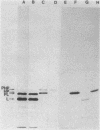
A fusion between the pCloDF13-derived bacteriocin release protein and beta-lactamase was constructed to investigate the subcellular localization and posttranslational modification of the bacteriocin release protein in Escherichia coli. The signal sequence and 25 of the 28 amino acid residues of the mature bacteriocin release protein were fused to the mature portion of beta-lactamase. The hybrid protein (Mr, 31,588) was expressed in minicells and whole cells and possessed full beta-lactamase activity. Immunoblotting of subcellular fractions revealed that the hybrid protein is present in both the cytoplasmic and outer membranes of E. coli. Radioactive labeling experiments in the presence or absence of globomycin showed that the hybrid protein is modified with a diglyceride and fatty acids and is processed by signal peptidase II, as is the murein lipoprotein. The results indicated that the pCloDF13-encoded bacteriocin release protein is a lipoprotein which is associated with both membranes of E. coli cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun V. Covalent lipoprotein from the outer membrane of Escherichia coli. Biochim Biophys Acta. 1975 Oct 31;415(3):335–377. doi: 10.1016/0304-4157(75)90013-1. [DOI] [PubMed] [Google Scholar]
- Brockman R. W., Heppel L. A. On the localization of alkaline phosphatase and cyclic phosphodiesterase in Escherichia coli. Biochemistry. 1968 Jul;7(7):2554–2562. doi: 10.1021/bi00847a016. [DOI] [PubMed] [Google Scholar]
- Cavard D., Lloubès R., Morlon J., Chartier M., Lazdunski C. Lysis protein encoded by plasmid ColA-CA31. Gene sequence and export. Mol Gen Genet. 1985;199(1):95–100. doi: 10.1007/BF00327516. [DOI] [PubMed] [Google Scholar]
- Choi D. S., Yamada H., Mizuno T., Mizushima S. Trimeric structure and localization of the major lipoprotein in the cell surface of Escherichia coli. J Biol Chem. 1986 Jul 5;261(19):8953–8957. [PubMed] [Google Scholar]
- Chopra I., Shales S. W. Comparison of the polypeptide composition of Escherichia coli outer membranes prepared by two methods. J Bacteriol. 1980 Oct;144(1):425–427. doi: 10.1128/jb.144.1.425-427.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S. T., Saint-Joanis B., Pugsley A. P. Molecular characterisation of the colicin E2 operon and identification of its products. Mol Gen Genet. 1985;198(3):465–472. doi: 10.1007/BF00332940. [DOI] [PubMed] [Google Scholar]
- De Graaf F. K., Oudega B. Production and release of cloacin DF13 and related colicins. Curr Top Microbiol Immunol. 1986;125:183–205. doi: 10.1007/978-3-642-71251-7_11. [DOI] [PubMed] [Google Scholar]
- Dev I. K., Harvey R. J., Ray P. H. Inhibition of prolipoprotein signal peptidase by globomycin. J Biol Chem. 1985 May 25;260(10):5891–5894. [PubMed] [Google Scholar]
- Dougan G., Sherratt D. The transposon Tn1 as a probe for studying ColE1 structure and function. Mol Gen Genet. 1977 Mar 7;151(2):151–160. doi: 10.1007/BF00338689. [DOI] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakkaart M. J., Veltkamp E., Nijkamp H. J. Protein H encoded by plasmid Clo DF13 involved in lysis of the bacterial host. I. Localisation of the gene and identification and subcellular localisation of the gene H product. Mol Gen Genet. 1981;183(2):318–325. doi: 10.1007/BF00270635. [DOI] [PubMed] [Google Scholar]
- Hayashi S., Wu H. C. Biosynthesis of Bacillus licheniformis penicillinase in Escherichia coli and in Bacillus subtilis. J Bacteriol. 1983 Nov;156(2):773–777. doi: 10.1128/jb.156.2.773-777.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inukai M., Takeuchi M., Shimizu K., Arai M. Mechanism of action of globomycin. J Antibiot (Tokyo) 1978 Nov;31(11):1203–1205. doi: 10.7164/antibiotics.31.1203. [DOI] [PubMed] [Google Scholar]
- Jakes K. S., Zinder N. D. Plasmid ColE3 specifies a lysis protein. J Bacteriol. 1984 Feb;157(2):582–590. doi: 10.1128/jb.157.2.582-590.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadonaga J. T., Gautier A. E., Straus D. R., Charles A. D., Edge M. D., Knowles J. R. The role of the beta-lactamase signal sequence in the secretion of proteins by Escherichia coli. J Biol Chem. 1984 Feb 25;259(4):2149–2154. [PubMed] [Google Scholar]
- Konisky J. Colicins and other bacteriocins with established modes of action. Annu Rev Microbiol. 1982;36:125–144. doi: 10.1146/annurev.mi.36.100182.001013. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Luirink J., de Graaf F. K., Oudega B. Uncoupling of synthesis and release of cloacin DF13 and its immunity protein by Escherichia coli. Mol Gen Genet. 1987 Jan;206(1):126–132. doi: 10.1007/BF00326547. [DOI] [PubMed] [Google Scholar]
- Luirink J., van der Sande C., Tommassen J., Veltkamp E., De Graaf F. K., Oudega B. Effects of divalent cations and of phospholipase A activity on excretion of cloacin DF13 and lysis of host cells. J Gen Microbiol. 1986 Mar;132(3):825–834. doi: 10.1099/00221287-132-3-825. [DOI] [PubMed] [Google Scholar]
- Mooi F. R., Harms N., Bakker D., de Graaf F. K. Organization and expression of genes involved in the production of the K88ab antigen. Infect Immun. 1981 Jun;32(3):1155–1163. doi: 10.1128/iai.32.3.1155-1163.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J. B., Caulfield M. P., Lampen J. O. Lipoprotein nature of Bacillus licheniformis membrane penicillinase. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3511–3515. doi: 10.1073/pnas.78.6.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka A., Nomura N., Morita M., Sugisaki H., Sugimoto K., Takanami M. Nucleotide sequence of small ColE1 derivatives: structure of the regions essential for autonomous replication and colicin E1 immunity. Mol Gen Genet. 1979 May 4;172(2):151–159. doi: 10.1007/BF00268276. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Palva E. T., Hirst T. R., Hardy S. J., Holmgren J., Randall L. Synthesis of a precursor to the B subunit of heat-labile enterotoxin in Escherichia coli. J Bacteriol. 1981 Apr;146(1):325–330. doi: 10.1128/jb.146.1.325-330.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P., Schwartz M. A genetic approach to the study of mitomycin-induced lysis of Escherichia coli K-12 strains which produce colicin E2. Mol Gen Genet. 1983;190(3):366–372. doi: 10.1007/BF00331060. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P., Schwartz M. Colicin E2 release: lysis, leakage or secretion? Possible role of a phospholipase. EMBO J. 1984 Oct;3(10):2393–2397. doi: 10.1002/j.1460-2075.1984.tb02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P., Schwartz M. Expression of a gene in a 400-base-pair fragment of colicin plasmid ColE2-P9 is sufficient to cause host cell lysis. J Bacteriol. 1983 Oct;156(1):109–114. doi: 10.1128/jb.156.1.109-114.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P. The ins and outs of colicins. Part I: Production, and translocation across membranes. Microbiol Sci. 1984 Oct;1(7):168–175. [PubMed] [Google Scholar]
- Suit J. L., Fan M. L., Sabik J. F., Labarre R., Luria S. E. Alternative forms of lethality in mitomycin C-induced bacteria carrying ColE1 plasmids. Proc Natl Acad Sci U S A. 1983 Jan;80(2):579–583. doi: 10.1073/pnas.80.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga H., Wu H. C. Studies on the modification and processing of prolipoprotein in Escherichia coli. Effects of structural alterations in prolipoprotein on its maturation in wild type and lpp mutants. J Biol Chem. 1984 May 25;259(10):6098–6104. [PubMed] [Google Scholar]
- Tommassen J., Leunissen J., van Damme-Jongsten M., Overduin P. Failure of E. coli K-12 to transport PhoE-LacZ hybrid proteins out of the cytoplasm. EMBO J. 1985 Apr;4(4):1041–1047. doi: 10.1002/j.1460-2075.1985.tb03736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Tiel-Menkvled G. J., Rezee A., De Graaf F. K. Production and excretion of cloacin DF13 by Escherichia coli harboring plasmid CloDF13. J Bacteriol. 1979 Nov;140(2):415–423. doi: 10.1128/jb.140.2.415-423.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R. J., Lau P. C., Vernet T., Visentin L. P. Characterization and nucleotide sequence of a colicin-release gene in the hic region of plasmid ColE3-CA38. Gene. 1984 Jul-Aug;29(1-2):175–184. doi: 10.1016/0378-1119(84)90178-1. [DOI] [PubMed] [Google Scholar]
- Witholt B., Boekhout M., Brock M., Kingma J., Heerikhuizen H. V., Leij L. D. An efficient and reproducible procedure for the formation of spheroplasts from variously grown Escherichia coli. Anal Biochem. 1976 Jul;74(1):160–170. doi: 10.1016/0003-2697(76)90320-1. [DOI] [PubMed] [Google Scholar]
- Wu H. C., Tokunaga M. Biogenesis of lipoproteins in bacteria. Curr Top Microbiol Immunol. 1986;125:127–157. doi: 10.1007/978-3-642-71251-7_9. [DOI] [PubMed] [Google Scholar]
- Yamada M., Miki T., Nakazawa A. Translocation of colicin E1 through cytoplasmic membrane of Escherichia coli. FEBS Lett. 1982 Dec 27;150(2):465–468. doi: 10.1016/0014-5793(82)80790-4. [DOI] [PubMed] [Google Scholar]
- Yamada M., Nakazawa A. Factors necessary for the export process of colicin E1 across cytoplasmic membrane of Escherichia coli. Eur J Biochem. 1984 Apr 16;140(2):249–255. doi: 10.1111/j.1432-1033.1984.tb08095.x. [DOI] [PubMed] [Google Scholar]