Abstract
Differences between rodent and human airway cell biology have made it difficult to translate recombinant adeno-associated virus (rAAV)-mediated gene therapies to the lung for cystic fibrosis (CF). As new ferret and pig models for CF become available, knowledge about host cell/vector interactions in these species will become increasingly important for testing potential gene therapies. To this end, we have compared the transduction biology of three rAAV serotypes (AAV1, 2 and 5) in human, ferret, pig and mouse-polarized airway epithelia. Our results indicate that apical transduction of ferret and pig airway epithelia with these rAAV serotypes closely mirrors that observed in human epithelia (rAAV1> rAAV2 ≅ rAAV5), while transduction of mouse epithelia was significantly different (rAAV1 > rAAV5 ≫ rAAV2). Similarly, ferret, pig and human epithelia also shared serotype-specific differences in the polarity (apical vs basolateral) and proteasome dependence of rAAV transduction. Despite these parallels, N-linked sialic acid receptors were required for rAAV1 and rAAV5 transduction of human and mouse airway epithelia, but not ferret or pig airway epithelia. Hence, although the airway tropisms of rAAV serotypes 1, 2 and 5 are conserved better among ferret, pig and human as compared to mouse, viral receptors/co-receptors appear to maintain considerable species diversity.
Keywords: AAV, species, tropism, sialic acid, lung, cystic fibrosis
Recombinant adeno-associated virus (rAAV) features the ability to transduce multiple tissue types with low immunogenicity, a finding that has led to considerable interest in this vector as a potential agent for gene therapy. In the context of cystic fibrosis (CF) lung disease, rAAV-based vectors have been tested successfully for the functional correction of CFTR in vitro1–3, and pre-clinical studies in non-human primates have demonstrated efficient CFTR transgene expression in the airway epithelium using rAAV2,4,5 supporting clinical testing with this serotype. However, despite these encouraging preclinical studies, clinical trials with rAAV2 have yet to demonstrate detectable rAAV2 transduction (based on expression of transgene-derived CFTR mRNA) in the airways of CF patients.6,7 These findings have raised concerns about the type of pre-clinical models used to select rAAV serotypes for clinical applications in the lung. Indeed, recent studies comparing the transduction of airway epithelia derived from Old World non-human primates (NHP) and humans by rAAV1, 2 and 5 vectors demonstrated considerable differences in the tropisms of these three serotypes.8 For example, rAAV2 transduces Old World NHP airway epithelia most effectively, with an efficiency 500-fold greater than that in human airway epithelia. In contrast, rAAV1 transduces human airway epithelia ~100-fold more effectively than rAAV2 and 5, while in Old World NHP airway epithelia, rAAV1 was significantly less efficient (10- to 15-fold) than rAAV2 and 5. The findings for rAAV2 are consistent with the discrepancies between pre-clinical data gathered in Old World NHPs and the results of clinical trials using the same serotype. In the light of these findings, it is presently unclear which species may be the most appropriate animal model to test rAAV-mediated gene therapies for CF lung disease.
The testing of gene therapies intended for the treatment of CF lung disease has been hindered by the lack of an appropriate surrogate CF animal model. Although CF mouse models have been useful for dissecting many aspects of CF pathophysiology, they have failed to reproduce the natural progression of human CF lung disease.9,10 Furthermore, the tropisms of several rAAV serotypes for the mouse airway epithelium appear to differ from those in human.11 With the generation of gene-targeted heterozygous CFTR knockout pigs12 and similar progress being made toward the development of CFTR knockout ferrets,13,14 more appropriate animal models for testing CF lung gene therapies may soon be available. In contrast to mice, pigs and ferrets have conducting airway epithelial cell types similar to those in humans15–18 suggesting that they may indeed be better models for gene therapy to the airway. However, given the divergence in rAAV tropisms between Old World NHP and human airway epithelia (which are very closely conserved from a cell biology and electrophysiologic standpoint8), the utility of the pig and ferret to test airway-directed gene therapies with rAAV remains unclear.
Airway epithelia from various species including human, Old-World NHP monkey, pig, ferret and mouse have been polarized successfully in vitro, and the resulting cultures have been shown to exhibit electro-physiologic properties similar to those of the respective native airway epithelia.8,19–21 Importantly, previous studies on rAAV transduction in mouse have suggested that in vitro-polarized airway epithelia elicit the same species-specific tropisms for rAAV2 and 5 that are seen in vivo.11 In the present study, we sought to evaluate the comparative biology of rAAV transduction in ferret, pig and human airway epithelia for three commonly used serotypes (1, 2 and 5), as a first step in analyzing the suitability of these rAAV serotypes for modeling gene therapy in CF pig and ferret models.
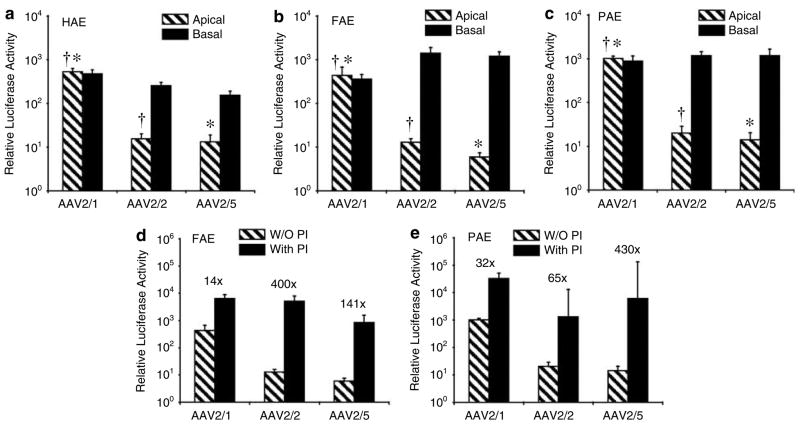
Using an AAV2 proviral vector encoding a cytomegalovirus promoter-driven luciferase transgene, we generated pseudotyped rAAV2/1, rAAV2/2 and rAAV2/5 viruses with capsid serotypes 1, 2 or 5, and investigated their ability to transduce air–liquid interface (ALI) cultures derived from human, pig and ferret tracheal epithelia. Results from these studies revealed extremely similar transduction profiles for the three species following apical infection (Figures 1a–c); in all cases, rAAV2/1 gave rise to ~50-fold greater transgene expression than rAAV2/2 and rAAV2/5. These findings are in stark contrast to findings in Old World NHPs, where rAAV2/2 is the most efficient serotype for apical infection of airway epithelia.8 Serotype-specific polarity of transduction was also conserved between human, pig and ferret airway epithelia; in each species, both rAAV2/2 and rAAV2/5 transduced the basolateral surface ~50-to 200-fold more efficiently than the apical surface, and rAAV2/1 lacked a polarity preference (Figures 1a–c). This polarity preference for these serotypes was also quantitatively different from that observed in Old World NHPs8 and mouse11 airway epithelia.
Figure 1.
Comparative biology of recombinant adeno-associated virus (rAAV) transduction in human, ferret and pig airway epithelia. (a–c) Polarized air–liquid interface (ALI) airway epithelial cultures from human (HAE), ferret (FAE) and pig (PAE) were generated from tracheal tissue as described previously.21 Differentiated cultures were infected with rAAV luciferase virus pseudotyped in serotype 1, 2 or 5 capsid containing an rAAV2 viral genome (rAAV2/1, rAAV2/2, rAAV2/5). Viruses were generated using a triple plasmid transfection method as described previously.22 Infections were performed by applying 2.0 × 103 particles per cell (2 × 109 total particles per well) to either the apical or basal surface for 16 h as described previously.11 Relative luciferase activity was measured at 3 days post-infection. (d, e) ALI cultures were apically infected with 2.0 × 103 particles per cell in the presence or absence of 40 μM N-acetyl-L-leucyl-L-leucyl-L-norleucine and 5 μM doxorubicin for 16 h, as described previously22 and luciferase activity was measured at 3 days post-infection. Proteasome inhibitors (PI) were removed after 16 h following viral infection. Numbers above each data set represent the fold induction in the presence of proteasome inhibitor. Data in all panels represent the mean (± s.e.m.) relative luciferase activity (per well) from three independent experiments (N = 12 transwells for each experimental point). †,* marked comparisons indicate signficant difference as determined by the Student’s t-test.
Previous studies have demonstrated that the low level of apical transduction of human airway epithelia with rAAV2/2 and rAAV2/5 results from a post-entry, ubiquitin/proteasome-dependent block in nuclear uptake of virus.22–24 Transient treatment with proteasome inhibitors at the time of infection can significantly augment rAAV2/2 and rAAV2/5 apical transduction of human airway epithelia (~500- to 1000-fold) by enhancing nuclear uptake of virus.23 However, this proteasome-dependent barrier plays less of a role in apical transduction of human airway epithelia by rAAV2/1.22 Species-specific differences in the magnitude of the proteasome-dependent blocks in intracellular processing of various rAAV serotypes also appear to account for the tropism of a given serotype for apical transduction of human, Old World NHP and mouse airway epithelia.8,11,22 In this context, proteasome inhibitor-dependent transduction with rAAV can be used to assess biological conservation of host cell/vector interactions that influence the efficiency of transduction in a give species.
To investigate whether rate-limiting aspects of the ubiquitin/proteasome pathway are also important for apical rAAV transduction of ferret and pig airway epithelia, we compared rAAV transduction in ALI cultures in the absence and presence of the proteasome-modulating agents N-acetyl-L-leucyl-L-leucyl-L-norleucine and doxorubicin. As reported previously for human airway epithelia,22 apical transduction of ferret epithelia with rAAV2/2 and rAAV2/5 was more significantly enhanced in the presence of proteasome inhibitors than was transduction with rAAV2/1 (Figure 1d). rAAV2/1 transduction in pig epithelia was also the least affected by proteasome inhibitors; however, in this setting only rAAV2/5 reached a significantly higher level of induction in comparison to rAAV2/1 (Figure 1e). These findings support the notion that the extent of intracellular proteasome-dependent barriers for the three serotypes tested are most conserved between the airway epithelia of ferret and human.
Given the relatively similar transduction profiles of human, pig and ferret airway epithelia by rAAV2/1, rAAV2/2 and rAAV2/5, we next sought to determine whether known virus-binding receptors for these serotypes might be conserved. To this end, we first evaluated the localization of membrane-associated heparin sulfate proteoglycan and 2,3-linked sialic acid, which serve as binding receptors for rAAV2 and rAAV5, respectively.25,26 Consistent with its localization in human airway epithelia,11,27 heparin sulfate proteoglycan was observed predominantly at the basal membranes of both ferret and pig ALI cultures, as well as in native tracheal airway tissue from both species (Figures 2a and b). Also consistent with findings from human airway cultures,11,25 Maackia amurensis lectin-binding to 2,3-linked sialic acid was detected on both apical and basolateral surfaces of pig and ferret ALI cultures, although it was more abundant on the basolateral surface and throughout the submucosa of the native tracheas from these species (Figures 2a and b). Although the extent to which these receptors contribute to rAAV2 and rAAV5 infection of human, ferret and pig airway epithelia remains unclear, their conserved patterns of expression suggest that co-receptor pathways that promote efficient basolateral transduction in these species with these two serotypes might also be conserved.
Figure 2.
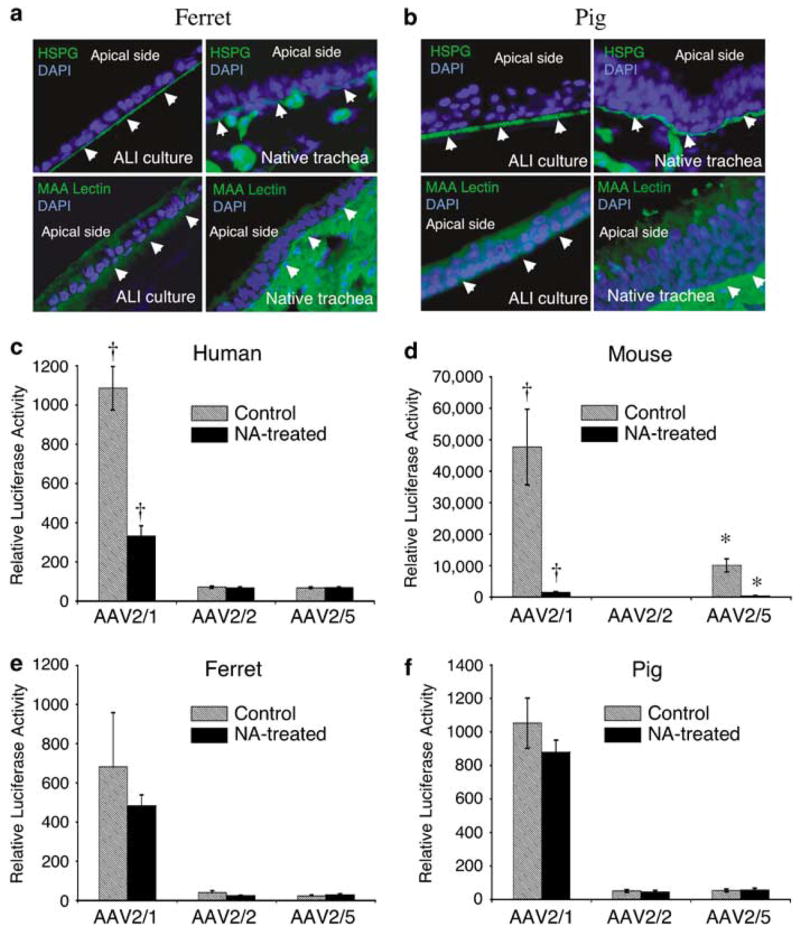
Comparative analysis of candidate viral-binding receptors in human, mouse, ferret and pig airway epithelia. (a, b) Immunostaining for heparan sulfate proteoglycan (HSPG) and 2,3-linked sialic acid using the Maackia amurensis (MAA) lectin on sections from ferret and pig airway epithelial ALI cultures (left panel) and native tracheas (right panel), as described previously for human and mouse ALI cultures and tracheal tissue.11 Arrowheads mark the basolateral side of each epithelium and 4’,6-diamidino-2-phenylindole demarcates nuclei. Antibodies used included rat anti-HSPG monoclonal antibody followed by fluoroisothiocyanate (FITC)-labeled anti-rat secondary antibody, and FITC-labeled MAA lectin. (c–f) ALI epithelial cultures from human, mouse, ferret and pig were pretreated with neuraminidase (NA-treated) to remove sialic acid from the apical surfaces of airway epithelial cultures, or with Ultraser G (USG) medium as a control (untreated), prior to infection with virus. Specifically, 200 μl of either USG medium containing 50 mU ml−1 of type III NA from Vibrio cholerae (Sigma, Santa Louis, MO, USA), or USG medium alone, was added to the apical side of the cultures and left there for 2 h (at 37 °C). NA was then removed and cultures were washed three times with phosphate-buffered saline. This was followed by apical infection at a multiplicity of infection of 2.0 × 103 particles per cell (2 × 109 total particles per well) with the indicated serotypes recombinant adeno-associated virus luciferase virus for 1 h at 4 °C. Cultures were then shifted to a 37 °C incubator and the efficiency of transduction was evaluated by measuring luciferase activity at 7 days post-infection. Data represent the means (± s.e.m.) of the relative luciferase activity (per well) from three independent experiments (N = 12). †,* marked comparisons indicate significant difference as determined by the student’s t-test.
Given that apical infection with rAAV is most therapeutically relevant, we sought to focus studies on the biology of potential apical receptor pathways for rAAV1. Cell surface α2,3 and α2,6 N-linked sialic acids are two potential viral-binding receptors for rAAV1.28 Additionally, α2,3 N-linked sialic acid is known to act as a receptor for AAV5.29 In light of these findings, we tested whether N-linked sialic acids might serve as receptors for rAAV2/1 binding and transduction of human, mouse, ferret and pig airway epithelia. To this end, we evaluated the efficiency of rAAV binding and transduction following neuraminidase (NA) treatment to remove N-linked sialic acids from the apical surfaces of epithelia prior to infection.28,29 A comparison of NA-sensitive apical transduction of human, mouse, ferret and pig airway epithelia demonstrated considerable diversity in the requirements for N-linked sialic acid to mediate rAAV1, 2 and 5 transduction. In human epithelia, removal of N-linked sialic acids inhibited significantly only rAAV2/1 transduction (Figure 2c). In contrast, both rAAV2/1 and rAAV2/5 required N-linked sialic acids for the apical transduction of mouse airway epithelia (Figure 2d). These findings are consistent with the notion that N-linked sialic acid serves as a rAAV2/5 receptor in mouse epithelia.30 Interestingly, none of the serotypes required N-linked sialic acids for the transduction of ferret and pig airway epithelia from the apical surface (Figures 2e and f). Although rAAV2/1 transduces human, pig and ferret airway epithelia with equal efficiency, these data suggest that pig and ferret differ from human with respect to the type of co-receptor that facilitates apical transduction.
Given the divergent requirements for N-linked sialic acids in rAAV transduction of human, pig, ferret and mouse epithelia, we sought to evaluate the extent to which rAAV binding to the apical surface was influenced by NA treatment. rAAV binding to the apical surface at 4 °C for 1 h was evaluated by both Southern blotting and quantitative PCR (assessment of viral genomes) (Figure 3). In the absence of NA treatment, the extent of surface binding by the various rAAV serotypes in human and pig epithelia did not correlate with the level of transduction; in both species, rAAV2/5 bound most effectively although rAAV2/1 most effectively transduced epithelia from these species (compare Figures 2c and f with Figure 3). In contrast, in ferret and mouse, the binding of rAAV2/1 and rAAV2/2 to the epithelia did correlate with the level of transduction, while that of rAAV2/5 did not (compare Figures 2d and e with Figure 3). These findings support the notion that the binding receptors and co-receptors required for efficient transduction of a given rAAV serotype differ from one species to another. NA-induced changes in rAAV binding correlated with changes in rAAV transduction only in the case of the mouse epithelium; NA treatment reduced binding for rAAV2/1 and rAAV2/5, and also significantly reduced transduction (Figures 2d and 3). Paradoxically, in human epithelia, NA treatment resulted in an increase in rAAV2/1 binding while it significantly reduced transduction (Figures 2 and 3). NA treatment of ferret and pig epithelia had no observable effect on rAAV transduction, nor did it affect rAAV binding for any serotype except rAAV2/5 in ferret epithelia (Figures 2e and 3). Cumulatively, these findings support the notion that binding receptors for the various rAAV serotypes differ considerably across species. Although α2,3 and α2,6 N-linked sialic acid appear to be required for rAAV1 and/or rAAV5 transduction of human and mouse epithelia, they are not required in ferret and pig epithelia. Interestingly, in some instances, removal of this receptor type appears to enhance the interactions between rAAV interactions and other binding receptors on the cell surface that may be functionally silent in the context of productive infection. Such findings may reflect the diversity of the receptors and co-receptors that are likely present on the surfaces of airway epithelia of different species and the combinations that can give rise to effective transduction.
Figure 3.
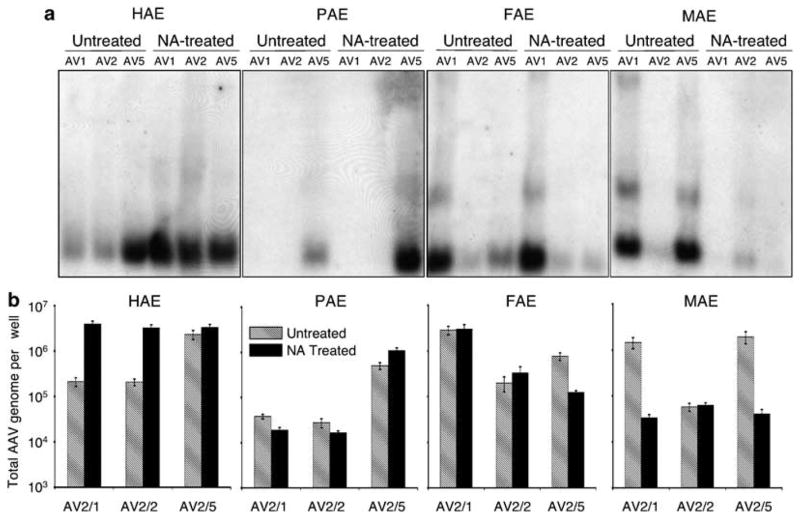
Comparative analysis of N-linked sialic acid-binding requirements for various recombinant adeno-associated virus (rAAV) serotypes. ALI cultures from human (HAE), pig (PAE), ferret (FAE) and mouse (MAE) were treated with neuraminidase (NA-treated) or Ultraser G control medium (untreated) prior to infection. This was followed by application of rAAV luciferase virus to the apical surface at a multiplicity of infection of 2.0 × 103 particles per cell (2 × 109 total particles per well) at 4 °C for 1 h (as described in Figure 2). The infected cultures were washed following virus binding, and cells were harvested for analysis of the viral genome. (a) The extracted viral DNA was detected by Southern blotting using a 32P-labeled luciferase DNA probe as described previously.22,31 (b) Quantitative analyses of rAAV genomes following the above infection protocol used Taqman PCR. Plotted are the means (± s.e.m.) of the numbers of rAAV viral genomes (per well) from three independent experiments (N = 12). The forward primer (5′-TTTTTGAAGCGAAGGTTGTGG-3′), and the reverse primer (5′-CACACACAGTTCGCCTCTTTG-3′) were designed to amplify a 32-bp fragment in the luciferase-coding region of rAAVLuc. The Taqman probe (5′-ATCTGGATACCG GGAAAACGCTGGGCGTTAAT-3′) was tagged with FAM dye (6-carboxyfluorescein) at its 5′-end to serve as the reporter, and with TAMRA dye (6-carboxytetramethylrhodamine) at the 3′-end to serve as the quencher.32 Series dilution of rAAVLuc was used to generate the standard curve, and was only accepted as linear when the slope fell to between −3.74 and −3.32 and the correlation coefficient was above 0.99.32
The pre-clinical development of gene therapies for CF has thus far been hindered by the lack of CF animal models that reproduce accurately human CF lung pathogenesis. With the near completion of the development of the CF ferret and pig models,12–14 these species may soon become preferred models for testing gene therapies of CF lung disease. Our results demonstrate that ferret, pig and human airway epithelia exhibit similar serotype preferences for vectors of the rAAV2/1, rAAV2/2 and rAAV2/5 serotypes, and hence ferret and pig may be useful pre-clinical models for testing rAAV-directed gene therapies to the airway. In this context, rAAV2/1 appears to be the best-suited rAAV serotype for gene delivery to the airway in these species. However, despite the high degree of conservation in the tropism of rAAV2/1 for ferret, pig and human airway epithelia, these species appear to differ in the receptors used for rAAV transduction. Hence, although rAAV2/1 may be suitable for modeling CF gene therapy in the pig and ferret, these species would not be preferred models for studying the mechanisms of rAAV transduction in the context of human biology.
Acknowledgments
This work was supported by NIH RO1 HL58340 (JFE), the Center for Gene Therapy (DK54759) and Targeted Genetics Corporation. We also gratefully acknowledge the Roy J Carver Chair in Molecular Medicine (JFE) and Dr Christine Blaumueller for editorial assistance.
References
- 1.Zhang LN, Karp P, Gerard CJ, Pastor E, Laux D, Munson K, et al. Dual therapeutic utility of proteasome modulating agents for pharmacogene therapy of the cystic fibrosis airway. Mol Ther. 2004;10:990–1002. doi: 10.1016/j.ymthe.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Sirninger J, Muller C, Braag S, Tang Q, Yue H, Detrisac C, et al. Functional characterization of a recombinant adeno-associated virus 5-pseudotyped cystic fibrosis transmembrane conductance regulator vector. Hum Gene Ther. 2004;15:832–841. doi: 10.1089/hum.2004.15.832. [DOI] [PubMed] [Google Scholar]
- 3.Liu X, Luo M, Zhang LN, Yan Z, Zak R, Ding W, et al. Spliceosome-mediated RNA trans-splicing with recombinant adeno-associated virus partially restores cystic fibrosis trans-membrane conductance regulator function to polarized human cystic fibrosis airway epithelial cells. Hum Gene Ther. 2005;16:1116–1123. doi: 10.1089/hum.2005.16.1116. [DOI] [PubMed] [Google Scholar]
- 4.Afione SA, Conrad CK, Kearns WG, Chunduru S, Adams R, Reynolds TC, et al. In vivo model of adeno-associated virus vector persistence and rescue. J Virol. 1996;70:3235–3241. doi: 10.1128/jvi.70.5.3235-3241.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conrad CK, Allen SS, Afione SA, Reynolds TC, Beck SE, Fee-Maki M, et al. Safety of single-dose administration of an adeno-associated virus (AAV)-CFTR vector in the primate lung. Gene Therapy. 1996;3:658–668. [PubMed] [Google Scholar]
- 6.Moss RB, Rodman D, Spencer LT, Aitken ML, Zeitlin PL, Waltz D, et al. Repeated adeno-associated virus serotype 2 aerosol-mediated cystic fibrosis transmembrane regulator gene transfer to the lungs of patients with cystic fibrosis: a multicenter, double-blind, placebo-controlled trial. Chest. 2004;125:509–521. doi: 10.1378/chest.125.2.509. [DOI] [PubMed] [Google Scholar]
- 7.Flotte TR, Zeitlin PL, Reynolds TC, Heald AE, Pedersen P, Beck S, et al. Phase I trial of intranasal and endobronchial administration of a recombinant adeno-associated virus serotype 2 (rAAV2)-CFTR vector in adult cystic fibrosis patients: a two-part clinical study. Hum Gene Ther. 2003;14:1079–1088. doi: 10.1089/104303403322124792. [DOI] [PubMed] [Google Scholar]
- 8.Liu X, Luo M, Trygg C, Yan Z, Lei-Butters DC, Smith CB, et al. Biological differences in rAAV transduction of human and Old World non-human primate airway epithelia. Mol Ther. 2007 doi: 10.1038/sj.mt.6300277. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grubb BR, Boucher RC. Pathophysiology of gene-targeted mouse models for cystic fibrosis. Physiol Rev. 1999;79:S193–S214. doi: 10.1152/physrev.1999.79.1.S193. [DOI] [PubMed] [Google Scholar]
- 10.Scholte BJ, Davidson DJ, Wilke M, De Jonge HR. Animal models of cystic fibrosis. J Cyst Fibros. 2004;3(Suppl 2):183–190. doi: 10.1016/j.jcf.2004.05.039. [DOI] [PubMed] [Google Scholar]
- 11.Liu X, Yan Z, Luo M, Engelhardt JF. Species-specific differences in mouse and human airway epithelial biology of recombinant adeno-associated virus transduction. Am J Respir Cell Mol Biol. 2006;34:56–64. doi: 10.1165/rcmb.2005-0189OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogers C, Hao Y, Rokhlina T, Yan Z, Engelhardt J, Prather R, et al. Gene targeting of pig CFTR: progress toward a large animal model of cystic fibrosis. Pediatr Pulmonol. 2006;(Suppl 29):281. [Google Scholar]
- 13.Li Z, Engelhardt JF. Progress toward generating a ferret model of cystic fibrosis by somatic cell nuclear transfer. Reprod Biol Endocrinol. 2003;1:83. doi: 10.1186/1477-7827-1-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Z, Sun X, Chen J, Liu X, Wisely SM, Zhou Q, et al. Cloned ferrets produced by somatic cell nuclear transfer. Dev Biol. 2006;293:439–448. doi: 10.1016/j.ydbio.2006.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeffery PK. Morphologic features of airway surface epithelial cells and glands. Am Rev Respir Dis. 1983;128:S14–S20. doi: 10.1164/arrd.1983.128.2P2.S14. [DOI] [PubMed] [Google Scholar]
- 16.Leigh MW, Gambling TM, Carson JL, Collier AM, Wood RE, Boat TF. Postnatal development of tracheal surface epithelium and submucosal glands in the ferret. Exp Lung Res. 1986;10:153–169. doi: 10.3109/01902148609061490. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Zhang Y, Amberson A, Engelhardt JF. New models of the tracheal airway define the glandular contribution to airway surface fluid and electrolyte composition. Am J Respir Cell Mol Biol. 2001;24:195–202. doi: 10.1165/ajrcmb.24.2.3918. [DOI] [PubMed] [Google Scholar]
- 18.Pavelka M, Ronge HR, Stockinger G. [Comparative study of tracheal epithelium of different mammals] Acta Anat (Basel) 1976;94:262–282. [PubMed] [Google Scholar]
- 19.Karp PH, Moninger TO, Weber SP, Nesselhauf TS, Launspach JL, Zabner J, et al. An in vitro model of differentiated human airway epithelia. Methods for establishing primary cultures. Methods Mol Biol. 2002;188:115–137. doi: 10.1385/1-59259-185-X:115. [DOI] [PubMed] [Google Scholar]
- 20.Davidson DJ, Kilanowski FM, Randell SH, Sheppard DN, Dorin JR. A primary culture model of differentiated murine tracheal epithelium. Am J Physiol Lung Cell Mol Physiol. 2000;279:L766–L778. doi: 10.1152/ajplung.2000.279.4.L766. [DOI] [PubMed] [Google Scholar]
- 21.Liu X, Luo M, Zhang L, Ding W, Yan Z, Engelhardt JF. Bioelectric properties of chloride channels in human, pig, ferret, and mouse airway epithelia. Am J Respir Cell Mol Biol. 2007;36:313–323. doi: 10.1165/rcmb.2006-0286OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan Z, Lei-Butters DC, Liu X, Zhang Y, Zhang L, Luo M, et al. Unique biologic properties of recombinant AAV1 transduction in polarized human airway epithelia. J Biol Chem. 2006;281:29684–29692. doi: 10.1074/jbc.M604099200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan Z, Zak R, Luxton GW, Ritchie TC, Bantel-Schaal U, Engelhardt JF. Ubiquitination of both adeno-associated virus type 2 and 5 capsid proteins affects the transduction efficiency of recombinant vectors. J Virol. 2002;76:2043–2053. doi: 10.1128/jvi.76.5.2043-2053.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duan D, Yue Y, Yan Z, Yang J, Engelhardt JF. Endosomal processing limits gene transfer to polarized airway epithelia by adeno-associated virus. J Clin Invest. 2000;105:1573–1587. doi: 10.1172/JCI8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walters RW, Yi SM, Keshavjee S, Brown KE, Welsh MJ, Chiorini JA, et al. Binding of adeno-associated virus type 5 to 2,3-linked sialic acid is required for gene transfer. J Biol Chem. 2001;276:20610–20616. doi: 10.1074/jbc.M101559200. [DOI] [PubMed] [Google Scholar]
- 26.Summerford C, Samulski RJ. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duan D, Yue Y, Engelhardt JF. Response to ‘polarity influences the efficiency of recombinant adeno-associated virus infection in differentiated airway epithelia’. Hum Gene Ther. 1999;10:1553–1557. doi: 10.1089/10430349950017888. [DOI] [PubMed] [Google Scholar]
- 28.Wu Z, Miller E, Agbandje-McKenna M, Samulski RJ. Alpha2,3 and alpha2,6 N-linked sialic acids facilitate efficient binding and transduction by adeno-associated virus types 1 and 6. J Virol. 2006;80:9093–9103. doi: 10.1128/JVI.00895-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seiler MP, Miller AD, Zabner J, Halbert CL. Adeno-associated virus types 5 and 6 use distinct receptors for cell entry. Hum Gene Ther. 2006;17:10–19. doi: 10.1089/hum.2006.17.10. [DOI] [PubMed] [Google Scholar]
- 30.Walters RW, Pilewski JM, Chiorini JA, Zabner J. Secreted and transmembrane mucins inhibit gene transfer with AAV4 more efficiently than AAV5. J Biol Chem. 2002;277:23709–23713. doi: 10.1074/jbc.M200292200. [DOI] [PubMed] [Google Scholar]
- 31.Liu X, Yan Z, Luo M, Zak R, Li Z, Driskell RR, et al. Targeted correction of single-base-pair mutations with adeno-associated virus vectors under nonselective conditions. J Virol. 2004;78:4165–4175. doi: 10.1128/JVI.78.8.4165-4175.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ding W, Zhang LN, Yeaman C, Engelhardt JF. rAAV2 traffics through both the late and the recycling endosomes in a dose-dependent fashion. Mol Ther. 2006;13:671–682. doi: 10.1016/j.ymthe.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]