Abstract
The function of vasa vasorum is both to deliver nutrients and oxygen to arterial and venous walls and to remove “waste” products, either produced by cells in the wall or introduced by diffusional transport through the endothelium of the artery or vein. Although the relationship between changes in vasa vasorum characteristics and the development of atheromatous plaques is well documented, the role of vasa vasorum, especially in terms of their appearance and disappearance in disease processes such as atherosclerosis, are still not clearly understood in terms of their being causative or merely reactive. However, even if their proliferation is merely reactive, these new microvessels may be a source of disease progression by virtue of endothelial impairment and as a pathway for monocytic cells to migrate to sites of early disease. As both these features are aspects of the vasa vasorum function, this Review focuses on the following issues: 1) acute modulation of vasa vasorum patency due to surrounding compressive forces within vessel wall and due to variable tone in the smooth muscle within proximal vasa vasorum and 2) chronic angiogenic responses due to local cytokine accumulations such as occur in the wall of arteries in the presence of hypertension, hypercholesterolemia, accumulation of lipids, extravasated blood products (e.g., red blood cells, macrophages, inflammatory products) which attract monocytes, and response of vasa vasorum to pharmacological stimuli.
Keywords: Angiogenesis, Arteries, Atherosclerosis, Coronary disease, Inflammation
1. Introduction
Vasa vasorum consist of small arteries which enter the vascular wall either from the abluminal surface (vasa vasorum externa) or from the luminal surfaces (vasa vasorum interna) and then arborize to the outer media. Venous vasa vasorum drain a network of capillaries/venules laid down around the outer media to veins in close proximity to the arteries. In humans, vessels with walls less than 29-cell layers thick [1] normally do not have vasa [2] and, in general, vessels less than 0.5 mm lumen diameter [3] (all normal vessels in mice and intramyocardial vessels in humans) do not have vasa vasorum. In larger vessels diffusion of solutes to the media from the vessel lumen is supplemented by vasa vasorum [4,5].
The vasa vasorum have been the subject of considerable interest for more than a century [6] because of their possible role in, atherogenesis [7-12], coronary interventions [13-15], and in response to risk factors for atherosclerosis, such as hypercholesterolemia [16-20] and hypertension [21-23]. Although many studies have described the anatomy of the vasa vasorum in a qualitative manner [24-28], the more recently described detailed 3D architecture of the vasa vasorum network (due to the availability of micro-CT imaging capabilities [29,30]) has made more quantitative information available. In addition, it is becoming increasingly clear that vasa vasorum are dynamic in that they can be transiently compressed by the surrounding arterial wall and/or undergo vasodilation and vasoconstriction as well as increase in number (e.g., angiogenesis). Thus, these capabilities of the vasa vasorum underscore their dynamic role in the regulation of vascular wall perfusion.
2. Anatomy and Function of Vasa Vasorum
2.1 Vasa Vasorum Branching Geometry
The vasa vasorum have been shown to function as end arteries [30], possibly due to the pressure distribution within the arterial wall which could compress most of the vasa vasorum. An arterial injection of the silicon polymer Microfil® results in high pressure in the arterial lumen and consequently within the arterial wall consistent with Lame’s Law [31]. This intramural pressure gradient can result in compression of some of the vasa vasorum. As illustrated in Figure 1, this phenomenon is demonstrated when Microfil® is injected into the concomitant vein so that the coronary artery is filled retrogradely via the intramyocardial capillaries and the arterial vasa vasorum are filled retrogradely via the venous vasa vasorum which empty directly into the concomitant vein and are therefore also exposed to the Microfil® injection pressure. Hence, the luminal pressure in the artery is much reduced, resulting in a less distended coronary arterial lumen and the compressive force within the arterial wall is proportionally decreased. This in turn results in less compression of the vasa vasorum in the arterial wall because they are both exposed to the increased pressure in the vasa vasorum as well as to the reduced intramural pressure. Consequently, the density of perfused (and therefore opacified) vasa vasorum under these conditions is increased.
Figure 1.
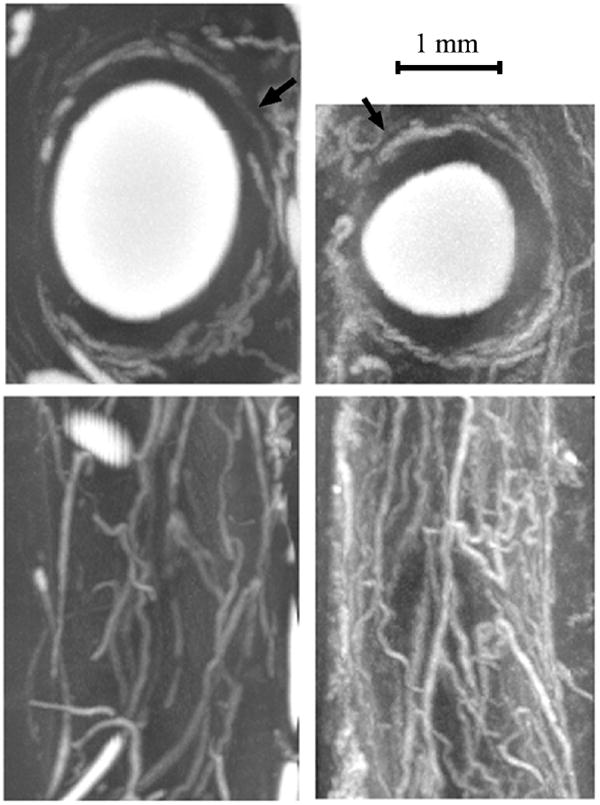
Top two panels are micro-CT transaxial images through a porcine coronary artery. The white regions are a radiopaque silicon-based polymer injected into the vascular lumen. In the left panel the polymer was injected into the coronary artery lumen at 100 mmHg pressure and in the right panel it was injected into the concomitant vein’s lumen at 100 mmHg pressure. The arrows point to opacified vasa vasorum around the main lumen. These conditions result in the larger coronary arterial lumen due to the higher intracoronary intralumenal pressure in the left upper panel and the increased number of vasa vasorum due to the reduced arterial lumen pressure resulting in a proportional decrease in the intramural pressure in the right panels. The lower panels are the corresponding axial projections of those arteries after the contrast in the main lumens was “removed” by image manipulation.
Due to the vasa vasorum both the vascular adventitia and, to a lesser extent, the media are brought into close proximity to blood cells and blood solutes. Because the blood pressure within the main arterial lumen is generally higher than the extravascular tissue pressure, the diffusion of the blood solutes would tend to be from the main lumen towards the adventitia, as described by Darcy’s Law [32] which links diffusion of a solute through a porous medium subjected to a pressure gradient. The only way that those solutes can leave the vessel wall is via the venous vasa vasorum and any lymphatics that may be present in the wall.
The branching structures of the vasa vasorum have also been explored [4,33,34], but little has been done to use that structure to explore the fluid dynamic conductive characteristics of the vasa vasorum [29,30,35].
An important consequence of the anatomic location and branching architecture of the vasa vasorum, which enter the arterial wall via the adventitia, is that flow through the vasa cannot proceed far into the media due to the compressive force (Pw) within the arterial wall, at location R, as described by Lamé’s Law [31]:
| (1) |
Where: Pℓ is the pressure in the coronary artery lumen, ‘a’ is radius of coronary artery lumen, ‘b’ is the radius of the outer adventitia, R is the radial distance (from the endothelial surface) within the coronary arterial wall.
This law indicates that the local compressive pressure within the vessel wall is equal to lumenal blood pressure in the sub-endothelial layer (i.e., R ≈ a) but falls-off hyperbolically towards to adventitia (i.e., R ≈ b). As a consequence, at the radial location at which the pressure inside the parent vessel wall exceeds the pressure in the vasa vasorum lumen (determined largely by pressure drop (ΔPi) along the vasa vasorum as described by Poiseuille’s Law [36]), no perfusion of the wall can occur closer to the main lumen.
| (2) |
Where: Pi is pressure in lumen of vasa vasorum at distance L from the origin of the vasa vasorum. Where: ‘ri’ is the radius of the ‘proximal’ vasa vasorum lumen and Li is the distance along the vasa vasorum to the point-of-interest within the wall.
The vascular resistance to flow in the vasa vasorum is high because the radii of the vasa vasorum are much smaller than the parent vessel lumen, hence the pressure within the distal vasa vasorum close to the media must be lower than the coronary arterial lumen pressure – hence this intima-medial zone must be where the compressive pressure within the wall can exceed the blood pressure within the vasa vasorum at that location. However, these considerations assume steady state. It is conceivable that as the systolic pressure pulse progresses along the vasa vasorum its arrival in the terminal vasa vasorum is delayed relative to the transient increase of systolic pressure propagated within the arterial wall. Consequently, the extent of transmural perfusion by vasa vasorum may be underestimated by a steady-state assumption.
Darcy’s Law [32] describes the driving force for diffusion of extra-vascular solutes to migrate across a vessel wall in the direction of the pressure gradient within the wall.
| (3) |
Where: fℓ and fvv are the fluxes of solutes from the coronary arterial lumen and into the venous vasa vasorum respectively. Area ℓ is the coronary arterial endothelial surface area across which solute flux occurs and Areavv being the area of the vasa endothelial surface within the wall at distance R within the wall. T is the distance from the endothelium towards the outer surface of the adventitia (i.e., R-a).
This law implies that for the venous vasa vasorum to match the flux across the main lumen’s endothelium, the venous vasa vasorum endothelial permeability surface area product must be large enough to cope with this transmural flux from the main lumen. Thus, ligating venous vasa vasorum should result in build-up of solutes such as fatty compounds [7,11,37].
As veins also have vasa vasorum [38,39], it is of interest that veins generally do not develop atherosclerosis except when they are exposed to increased lumen pressure. This is observed when they are used as arterial shunts (such as saphenous vein bypass grafts [40-42]) which often undergo accelerated intimal thickening and plaque formation after three years. Although the accelerated venous atherosclerosis may in part be due to damage to the vasa vasorum at the time of harvesting and transplant of the vein segment, the combination of the presence of vasa and high intra-vascular blood pressure [42,43] or very high plasma lipid concentrations [44] appear to be necessary for development of atherosclerotic plaques.
The “footprints” of coronary vasa vasorum perfusion territories (Figure 2) have also been studied [30]. Microembolization reduced vasa vasorum densities significantly and increased the size of low-vasa-vasorum-density territories. Consequently, under normal conditions coronary vasa vasorum are functional end-arteries, even though they may end up being connected via an anatomic plexus. This characteristic may have a significant impact on the spatial distribution of perfusion and drainage of the coronary vessel wall. If the heterogeneous distribution of both coronary atherosclerosis and of the vasa vasorum along the coronary vascular tree are anatomically coincident, this would more directly support the potential role of the vasa vasorum in the disease process.
Figure 2.
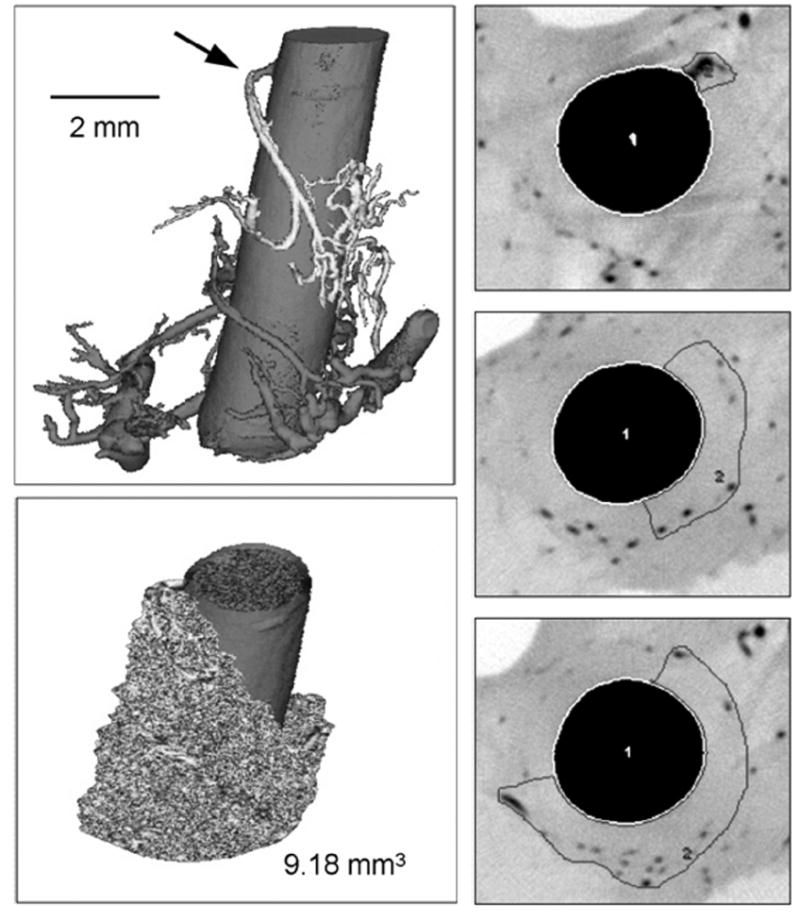
Upper left panel: Volume-rendered micro-CT image of a porcine right coronary artery (20 μm cubic voxel size). The origin of the vasa vasorum interna from the main coronary artery lumen is indicated by the arrow. Right panels: grayscale-inverted μ-CT cross-sections with areas of perfusion. These areas of perfusion enclose the vasa vasorum branches that belong to the tree in the upper left panel. After adding the areas of perfusion in all contiguous cross-section images, the volume of vessel wall perfused can be assessed (lower left panel). [With permission from Gössl M et al., Ref. 29].
2.2 Physiologic Reactivity of Vasa Vasorum
The proximal vasa vasorum display a regularly layered vascular structure of endothelial cells, vascular smooth muscle cells, and surrounding connective tissue. These characteristics are important since they imply that the vasa vasorum may regulate their own tone and vascular perfusion [5,45,46] in a manner similar to small coronary arteries, as was demonstrated by Scotland et al., [47,48].
Vasa vasorum, isolated from porcine aorta, respond to the endothelium-dependent vasodilators substance P and bradykinin similar to the host vessel response. Although vascular reactivity of the vasa vasorum in different vascular beds is not known, any differences in reactivity conceivably contribute to the different susceptibilities of different vascular beds, (such as coronary versus peripheral arteries) to atherosclerosis [49-51].
2.3 Role of Vasa Vasorum in Solute Transport into and from Arterial Wall
The transport physiology of aortic vasa vasorum has been explored using radiolabeled microsphere-based estimates of perfusion [5] as well as the oxygenation of the arterial wall by direct measurement [52,53] and by model-based analysis of oxygen tension [54]. These approaches show a nadir of oxygen tension of approximately 10 mmHg at about 300 μm from the lumen. Lipid transport by vasa vasorum into the parent vessel wall has been shown to be about 30% of the transintimal transport in rabbit aorta [55].
3. Role of Vasa Vasorum in Atherosclerosis
Several interacting feedback loops that plausibly involve vasa vasorum during the development of atherosclerotic plaques are provided in (Figure 3). The relative magnitude and timing of the activation and/or suppression of these feedback loops would largely determine, 1) the rate of development of plaques [56] and 2) the stability of those plaques [57].
Figure 3.
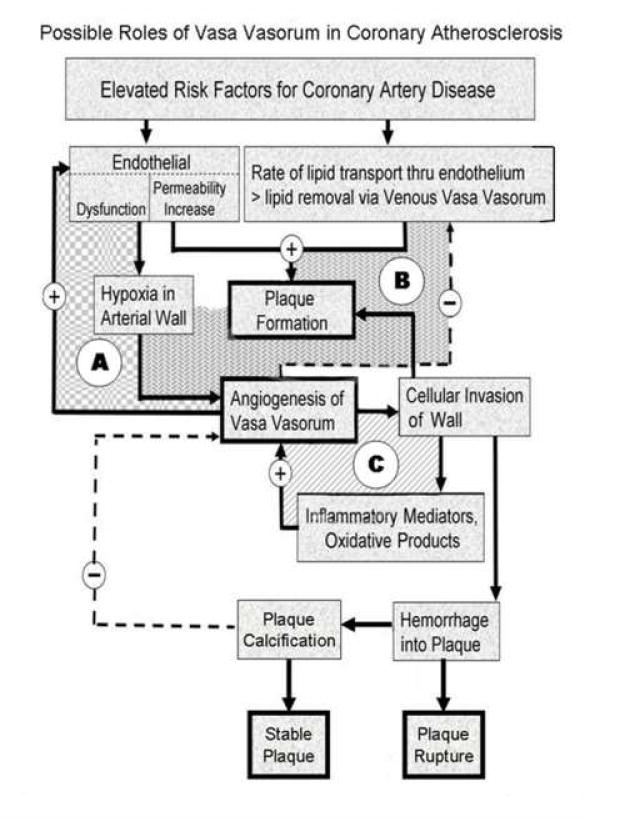
This is a flow chart of the proposed cascade of events triggered by increased risk factors (such as elevated plasma lipids) and altered endothelial function. There are positive feedback loops (e.g., A, C) which result in progressive plaque formation and ultimately plaque rupture or plaque calcification. The negative feedback loops (e.g., B) involve the salutary effect of new vasa vasorum formation and the anti-angiogenesis association of plaque stabilization with calcification. The relative magnitude and time of onset of these various reactions to the initial stimuli determine the specific course followed (i.e., relentless progression vs. self limiting) in any one individual case. (See text for more detailed discussion).
3.1 Modification of Transport into and out of Vascular Wall
That endothelial injury and dysfunction are early features of atherosclerosis is supported by numerous experimental findings [58]. However, the hypothesis that “injured” endothelium is a causative factor begs the question as to why early atherosclerosis is not observed in small arteries, in the outer media (where vasa vasorum endothelium is present) or in undisturbed veins.
Studies involving measurement of the transport of lipids and albumin and dyes into the wall from the main lumen [44,59] generally do not consider the possible role of vasa vasorum [60] but focused exclusively on the influx of solute, endothelial permeability and local solute residence times or heat removal/delivery. Moreover, these studies also did not address the possibility that there may be circumferential variations in transmural pressure gradient. Thus, a region of wall that is not supported at its abluminal surface would have a larger pressure gradient (which would drive diffusion into the wall via Darcy’s Law) than would wall that is supported on its abluminal surface.
The reason veins and the normal pulmonary artery do not develop atherosclerosis may be due to the fact that the transmural flux of solute (as described by Darcy’s Law) is diminished due to the low venous and pulmonary artery lumen pressures. In addition, with these lumen pressures generally being lower than the pressure within the arterial vasa vasorum, these vasa vasorum may never be compressed during the entire cardiac cycle, thereby maintaining adequate flow in the vasa vasorum. Nonetheless, the need for a high density of external vasa vasorum in vein walls is likely due to the fact that the venous (unlike arterial) blood in the main lumen provides little, if any, oxygen to the wall via transendothelial diffusion from the main lumen.
The early stages of histologically detectable atherogenesis have been shown to involve increased transport of low density lipoprotein (LDL) across the endothelium, preceding cellular infiltration/proliferation in the arterial wall [61-64]. Because of endothelial dysfunction in arterial vasa vasorum, delivery of LDL (and probably oxidized and inflammatory products) may occur at a rate greater than can be removed by venous vasa vasorum [17]. In addition to the increased delivery (or impaired removal) of LDL from the arterial wall by the arterial vasa vasorum [65], an early major focus on the vasa vasorum’s possible role in atherogenesis has been the oxygen delivery [66-68]. Low oxygen tension has been shown to accelerate atherogenesis and interfere with LDL transport [69]. Moreover, fatty streaks, especially in diabetic situations, have been shown to increase oxygen demand [70]. We speculate that these data suggest the presence of a positive feedback process so that reduced perfusion from the vasa vasorum results in local hypoxia and in increased accumulation of fatty substances in the intima/media, which, in turn, results in further local hypoxia due to the increased local oxygen consumption.
3.2 Modification of Vasa Vasorum Function by Neovascularization
Despite the fact that atherosclerosis encroaches on the arterial lumen only at its late stage, the majority of research efforts continue to focus on the luminal side of the vascular wall. Recent evidence, however, suggests that the adventitia may play a significant role in maintaining vessel integrity, and may contribute to the initiation and/or progression of certain types of vascular disease [71,72]. Indeed, experimental studies demonstrated that manipulation of the adventitia, and more specifically of the vasa vasorum, such as handling of the vessels at surgery or deposits of talcum powder from the gloves, could lead to atherosclerotic changes of the intima [7,11,12, 73,74]. Atherosclerotic lesion formation is associated with neovascularization of the vasa vasorum [30,75,76] as illustrated in (Figure 4).
Figure 4.
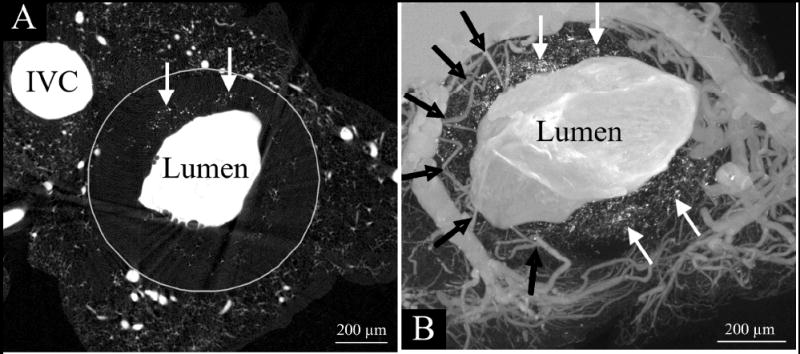
Micro-CT transaxial images of a double knockout mouse (LDL-/-, apoE-/-) aorta after it was injected with a radiopaque silicon polymer. The aortic lumen (large white area) is irregular rather than round because of the atherosclerotic plaques encroaching into the lumen. The small vessels around the lumen are vasa vasorum. Note that unlike the “clear” zone between the vasa vasorum and the main lumen in the normal artery (upper panels of Fig. 1), the black arrows in this image show vasa vasorum entering the plaque area close to the main lumen surface. These are the newly formed vasa vasorum in response to the plaque formation process. Also, the white arrows point to isolated punctate opacities in the region of the plaques. These were shown to be small accumulations of iron and calcium, presumably the remains of red blood cells. [Abridged version of Figure 3 with permission from Langheinrich AC et al., Ref. 109].
A number of autopsy-based studies highlighted that this neovascularization process occurs in the neointima, which progresses with and determines plaque extent [77]. The latter aspect was underscored by an experimental study in apoE-deficient mice, showing that anti-angiogenic therapy not only reduced plaque neovascularization but eventually plaque growth [78]. Moreover, the inhibition of angiogenesis was associated with a reduction of macrophages in the plaque and around the vasa vasorum [79-81].
3.3 Alteration of Vasa Vasorum Endothelial Function by Disease
Hypercholesterolemia and hypertension are associated with impaired endothelial function and an increase in vascular inflammation such as indicated by increased expression of the nuclear transcription factor kappaB (NF-κB) [82]. In addition, an increase of NF-κB has been shown to cause enhanced endothelial cell apoptosis [83]. Endothelial dysfunction and an increase in the vascular tone of the vasa vasorum may be enhanced in these states due to the enhanced inflammation and the reduction in the bioavailability of nitric oxide, secondary to the increase in endogenous oxidative stress. Eventually, these alterations in the balance of vasoreactive factors and endothelial cell function may lead to the functional reduction in blood flow to the vascular wall and local hypoxia potentially resulting in vasa vasorum neovascularization to meet the perfusion needs of the arterial wall. The imbalance between vascular nutrient supply and demand might even be worsened by an enhanced metabolism and/or size of the vascular wall upon exposure to a cardiovascular risk factor. This relates to the hypoxia or anoxemia theory of atherosclerosis [84]. Indeed, recent studies in hypertensive rats demonstrated increase in hypoxia-inducible factor 1 alpha (HIF-1α) and VEGF expression in the aorta, which was subsequently followed by increase in vasa vasorum density around the aorta [23]. A similar increase in HIF-1α and VEGF has been demonstrated in coronary arteries in hypercholesterolemic pigs [85]. This study also indicated that the reversibility of endothelial dysfunction at the early stages of atherosclerosis was associated with a parallel reduction in the coronary vasa vasorum spatial density (neovascularization). Vasa vasorum neovascularization has also been shown to precede the development of atherosclerotic lesion and even the impairment of endothelium-dependent vaso-relaxation, a hallmark of early atherosclerosis [17]. Hence, there seems to be an interaction between the pathophysiologic state of the vessel and the vasa vasorum spatial density, which is a dynamic, not a static, process.
The reduction in vascular wall hypoxia coincides with the decrease in the expression of pro-angiogenic factors in the coronary arterial wall [18,19,86]. Thus, preservation of endothelial function of the vasa vasorum and thereby preservation of adventitial blood supply might be a common mechanism of the neovascularization in different vascular beds.
Elevated plasma lipid concentration [87] and coronary artery luminal endothelium damage have been shown to be major factors in the initiation and progression of atherogenesis [88,89]. However, it is plausible that the coronary artery vasa vasorum have an aggregate endothelial surface area that is comparable in size to the host vessel’s luminal endothelium, especially in early stages of atherosclerosis, when there is increased density of vasa vasorum [90,91]. Hence, disparity between the luminal and vasa vasorum endothelial surfaces areas may be an important factor. In addition, it seems possible that vasa vasorum blood flow may be selectively reduced by increased smooth muscle tone in proximal vasa vasorum due to reduced endothelial transduction function, infection, inflammation or thrombosis. This would result in hypoxia and reduced removal of substances from the media, which must now accumulate. Despite this possible inequality in anatomic endothelial surface areas it is not clear if the functional surface areas and endothelial permeabilities (i.e., permeability surface area products) at the two sites are comparable and equally susceptible to damage or loss of function. Such mismatch of endothelial function would seem plausible [92]. Importantly, however, the “host artery” endothelium has a high pressure driving substances into the intima whereas the lower luminal pressure in the vasa vasorum (due to the small diameter of these vessels which causes a pressure drop as described by Poiseuille’s Law) creates a pressure gradient which favors solute transport from host artery lumen towards the adventitia as described by Darcy’s Law. Indeed, that it is more likely that substances diffuse into the vasa from the media, rather than the opposite, has been shown by the clearance of radiolabeled molecules [75,93,94].
One of the possible consequences of infiltration of lipids into the subintima or media is that vasa proliferate due to the angiogenic stimulus they generate via the concomitant oxidative stress [95]. Specific angiogenic factors have been shown to play a role in the proliferation of vasa vasorum [93,96-98]. However, the angiogenesis may just not be enough to meet the need for increased endothelial surface area product of the vasa vasorum.
3.4 Role of Vasa Vasorum as a Portal for Cellular Invasion of the Arterial Wall
Neovascularization of the vasa vasorum could conceivably function as a conduit for entry of macrophages and inflammatory factors that may potentially promote the progression of the disease and angiogenesis [79]. Moreover, as increased endothelial permeability and fragility are cardinal features of pathological neovascularization [99] pro-atherogenic cellular and soluble plasma components may enter the vessel wall more easily through ruptured and/or leaky vasa vasorum thereby further enhancing the progression of atherosclerosis [100]. Indeed, there is an increased influx to, as well as a decreased drainage from, the coronary vessel wall in the porcine model of hypercholesterolemia [101]. Pathological and experimental studies are consistent with the contention that vasa vasorum hemorrhage may be a key factor in the development of unstable atherosclerotic lesions [102,103].
Moreno et al., [104] demonstrated that neovascularization, as manifested by the localized appearance of microvessels, is increased in ruptured plaques in the human aorta. Furthermore, they could demonstrate that microvessel density is increased in lesions with inflammation, with intraplaque hemorrhage, and in thin-cap fibroatheromas. A recent study by Langheinrich et al., [76] demonstrates the association among different advanced atherosclerotic lesions, adventitial vasa vasorum neovascularization and adventitial inflammation in apoE-/-/LDL-/- double knockout mice.
4. Summary
The role of vasa vasorum in maintaining the integrity of the walls of vessels more than 0.5 mm in diameter is not fully understood, although they clearly are present when the wall is thicker than can be maintained viable by diffusion of solutes from the lumen alone. There is clearly a strong association between the density of vasa vasorum in an arterial vessel wall and severity of plaque formation, but it is still not clear whether the vasa vasorum play a causative or merely reactive role. The latter possibility is complicated by the possibility that the development of new vasa vasorum is too late and/or that the new vasa vasorum serve as conduit which facilitates cellular invasion of the vessel wall and thereby impact on the type of plaque formed. Table 1 summarizes literature references that address the possible roles of vasa vasorum in atherosclerosis.
Table 1.
| Evidence Suggesting the Role of the Vasa Vasorum in Atherosclerosis | References |
|---|---|
| Distribution | |
| Higher vasa vasorum density in vascular beds more prone for developing atherosclerosis. | 51, 105 |
| Higher density of vasa vasorum in the proximal segments of the vessels than in the distal segments. | 106 |
| Heterogeneous distribution of atherosclerotic plaques. | 29 |
| Relationship to coronary artery disease risk factor and reversibility | |
| Induction: Increase in vasa vasorum density in models of atherosclerosis and vascular injury. |
14, 16
17, 78 |
| Reversibility: Inhibition of vasa vasorum angiogenesis attenuates the progression of atherosclerosis. |
18, 56, 57,
78, 79, 82 |
| Relationship with cardiovascular events | |
| Vasa vasorum neovascularization and hemorrhage contributing to plaque rupture and cardiovascular events. |
107, 108,
102, 104 |
Acknowledgments
This manuscript was supported in part by NIH grants, HL65342 and EB000305. We also want to thank Ms. Mara Lukenda for typing and coordinating this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wolinsky H, Glagov S. Nature of species differences in the medial distribution of aortic vasa vasorum in mammals. Cir Res. 1967;20:409–21. doi: 10.1161/01.res.20.4.409. [DOI] [PubMed] [Google Scholar]
- 2.Okuyama K, Yagimuna G, Takahashi T, Sasaki H, Mori S. The development of vasa vasorum of the human aorta in various conditions. A morphometric study. Archives of Pathol and Lab Medicine. 1988;112:721–5. [PubMed] [Google Scholar]
- 3.Geiringer E. Intimal vascularization and atherosclerosis. J Pathol Bact. 1951;63:201–11. doi: 10.1002/path.1700630204. [DOI] [PubMed] [Google Scholar]
- 4.Zamir M, Silver MD. Vasculature in the walls of human coronary arteries. Arch Pathol Lab Med. 1985;109:659–62. [PubMed] [Google Scholar]
- 5.Heistad DD, Marcus ML. Role of vasa vasorum in nourishment of the aorta. Blood Vessels. 1979;16:225–38. doi: 10.1159/000158209. [DOI] [PubMed] [Google Scholar]
- 6.Koester W. Endarteritis and arteritis. Berl Klin Wochenschr. 1876;13:454–5. [Google Scholar]
- 7.Nakata Y, Shionoya S. Vascular lesion due to obstruction of the vasa vasorum. Nature. 1966;212:1258–9. doi: 10.1038/2121258a0. [DOI] [PubMed] [Google Scholar]
- 8.Mann FD. Vasa vasorum and coronary atherosclerosis. Lancet. 1978;1:1319–20. doi: 10.1016/s0140-6736(78)91313-2. [DOI] [PubMed] [Google Scholar]
- 9.Barger AC, Beeuwkes R, III, Lainey LL, Silverman KJ. Hypothesis: vasa vasorum and neovascularization of human coronary arteries. A possible role in the pathophysiology of atherosclerosis. N Engl J Med. 1984;310:175–7. doi: 10.1056/NEJM198401193100307. [DOI] [PubMed] [Google Scholar]
- 10.Barger AC, Beeuwkes R., III Rupture of coronary vasa vasorum as a trigger of acute myocardial infarction. Am J Cardiol. 1990;66:41G–3G. doi: 10.1016/0002-9149(90)90394-g. [DOI] [PubMed] [Google Scholar]
- 11.Barker SG, Talbert A, Cottam S, Baskerville PA, Martin JF. Arterial intimal hyperplasia after occlusion of the adventitial vasa vasorum in the pig. Arterioscler Thromb. 1993;13:70–7. doi: 10.1161/01.atv.13.1.70. [DOI] [PubMed] [Google Scholar]
- 12.Barker SG, Tilling LC, Miller GC, Beesley JE, Fleetwood G, Stavri GT, et al. The adventitia and atherogenesis: removal initiates intimal proliferation in the rabbit which regresses on generation of a ‘neoadventitia’. Atherosclerosis. 1994;105:131–44. doi: 10.1016/0021-9150(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 13.Cragg AH, Einzig S, Rysavy JA, Castaneda-Zuniga WR, Borgwardt B, Amplatz K. The vasa vasorum and angioplasty. Radiology. 1983;148:75–80. doi: 10.1148/radiology.148.1.6222396. [DOI] [PubMed] [Google Scholar]
- 14.Kwon HM, Sangiorgi G, Ritman EL, Lerman A, McKenna C, Virmani R, et al. Adventitial vasa vasorum in balloon injured coronary arteries: visualization and quantitation by a microscopic three-dimensional computed tomography technique. J Am Coll Cardiol. 1998;32:2072–9. doi: 10.1016/s0735-1097(98)00482-3. [DOI] [PubMed] [Google Scholar]
- 15.Sanada JI, Matsui O, Yoshikawa J, Matsuoka T. An experimental study of endovascular stenting with special reference to the effects on the aortic vasa vasorum. Cardiovasc Intervent Radiol. 1998;21:45–9. doi: 10.1007/s002709900210. [DOI] [PubMed] [Google Scholar]
- 16.Kwon HM, Sangiorgi G, Ritman EL, McKenna C, Holmes DR, Jr, Schwartz RS, et al. Enhanced coronary vasa vasorum neovascularization in experimental hypercholesterolemia. J Clin Invest. 1998;101:1551–6. doi: 10.1172/JCI1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herrmann J, Lerman LO, Rodriguez-Porcel MG, Holmes DR, Jr, Richardson DM, Ritman EL, et al. Coronary vasa vasorum neovascularization precedes epicardial endothelial dysfunction in experimental hypercholesterolemia. Cardiovasc Res. 2001;51:762–6. doi: 10.1016/s0008-6363(01)00347-9. [DOI] [PubMed] [Google Scholar]
- 18.Herrmann J, Best PJ, Ritman EL, Holmes DR, Lerman LO, Lerman A, et al. Chronic endothelin receptor antagonism prevents coronary vasa vasorum neovascularization in experimental hypercholesterolemia. J Am Coll Cardiol. 2002;39:1555–61. doi: 10.1016/s0735-1097(02)01798-9. [DOI] [PubMed] [Google Scholar]
- 19.Wilson SH, Simari RD, Best PJ, Peterson TE, Lerman LO, Aviram M, et al. Simvastatin preserves coronary endothelial function in hypercholesterolemia in the absence of lipid lowering. Arterioscler Thromb Vasc Biol. 2001;21:122–8. doi: 10.1161/01.atv.21.1.122. [DOI] [PubMed] [Google Scholar]
- 20.Cheema AN, Hong T, Nili N, Segev A, Moffat JG, Lipson KE, et al. Adventitial microvessel formation after coronary stenting and the effects of SU11218, a tyrosine kinase inhibitor. J Am Coll Cardiol. 2006;47:1067–75. doi: 10.1016/j.jacc.2005.08.076. [DOI] [PubMed] [Google Scholar]
- 21.Marcus ML, Heistad DD, Armstrong ML, Abboud FM. Effects of chronic hypertension on vasa vasorum in the thoracic aorta. Cardiovasc Res. 1985;19:777–81. doi: 10.1093/cvr/19.12.777. [DOI] [PubMed] [Google Scholar]
- 22.Kai H, Kuwahara F, Tokuda K, Shibata R, Kusaba K, Niiyama H, et al. Coexistence of hypercholesterolemia and hypertension impairs adventitial vascularization. Hypertension. 2002;39:455–9. doi: 10.1161/hy0202.103001. [DOI] [PubMed] [Google Scholar]
- 23.Kuwahara F, Kai H, Tokuda K, Shibata R, Kusaba K, Tahara N, et al. Hypoxia-inducible factor-1alpha/vascular endothelial growth factor pathway for adventitial vasa vasorum formation in hypertensive rat aorta. Hypertension. 2002;39:46–50. doi: 10.1161/hy1201.097200. [DOI] [PubMed] [Google Scholar]
- 24.Winternitz MD, Thomas RM, LeCompte PM. The Biology of Arteriosclerosis. Springfield, IL: Charles C. Thomas Publisher; 1938. [Google Scholar]
- 25.Schoenenberger F, Mueller A. Ueber die Vaskularisierung der Rinderaortenwand. Helvet Physiol Pharmacol Acta. 1960;18:136–50. [PubMed] [Google Scholar]
- 26.Nakata Y, Shionoya S, Tamura J, Hirabayashi N. Vasa vasorum and vascular lesions in the human abdominal aorta. Vasa. 1977;6:255–8. [PubMed] [Google Scholar]
- 27.Stefanadis C, Vlachopoulos C, Karayannacos P, Boudoulas H, Stratos C, Filippides T, et al. Effect of vasa vasorum flow on structure and function of the aorta in experimental animals. Circulation. 1995;91:2669–78. doi: 10.1161/01.cir.91.10.2669. [DOI] [PubMed] [Google Scholar]
- 28.Angouras D, Sokolis DP, Dosios T, Kostomitsopoulos N, Boudoulas H, Skalkeas G, et al. Effect of impaired vasa vasorum flow on the structure and mechanics of the thoracic aorta: implications for the pathogenesis of aortic dissection. Eur J Cardiothorac Surg. 2000;17:468–73. doi: 10.1016/s1010-7940(00)00382-1. [DOI] [PubMed] [Google Scholar]
- 29.Gössl M, Rosol M, Malyar NM, Fitzpatrick LA, Beighley PE, Zamir M, et al. Functional and hemodynamic characteristics of vasa vasorum in the walls of porcine coronary arteries. Anat Rec. 2003;272A:526–37. doi: 10.1002/ar.a.10060. [DOI] [PubMed] [Google Scholar]
- 30.Gössl M, Malyar NM, Rosol M, Beighley PE, Ritman EL. Impact of coronary vasa vasorum functional structure on coronary vessel wall perfusion distribution. Am J Physiol (Heart Circ Physiol) 2003;285:H2019–26. doi: 10.1152/ajpheart.00399.2003. [DOI] [PubMed] [Google Scholar]
- 31.Den Hartog JP. Strength of Materials. New York: Dover Publications, Inc.; 1949. p. 323. [Google Scholar]
- 32.Bear J. Dynamics of Fluids in Porous Media. New York: Dover Publications, Inc.; 1972. p. 764. [Google Scholar]
- 33.Phillips GD, Stone AM, Schultz JC, Whitehead RA, Jones BD, Goodkin ML, et al. Age-related alterations in the morphology of femoral artery vasa vasorum in the rat. Mech Ageing Dev. 1995;82:149–54. doi: 10.1016/0047-6374(95)01604-x. [DOI] [PubMed] [Google Scholar]
- 34.Okuyama K, Yoegashi H, Takahashi T, Sasaki H, Mori S. The three dimensional architecture of vasa vasorum in the wall of the human aorta. A computer aided reconstruction study. Arch Pathol Lab Med. 1988;112:726–30. [PubMed] [Google Scholar]
- 35.Gössl M, Zamir M, Ritman EL. Vasa vasorum growth in the coronary arteries of newborn pigs. Anat Embryol. 2004;208:351–7. doi: 10.1007/s00429-004-0400-7. [DOI] [PubMed] [Google Scholar]
- 36.Considini DM, editor. Van Nostrand’s Scientific Encyclopedia. 5. Van Nostrand Reinhold CY; NY: 1976. p. 2370. [Google Scholar]
- 37.Nakata Y, Shionoya S. An experimental study on the vascular lesions caused by obstruction of vasa vasorum (II). Special consideration on deposition of fat into vascular wall. Japanese Circ J. 1970;269:804–806. doi: 10.1253/jcj.34.1029. [DOI] [PubMed] [Google Scholar]
- 38.Heistad DD. Blood flow through vasa vasorum in arteries and veins: effect of luminal pO2. Am J Physiol (Heart Circ Physiol) 1986;250:H434–42. doi: 10.1152/ajpheart.1986.250.3.H434. [DOI] [PubMed] [Google Scholar]
- 39.Heistad DD, Marcus ML, Law EG, Armstrong ML, Ehrhardt JC, Abboud FM. Regulation of blood flow to the aortic media in dogs. J Clin Invest. 1978;62:133–40. doi: 10.1172/JCI109097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Motwani JG, Topol EJ. Aortocoronary saphenous vein graft disease. Pathogenesis and prevention. Circulation. 1998;97:916–31. doi: 10.1161/01.cir.97.9.916. [DOI] [PubMed] [Google Scholar]
- 41.Davies MG, Hagen P-O. Pathophysiology of vein graft failure: A review. Eur J Vasc Endovasc Surg. 1995;9:7–18. doi: 10.1016/s1078-5884(05)80218-7. [DOI] [PubMed] [Google Scholar]
- 42.Watelet J, Soury P, Menard JF, Plissonnier D, Peillon C, Lestrat JP, et al. Femoropopliteal Bypass: In situ or reversed vein grafts? Ten-year results of a randomized prospective study. Am Vasc Surg. 1997;11:510–19. doi: 10.1007/s100169900083. [DOI] [PubMed] [Google Scholar]
- 43.Dashwood MR, Anand R, Loesch A, Souza DS. Hypothesis: a potential role for the vasa vasorum in the maintenance of vein graft patency. Angiology. 2004;55:385–95. doi: 10.1177/000331970405500405. [DOI] [PubMed] [Google Scholar]
- 44.Tarbell JM. Mass transport in arteries and the localization of atherosclerosis. Ann Rev Biomed Eng. 2003;5:79–118. doi: 10.1146/annurev.bioeng.5.040202.121529. [DOI] [PubMed] [Google Scholar]
- 45.Scotland RS, Vallance PJ, Ahluwalia A. Endogenous factors involved in regulation of tone of arterial vasa vasorum: implications for conduit vessel physiology. Cardiovasc Res. 2000;46:403–11. doi: 10.1016/s0008-6363(00)00023-7. [DOI] [PubMed] [Google Scholar]
- 46.Heistad D, Marcus M, Martin J. Effects of neural stimuli on blood flow through vasa vasorum in dogs. Circ Res. 1979;45:615–20. doi: 10.1161/01.res.45.5.615. [DOI] [PubMed] [Google Scholar]
- 47.Scotland RS, Vallance P, Ahluwalia A. On the regulation of tone in vasa vasorum. Cardiovasc Res. 1999;41:237–45. doi: 10.1016/s0008-6363(98)00223-5. [DOI] [PubMed] [Google Scholar]
- 48.Scotland RS, Vallance PJT, Ahluwalia A. Endothelin alteres the reactivity of vasa vasorum: mechanisms and implications for conduit vessel physiology and pathophysiology. Br J Pharmocol. 1999;128:1229–34. doi: 10.1038/sj.bjp.0702930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nikol S, Pelisek J, Engelmann MG, Shimizu M, Fuchs A, Golda A, et al. Vascular endothelial growth factor (VEGF165) and its influence on angiogenesis versus arteriogenesis in different vascular beds. J Endovasc Ther. 2002;9:842–54. doi: 10.1177/152660280200900619. [DOI] [PubMed] [Google Scholar]
- 50.Nikol S, Engelmann MG, Pelisek J, Fuchs A, Golda A, Shimizu M, et al. Local perivascular application of low amounts of a plasmid encoding for vascular endothelial growth factor (VEGF165) is efficient for therapeutic angiogenesis in pigs. Acta Physiol Scand. 2002;176:151–9. doi: 10.1046/j.1365-201X.2002.01018.x. [DOI] [PubMed] [Google Scholar]
- 51.Galili O, Herrmann J, Woodrum J, Sattler KJ, Lerman LO, Lerman A. Adventitial vasa vasorum heterogeneity among different vascular beds. J Vasc Surg. 2004;40:529–35. doi: 10.1016/j.jvs.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 52.Crawford DW, Back LH, Cole MA. In vivo oxygen transport in the normal rabbit femoral arterial wall. J Clin Invest. 1980;65:1498–508. doi: 10.1172/JCI109815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buerk DG, Goldstick TK. Arterial wall oxygen consumption rates vary spatially. Am J Physiol (Heart Circ Physiol) 1982;243:H948–58. doi: 10.1152/ajpheart.1982.243.6.H948. [DOI] [PubMed] [Google Scholar]
- 54.Schneiderman G, Mockros LF, Goldstick TK. Effect of pulsatility on oxygen transport to the human arterial wall. J Biomechanics. 1982;15:849–58. doi: 10.1016/0021-9290(82)90050-1. [DOI] [PubMed] [Google Scholar]
- 55.Bratzler RL, Chisolm GM, Colten CK, Smith KA, Lees RS. The distribution of low density lipoproteins across the rabbit thoracic aorta in vivo. Atherosclerosis. 1977;28:289–307. doi: 10.1016/0021-9150(77)90177-0. [DOI] [PubMed] [Google Scholar]
- 56.Doyle B, Caplice N. Plaque neovascularization and antiangiogenic therapy for atherosclerosis. J Am Coll Cardiol. 2007;49:2073–80. doi: 10.1016/j.jacc.2007.01.089. [DOI] [PubMed] [Google Scholar]
- 57.Kolodgie FD, Narula J, Yuan C, Burke AP, Finn AV, Virmani R. Elimination of neoangiogenesis for plaque stabilization: is there a role for local drug therapy? J Am Coll Cardiol. 2007;49:2093–101. doi: 10.1016/j.jacc.2006.10.083. [DOI] [PubMed] [Google Scholar]
- 58.Ross R. The pathogenesis of atherosclerosis: An update. N Eng J Med. 1986;314:488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- 59.Goldstick TK, Dobrin PB. Arterial wall oxygen transport and its relationship to atherogenesis. In: Skalak R, Chien S, editors. Handbook of Bioengineering. Vol. 22. New York: McGraw-Hill Book Company; 1987. pp. 1–11. [Google Scholar]
- 60.Shou Y, Jan KM, Rumschitzki DS. Transport in rat vessel walls. I. Hydraulic conductivities of the aorta, pulmonary artery, and inferior vena cava with intact and denuded endothelia. Am J Physiol (Heart Circ Physiol) 2006;291:H2758–71. doi: 10.1152/ajpheart.00610.2005. [DOI] [PubMed] [Google Scholar]
- 61.Liu S-J, Jan K-M, Weinbaum S, Chien S. Transendothelial transport of low density lipoprotein in association with cell mitosis in rat aorta. Arteriosclerosis. 1989;9:230–6. doi: 10.1161/01.atv.9.2.230. [DOI] [PubMed] [Google Scholar]
- 62.Yin Y, Liu K-H, Weinbaum S, Chien S, Rumschitz DS. A model for the initation and growth of extra cellular lipid liposomes in arterial intima. Am J Physiol (Heart Circ Physiol 41) 1997;272:H1033–6. doi: 10.1152/ajpheart.1997.272.2.H1033. [DOI] [PubMed] [Google Scholar]
- 63.Wu C-H, Chi J-C, Jerug J-S, Lin SJ, Jan KM, Wang DL, et al. Transendothelial macromolecular transport in the aorta of spontaneously hypertensive rats. Hypertension. 1990;16:154–61. doi: 10.1161/01.hyp.16.2.154. [DOI] [PubMed] [Google Scholar]
- 64.Simionescu N, Vasile E, Lupu F, Popescu G, Simionescu M. Prelesional events in atherosclerosis. Am J Pathol. 1986;123:109–25. [PMC free article] [PubMed] [Google Scholar]
- 65.Adams CWM, Bayliss OB. The relationship between diffuse intimal thickening, medial enzyme failure and intimal lipid deposition in various human arteries. J Atherosclerosis Res. 1969;10:327–39. doi: 10.1016/s0368-1319(69)80036-0. [DOI] [PubMed] [Google Scholar]
- 66.Hueper WC. Arteriosclerosis: the anoxemia theory. Arch Pathol. 1944;38:162–81. [Google Scholar]
- 67.Hueper WC. Arteriosclerosis: the anoxemia theory. Vasotonia: B Hypertonia followed by constriction anoxemia; agents and Influences producing it. Arch Pathol. 1944;38:245–85. [Google Scholar]
- 68.Hueper WC. Arteriosclerosis: the anoxemia theory. Renal hypertension and essential hypertension. Arch Pathol. 1944;38:350–64. [Google Scholar]
- 69.Getz GS, Vesselinovitch D, Wissler RW. A dynamic pathology of atherosclerosis. Am J Med. 1969;46:657–73. [PubMed] [Google Scholar]
- 70.Morrison AD, Clements RS, Jr, Weingrad AT. Effects of elevated glucose concentrations on the metabolism of the aortic wall. J Clin Invest. 1972;51:3114–23. doi: 10.1172/JCI107138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wilcox JN, Scott NA. Potential role of the adventitia in arteritis and atherosclerosis. Int J Cardiol. 1996;54:S21–S35. doi: 10.1016/s0167-5273(96)02811-2. [DOI] [PubMed] [Google Scholar]
- 72.Barker SG, Beesley JE, Baskerville PA, Martin JF. The influence of the adventitia on the presence of smooth muscle cells and macrophages in the arterial intima. Eur J Vasc Endovasc Surg. 1995;9:222–7. doi: 10.1016/s1078-5884(05)80094-2. [DOI] [PubMed] [Google Scholar]
- 73.Booth RF, Martin JF, Honey AC, Hassall DG, Beesley JE, Moncada S. Rapid development of atherosclerotic lesions in the rabbit carotid artery induced by perivascular manipulation. Atherosclerosis. 1989;76:257–68. doi: 10.1016/0021-9150(89)90109-3. [DOI] [PubMed] [Google Scholar]
- 74.Martin JF, Booth RF, Moncada S. Arterial wall hypoxia following hyperfusion through the vasa vasorum is an initial lesion in atherosclerosis. Eur J Clin Invest. 1990;20:588–92. doi: 10.1111/j.1365-2362.1990.tb01905.x. [DOI] [PubMed] [Google Scholar]
- 75.Zhang Y, Cliff WJ, Schoefl GI, Higgins G. Immunohistochemical study of intimal microvessels in coronary atherosclerosis. Am J Pathol. 1993;143:164–72. [PMC free article] [PubMed] [Google Scholar]
- 76.Langheinrich AC, Michniewiz A, Sedding DG, Walker G, Beighley PE, Rau WS, et al. Correlation of vasa vasorum neovascularization and plaque progression in aortas of apoE-/-/LDL-/- double knockout mice. Arterioscler Throm Vasc Biol. 2006;26:347–52. doi: 10.1161/01.ATV.0000196565.38679.6d. [DOI] [PubMed] [Google Scholar]
- 77.Kumamoto M, Nakashima Y, Sueishi K. Intimal neovascularization in human coronary atherosclerosis: its origin and pathophysiological significance. Hum Pathol. 1995;26:450–6. doi: 10.1016/0046-8177(95)90148-5. [DOI] [PubMed] [Google Scholar]
- 78.Moulton KS, Heller E, Konerding MA, Flynn E, Palinski W, Folkman J. Angiogenesis inhibitors endostatin or TNP-470 reduce intimal neovascularization and plaque growth in apolipoprotein E-deficient mice. Circulation. 1999;99:1726–32. doi: 10.1161/01.cir.99.13.1726. [DOI] [PubMed] [Google Scholar]
- 79.Moulton KS, Vakili K, Zurakowski D, Soliman M, Butterfield C, Sylvin E, et al. Inhibition of plaque neovascularization reduces macrophage accumulation and progression of advanced atherosclerosis. Proc Natl Acad Sci USA. 2003;100:4736–41. doi: 10.1073/pnas.0730843100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gössl M, Versari D, Mannheim D, Lerman LO, Lerman A. Inhibition of vasa vasorum neovascularization prevents vessel wall inflammation and intimal hyperplasia in early atherosclerosis. Circulation. 2006 Oct. 31114(18 Suppl):186. Abstract. [Google Scholar]
- 81.Williams JK, Armstrong ML, Heistad DD. Vasa vasorum in atherosclerotic coronary arteries: responses to vasoactive stimuli and regression of atherosclerosis. Circ Res. 1988;62:515–23. doi: 10.1161/01.res.62.3.515. [DOI] [PubMed] [Google Scholar]
- 82.Wilson SH, Caplice NM, Simari RD, Holmes DR, Jr, Carlson PJ, Lerman A. Activated nuclear factor-kappaB is present in the coronary vasculature in experimental hypercholesterolemia. Atherosclerosis. 2000;148:23–30. doi: 10.1016/s0021-9150(99)00211-7. [DOI] [PubMed] [Google Scholar]
- 83.Matsushita H, Morishita R, Nata T, Aoki M, Nakagami H, Taniyama Y, et al. Hypoxia-induced endothelial apoptosis through nuclear factor-kappaB (NF-kappaB)-mediated bcl-2 suppression: In vivo evidence of the importance of NF-kappaB in endothelial cell regulation. Circ Res. 2000;86:974–81. doi: 10.1161/01.res.86.9.974. [DOI] [PubMed] [Google Scholar]
- 84.Gainer JL. Hypoxia and atherosclerosis: re-evaluation of an old hypothesis. Atherosclerosis. 1987;68:263–6. doi: 10.1016/0021-9150(87)90206-1. [DOI] [PubMed] [Google Scholar]
- 85.Wilson SH, Herrmann J, Lerman LO, Holmes DR, Jr, Napoli C, Ritman EL, et al. Simvastatin preserves the structure of coronary adventitial vasa vasorum in experimental hypercholesterolemia independent of lipid lowering. Circulation. 2002;105:415–8. doi: 10.1161/hc0402.104119. [DOI] [PubMed] [Google Scholar]
- 86.Best PJ, McKenna CJ, Hasdai D, Holmes DR, Jr, Lerman A. Chronic endothelin receptor antagonism preserves coronary endothelial function in experimental hypercholesterolemia. Circulation. 1999;99:1747–52. doi: 10.1161/01.cir.99.13.1747. [DOI] [PubMed] [Google Scholar]
- 87.Steinberg D. Lipoprotein and atherosclerosis. A look back and a look ahead. Arteriosclerosis. 1983;3:283–301. doi: 10.1161/01.atv.3.4.283. [DOI] [PubMed] [Google Scholar]
- 88.Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes (1 & 2) New England J Med. 1992;326:242–50. 310–8. doi: 10.1056/NEJM199201233260406. [DOI] [PubMed] [Google Scholar]
- 89.Busse R, Fleming I. Endothelial dysfunction in atherosclerosis. J Vasc Res. 1996;33:181–94. doi: 10.1159/000159147. [DOI] [PubMed] [Google Scholar]
- 90.Kahlon R, Shapero J, Gotlieb AI. Angiogenesis in atherosclerosis. Can J Cardiol. 1992;8:60–4. Review. [PubMed] [Google Scholar]
- 91.Heistad DD. Blood flow through vasa vasorum of coronary arteries in atherosclerotic monkeys. Arteriosclerosis. 1986;6:326–31. doi: 10.1161/01.atv.6.3.326. [DOI] [PubMed] [Google Scholar]
- 92.Rubin K, Hansson GK, Ronnstrand L, Claesson-Weksesh L, Heldin CH, Terracio L. Induction of B-type receptors for platelet derived growth factor in vascular inflammation: Possible implications for development of vascular proliferative lesions. Lancet. 1988;I:1353–6. doi: 10.1016/s0140-6736(88)92177-0. [DOI] [PubMed] [Google Scholar]
- 93.Nordestgaad BG, Hjelms E, Stender S, Kjeldsen K. Different efflux pathways for high and low density lipoproteins from porcine aortic intima. Arteriosclerosis. 1990;10:477–85. doi: 10.1161/01.atv.10.3.477. [DOI] [PubMed] [Google Scholar]
- 94.Werber AH, Heistad DD. Diffusional support of the thoracic aorta in atherosclerotic monkeys. Atherosclerosis. 1987;68:123–30. doi: 10.1016/0021-9150(87)90102-x. [DOI] [PubMed] [Google Scholar]
- 95.Tufro-McReddie A, Norwood VF, Aylor KW, Botkin SJ, Carey RM, Gomey RA. Oxygen regulates vascular endothelial growth factor-mediated vasculogenesis and tubulogenesis. Dev Biol. 1997;1B3:139–49. doi: 10.1006/dbio.1997.8513. [DOI] [PubMed] [Google Scholar]
- 96.SoRelle R. Two sides of same coin. Stop angiogenesis for cancer and encourage it for coronary artery disease. Circulation. 1998;98:383–4. doi: 10.1161/01.cir.98.5.383. [DOI] [PubMed] [Google Scholar]
- 97.Robert NE, Palade GE. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J Cell Sci. 1995;108:2369–79. doi: 10.1242/jcs.108.6.2369. [DOI] [PubMed] [Google Scholar]
- 98.D’Amato RJ, Loughnan MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc of Nat Acad Sciences USA. 1994;91:4082–5. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bates DO, Harper SJ. Regulation of vascular permeability by vascular endothelial growth factors. Vasc Pharmacol. 2002;39:225–37. doi: 10.1016/s1537-1891(03)00011-9. [DOI] [PubMed] [Google Scholar]
- 100.Nielsen LB. Atherogenecity of lipoprotein (a) and oxidized low density lipoprotein: insight from in vivo studies of arterial wall influx, degradation and efflux. Atherosclerosis. 1999;143:229–43. doi: 10.1016/s0021-9150(99)00064-7. [DOI] [PubMed] [Google Scholar]
- 101.Gössl M, Beighley PE, Malyar NM, Ritman EL. Role of vasa vasorum in transendothelial solute transport in the coronary vessel wall: A study with cryostatic micro-CT. Am J Physiol (Heart Circ Physiol) 2004;287:H2346–51. doi: 10.1152/ajpheart.00066.2004. [DOI] [PubMed] [Google Scholar]
- 102.Kolodgie FD, Gold HK, Burke AP, et al. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med. 2003;349:2316–25. doi: 10.1056/NEJMoa035655. [DOI] [PubMed] [Google Scholar]
- 103.O’Brien KD, McDonald TO, Chait A, Allen MD, Alpers CE. Neovascular expression of E-selectin, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 in human atherosclerosis and their relation to intimal leukocyte content. Circulation. 1996;93:672–82. doi: 10.1161/01.cir.93.4.672. [DOI] [PubMed] [Google Scholar]
- 104.Moreno PR, Purushothaman KR, Fuster V, Echeverri D, Truszczynska H, Sharma SK, et al. Plaque neovascularization is increased in ruptured athero-sclerotic lesions of human aorta: Implications for plaque vulnerability. Circulation. 2004;110:2032–8. doi: 10.1161/01.CIR.0000143233.87854.23. [DOI] [PubMed] [Google Scholar]
- 105.Galili O, Sattler KJ, Herrmann J, Woodrum J, Olson M, Lerman LO, et al. Experimental hypercholesterolemia differentially affects adventitial vasa vasorum and vessel structure of the left internal mammary and coronary arteries. J Thorac Cardiovasc Surg. 2005;129:767–72. doi: 10.1016/j.jtcvs.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 106.Gössl M, Versari D, Mannheim D, Ritman EL, Lerman LO, Lerman A. Increased spatial vasa vasorum density in the proximal LAD in hypercholesterolemia-Implications for vulnerable plaque-development. Atherosclerosis. 2007;192:246–252. doi: 10.1016/j.atherosclerosis.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 107.Rome JJ, Shoyani VF, Lugelman MY, Newman KD, Farb A, Virmani R, et al. Anatomic barriers influence the distribution of in vivo gene transfer into arterial wall. Modeling with microscopic tracer particles and verification with recombinant adenoviral vector. Arterioscler and Thromb. 1994;14:148–61. doi: 10.1161/01.atv.14.1.148. [DOI] [PubMed] [Google Scholar]
- 108.Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, Tulenko TN, et al. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol. 2005;25:2054–61. doi: 10.1161/01.ATV.0000178991.71605.18. [DOI] [PubMed] [Google Scholar]
- 109.Langheinrich AC, Michniewicz A, Sedding DG, Lai B, Jorgensen SM, Bohle RM, et al. Quantitative x-ray imaging of intraplaque hemorrhage in aortas of apoE-/-/LDL-/- double knockout mice. Invest Radiol. 2007;42:263–73. doi: 10.1097/01.rli.0000258085.87952.ea. [DOI] [PubMed] [Google Scholar]


