Abstract
While Bcl-2 plays an important role in cell apoptosis, its relationship to the orphan nuclear receptors remains unclear. Here we report that mouse embryonic fibroblast (MEF) cells prepared from TR4-deficient (TR4−/−) mice are more susceptible to UV-irradiation mediated apoptosis compared to TR4-Wildtype (TR4+/−) littermates. Substantial increasing TR4−/− MEF apoptosis to UV-irradiation was correlated to the down-regulation of Bcl-2 RNA and protein expression and collaterally increased caspase-3 activity. Furthermore, this TR4-induced Bcl-2 gene expression can be suppressed by co-transfection with TR4 coregulators, such as androgen receptor (AR) and receptor-interacting protein 140 (RIP140) in a dose-dependent manner. Together, our results demonstrate that TR4 might function as an apoptosis modulator through induction of Bcl-2 gene expression.
Keywords: TR4, Bcl-2, AR, caspase-3, apoptosis
INTRODUCTION
Members of the nuclear receptor superfamily are transcription factors that regulate gene expression important for growth, differentiation, and development. These nuclear receptors include receptors for steroids, thyroid, vitamin D3, retinoid, and a large number of orphan receptors with unknown ligands[1]. Among the nuclear receptor superfamily, TR2 and TR4 form a unique subfamily of orphan receptors, which is able to regulate the expression of target genes through binding to direct repeat (DR) of AGGTCA core motif with variable numbers of nucleotides [2–6]. TR4 is known to modulate the signaling pathway of various nuclear receptors, such as retinoic acid receptor (RAR), androgen receptor (AR), thyroid receptor (TR), and vitamin D3 receptor (VDR) through competition to DNA binding or inactivation by protein-protein interaction. Previous studies have demonstrated that androgen and AR signal could down-regulate Bcl-2 expression. However, this suppressive effect of androgen and AR in Bcl-2 expression has not been determined yet.
Cell proliferation and apoptosis play an important role in development and cell homeostasis. The proto-oncogene Bcl-2 was first identified by its over-expression in non-Hodgkins B cell lymphoma with chromosomal translocation[7]. Bcl-2 is a membrane-bound protein with a molecular weight of 26 kDa and is located in the nuclear, endoplasmic reticular membranes as well as outer mitochondrial membranes[8]. The anti-apoptotic function of Bcl-2 and the over-expression of Bcl-2 was found in many tumors indicates its oncogenic property[9]. Bcl-2 plays its anti-apoptotic role by maintaining mitochondrial integrity, thereby blocking cytochrome c releasing and serial intrinsic cascade. The consequence of this serial cascade is activation of the downstream effecter, caspase-3, which ultimately leads to cell death[10]. Previous reports showed that UV-induced apoptosis is attributed to activation of caspase-3 activity and is partially due to declination of Bcl-2 amount[11].
Two major promoter regions, P1 and P2, have been identified in the 5′ promoter region of the Bcl-2 gene. P1 is known as the promoter having a predominant role in B cells and many transcription factors, such as cAMP response element-binding protein (CREB), Sp1, and NFκB, have been known to regulate transcription of Bcl-2 via the P1 promoter. The second region, P2, is located about 1.3-kb downstream of the P1 promoter[12], however, the regulation of P2 is relatively unidentified.
Recently, we have established a TR4 knockout (TR4−/−) mouse model to study the physiological roles of TR4. The TR4−/− mice showed several important phenotypes including defects in motor coordination and aberrant cerebellar developments with increased neuronal apoptosis[13]. Furthermore, we found that loss of TR4 resulted in delayed adhesion and growth retardation of mouse embryonic fibroblast (MEF) cells in this study. This prompted us to investigate the cellular function of TR4 in cell apoptosis.
MATERIALS AND METHODS
Preparation of Mouse Embryonic Fibroblasts, UV-irradiation and Cell Culture
Primary TR4+/− and TR4−/− MEFs were isolated from embryonic (E) 14–15 days littermates and cultured in Dulbecco’s modified eagle’s medium (DMEM) plus 10% fetal bovine serum. TR4+/− and TR4−/− mice were housed and studied under the University Committee on Animal Resources–approved protocols in the animal facility of the University of Rochester Medical Center. COS-1 and H1299 cells were cultured in DMEM plus 10% heat-inactivated fetal calf serum. Early passage (2–4) MEFs, COS-1 or H1299 cells were then plated at a density of 5 × 105 cells per 6-cm dish, then 24 h later, cells were treated with UV-irradiation (200 J/m2, for MEF cells, 100 J/m2 for H1299; Stratalinker, Stratagene) or (100 J/m2) combined either with 10-μM ceramide (Sigma) or antisense-Bcl-2 (100 μM; Sigma) for another 24 h. Cell death was determined by flow cytometric analysis, MTT or changes of proteins by immunoblotting.
Plasmids
pCMX-TR4 has been previously described[4]. pEF-receptor-interacting protein 140 (RIP140) and pRC/RSV-CREB, were kindly provided by Dr. M. Parker (Institute of Reproductive and Developmental Biology, Imperial College, UK) and Dr. R. H. Goodman (the Vollum Institute, Oregon Health and Science University, USA), respectively. The chloramphenicol acetyltransferase (CAT) reporter plasmid, which contains Bcl-2 P1 promoter (−2852 bp to −1288 bp), was a gift from Dr. J. C. Reed (The Burnham Institute, USA).
Transient Transfection and CAT Assay
COS-1 and H1299 cells were transfected using a modified calcium phosphate precipitation method[14]. For CAT assays, 4 μg of CAT constructs were co-transfected with pCMX-TR4 or pCMX. β-galactosidase expression vector were used to normalize transfection efficiency in all the experiments.
Immunoblot Analysis
Proteins of total cell extracts (50 μg) were separated on 10% SDS-PAGE and transferred to Immobilin P transfer membrane (Millipore). Bcl-2 was resolved by enhanced chemiluminescence per manufacturer’s instructions (ECL detection system, Amersham) using anti-Bcl-2 monoclonal antbodies (DAKO) and horseradish peroxidase-conjugated secondary antibodies (Santa Cruz).
Determination of Gene Expression
Total RNA was isolated from different tissues using Trizol reagent (Invitrogen), and cDNA was synthesized using SuperScript™ II and random hexamer primers (Invitrogen). Quantitative reverse transcription-polymerase chain reaction (RT-PCR) was performed with iQ™ SYBR green supermix reagent using iCycler real-time PCR amplifier (Bio-Rad) according to the manufacturer’s protocols. Sequences of the primers used for RT-PCR are available upon request.
Detection of Apoptotic Cells
Cells were stained with 7-amino actinomycin D (7-AAD, BD PharMingen) for 30 min on ice. After washing with ice-cold PBS, cells were resuspended in binding buffer at a concentration of 105–106 cells/ml and incubated with 7-AAD (1:1,000 diluted) for 15 min on ice. Without washing, the cells were immediately analyzed. Single staining of 7-AAD was used to determine positive signal. 7-AAD-negative cells were defined as viable, 7-AAD-positive cells as apoptotic. Cells were analyzed by flow cytometric analyses using a dual-laser FACSCalibur flow cytometer (Becton Dickinson).
Caspase-3 activity assay
Cells at 5×105 were resuspended in 1 ml Permeafix (BD PharMingen) and incubated for 40 min at 20°C. The suspension was then centrifuged, and the pellet was washed twice with washing buffer (0.2 mM EDTA, 5% FCS in PBS). Labeling was performed by adding 100 μl washing buffer containing 5 μl polyclonal phycoerythrin–conjugated anti–active caspase-3 antibodies (BD PharMingen). The samples were gently stirred for 1 h, washed in washing buffer, and analyzed by FACS analysis.
RESULTS
Down-regulation of Bcl-2 gene expression in TR4 knockout (TR4−/−) MEF cells increases sensitivity to UV-irradiation
We have generated TR4−/− mice by insertion of an IRES β-gal MCI-Neo selection cassette between exons 4 and 5 of the TR4 gene[13]. The RT-PCR analysis of total RNAs from TR4+/− and TR4−/− MEF cells confirmed deletion of TR4 gene in exons 4 and 5 in homozygous TR4−/− MEF cells (Fig 1a). To investigate whether loss of TR4 affects MEF cell growth, MTT assays were carried out. TR4−/− MEF cells showed significant growth retardation as compared to TR4+/− MEF cells (Fig. 1b) with little influence on basal apoptotic rate (TR4+/−: TR4−/− = 3.38% vs. 2.61%). To determine whether loss of TR4 could affect cell survival upon apoptotic stimuli, we treated the cells with UV-irradiation, a well-known cell apoptotic challenge. TR4−/− MEF cells showed significantly increase in apoptosis upon UV-irradiation compared to TR4+/+ (13.2 ± 2.5% vs. 21.78 ± 0.53%)(Fig. 1c).
FIG. 1. TR4−/− MEF cells show increased sensitivity to UV treatment.
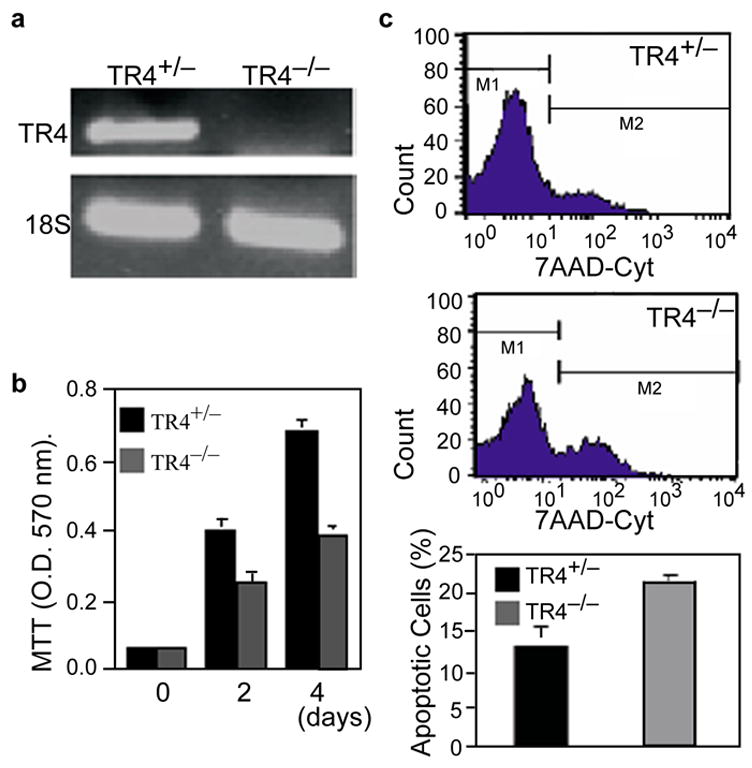
a, RT-PCR analysis of TR4+/− and homozygous TR4−/− MEF cells using specific primer amplifying exon 3 ~ 5 to distinguish TR4+/− and TR4−/−. b, Relative growth rate of TR4+/− and TR4−/− MEF cells. Values are the means ± S.D. of MTT assay readings from three independent wells. c, Flow cytometric measurement of MEF cell death induced by UV (200 J/cm2) as indicated. Cells were stained with 7-AAD and analyzed by FACSCalibur flow cytometer (Upper and middle panel). Live cells were gated as M1 and apoptotic cells were gated as M2 population in readings. Relative Percentage of apoptotic cells are shown in lowest panel as the mean ± S.D. of three individual experiments.
To determine whether TR4 mediated cell apoptosis is linked to Bcl-2 gene expression, we examined Bcl-2 mRNA in TR4+/− and TR4−/− MEF cells derived from same littermates. Q-PCR analysis showed that Bcl-2 mRNA levels in TR4−/− MEF cells were reduced to 50% of those in TR4+/− MEF cells (Fig. 2a). Basal levels of Bcl-2 protein in TR4−/− MEF cells are lower than those of TR4+/− MEF cells (Fig. 2b, lane 1 and 4), while Bax protein levels remain similar (Fig. 2b, middle panel, lane 1 vs. 4). To test if TR4 effects cell apoptosis through the regulation of Bcl-2 expression, we challenged MEF cells with either UV-irradiation (200 J/cm2) or sub-lethal UV-irradiaiton (100 J/cm2) with ceramide (10 μg/ml) co-treatment. After 24 hr treatments, Bcl-2 protein levels in TR4+/− (Fig. 2b, lanes 2 and 3) and TR4−/− (Fig. 2b, lanes 5 and 6) MEF cells were decreased. In addition, we also examined TR4 effect on cell survival upon UV-irradiation. As shown in Suppl. Fig. 1, TR4 partially rescued UV-mediated cell apoptosis and anti-Bcl2 dsDNA diminished the cell survival effect of TR4. Furthermore, Bcl-2 protein level of TR4−/− MEF cells was much lower than that of TR4+/− MEF cells (Fig. 2b, lane 5 and 2; Lane 6 and 3), suggesting that decreased Bcl-2 protein levels may contribute to enhanced apoptosis in TR4−/− MEF cells. Bcl-2 has been shown to inhibit caspase-3 mediated intrinsic pathway, which ultimately leads to cell death. To determine whether down-regulation of Bcl-2 expression in TR4−/− MEF cells causes to caspase activity, we compared caspase-3 activity between TR4+/− and TR4−/− MEF cells after UV-irradiation. As shown in Fig. 2c, TR4−/− MEF cells showed more than 2-fold increase of caspase-3 activity compared to that of TR4+/+ MEF cells, indicating that reduced Bcl-2 protein might result in an increase of caspase-3 activity in TR4−/− MEF cells. Since Bcl-2 mRNA level was reduced in TR4−/− MEF cells, we determined whether TR4 could induce Bcl-2 gene expression through the stimulation of Bcl-2 promoter region. pBcl-2-CAT, the plasmid containing Bcl-2 P1 promoter (−2852 bp to −1288 bp) linked to CAT reporter gene was co-transfected to COS-1 and H1299 cells with TR4 expression vector. As shown in Suppl. Fig. 2a, Bcl-2 promoter activity was up-regulated to around 14 fold in COS-1 and 7 fold in H1299 cells with TR4 transfection. Suppl. Fig. 2b also demonstrated a dose-dependent effect of TR4 on Bcl-2 promoter activity in COS-1 cells. These results strongly suggest that Bcl-2 expression could be directly regulated by TR4.
FIG. 2. Expression of the Bcl-2 gene is reduced in TR4−/− MEF cells.
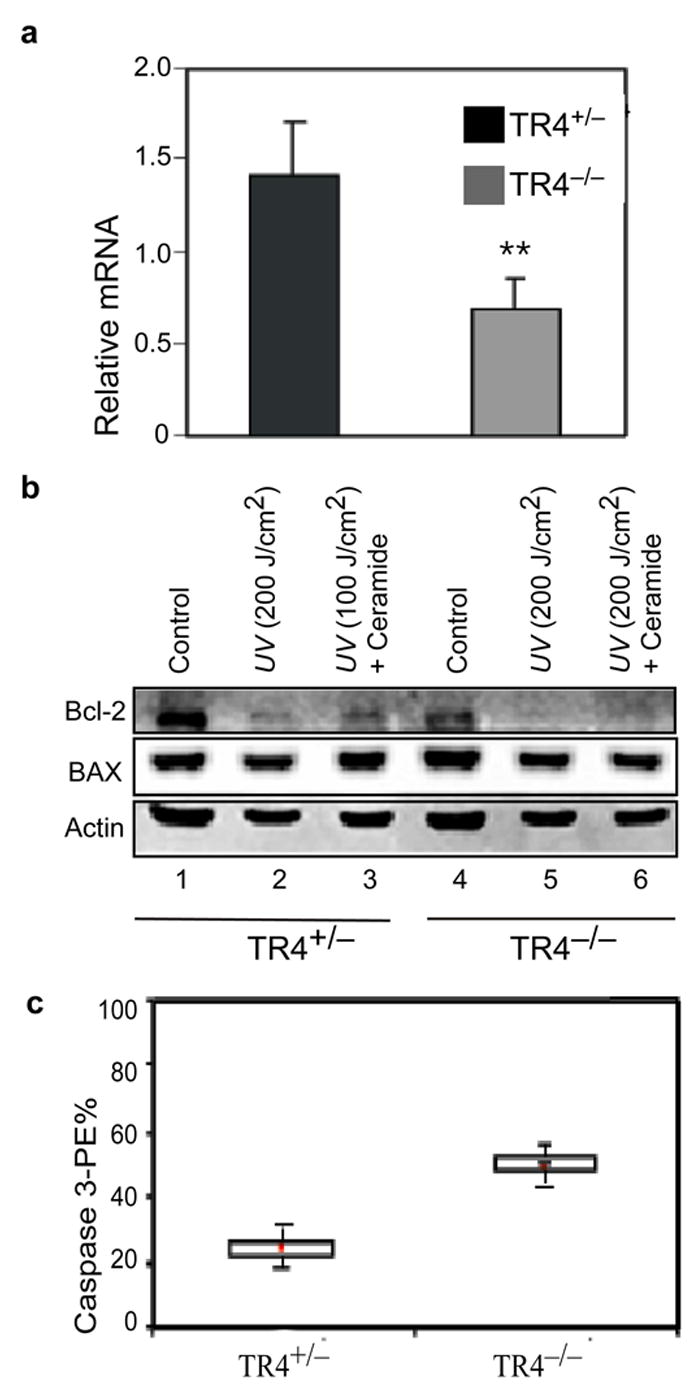
a, Bcl-2 mRNA expression in TR4−/− MEF cells. Data is showed as mean ± S.D. from four individual assays. **, P<0.01 as compared with TR4+/− MEF cells. b, Immunoblot analysis of Bcl-2 and Bax expression in TR4+/− and TR4−/− MEF cells under UV (200 J/m2) or combined treatment of UV (100 J/m2) and ceramide (10 μg/ml). Representative blotting shown here is from 2 individual experiments with similar result. c, Activation of Caspase-3 in UV-treated TR4+/− and TR4−/− MEF cells. Percentage of Caspase-3 positive cells are represented as the values of means ± S.D. from three independent sets of experiments and have reached to significant difference (p<0.05).
To determine whether this TR4 effect on Bcl-2 expression is through direct binding of TR4 to Bcl-2 promoter, we performed gel mobility shift assay. The specific region (−1562 to −1487bp) of Bcl-2 promoter was able to bind TR4 (Fig. 3c). Considering the band appeared only when TR4 and anti-TR4 antibodies were added, and not in the mock lysate with anti-TR4 antibodies or TR4 with unrelated monoclonal antibodies, we strongly believe this band is specific for TR4. We could not find any consensus sequence in this region; therefore, we assumed that there is TR4 binding sites with low affinities and these binding sites may cooperatively contribute to TR4 response. The specific region of −1562 ~ −1487bps were constructed into pGL3-Luciferase vector and examined for promoter activity in COS-1 cells with TR4 co-expression. TR4 can activate this reporter gene (Fig. 3d), suggesting that TR4 regulate Bcl-2 gene expression via direct binding to this promoter region.
FIG. 3. Positive regulation of Bcl-2 expression by TR4.
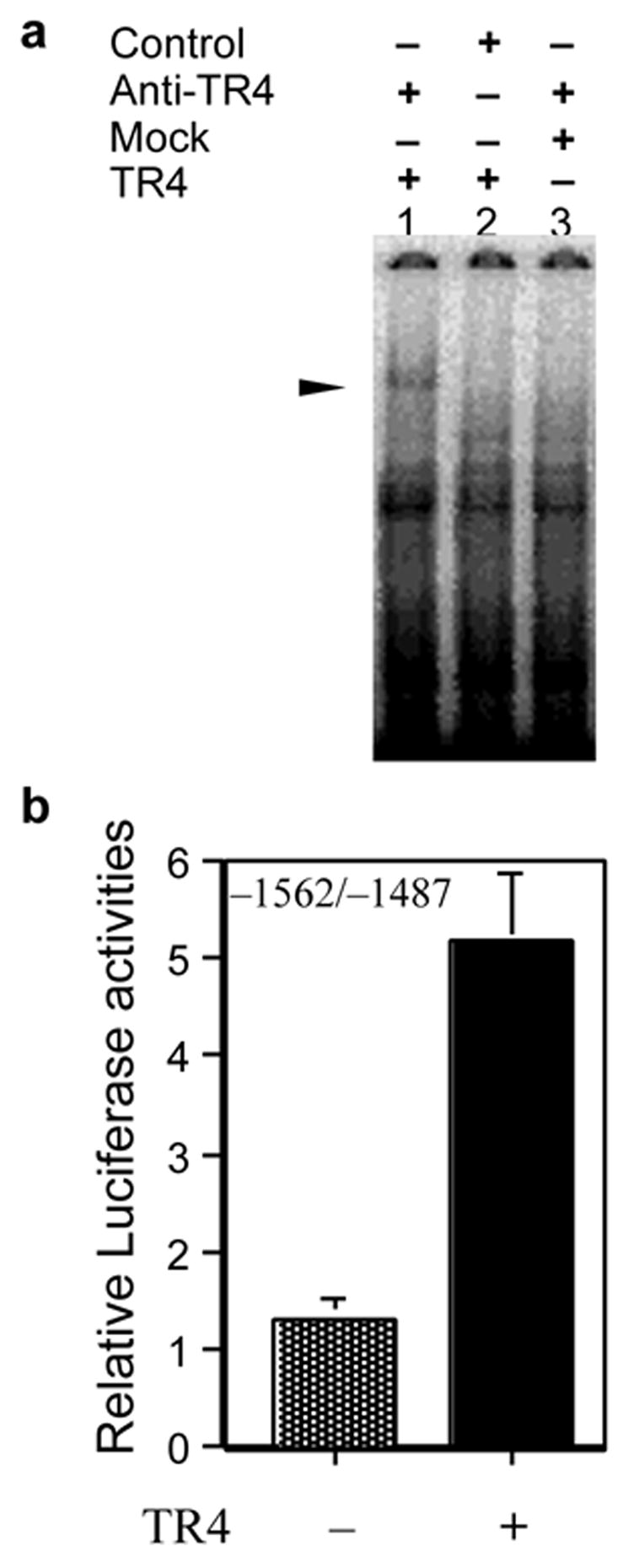
a, Induction of Bcl-2 gene is mediated by TR4 binding to Bcl-2 promoter. 32P-labeled Bcl-2 DNA fragment (−1562~−1487bp) was used in electrophoretic mobility shift assay without or with in vitro translated TR4 (lanes 1 and 2). Supershift of specific complex was induced by the presence of TR4 and anti-TR4 antibodies (lane 3) but was not by mock translated lysate and anti-TR4 antibodies (lane 1) or by TR4 with unrelated monoclonal antibodies (anti-RXR antibodies, lane 2). b, The sequence of −1562 ~ −1487 bp of Bcl-2 promoter is enough for the promoter activation by TR4. The pBcl-2-Luc containing −1562 ~ −1487 region of the Bcl-2 promoter with pCMX-TR4 or pCMX (1μg) was transfected to COS-1 cells. All results of reporter gene assays were expressed as means ± S.D. of three separate experiments.
TR4 coregulators, RIP140 and AR, modulate TR4 transcriptional activity of Bcl-2 promoter
RIP140 has been shown to be a common coregulator for several members of the nuclear receptor superfamily[15, 16]. We co-transfected RIP140, TR4 expression vector and pBcl-2-CAT into COS-1 cells to observe RIP140 effect. As shown in Fig. 4a, RIP140 suppressed TR4-mediated transcriptional activity in a dose-dependent manner. Recently, we reported that TR4 and AR have cross-suppressive effects on their transcriptional activities and this repression may go through TR4-AR heterodimer formation[14]. We were interested in determining whether AR also represses TR4-mediated transcriptional activity of Bcl-2 promoter. As shown in Fig. 4b, in the absence of DHT, basal Bcl-2 promoter activity was not affected by AR. While DHT alone in the absence of AR increased basal promoter activity about 2 fold, cotreatment of DHT and AR reduced Bcl-2 promoter activity to basal level. This may indicate that DHT alone may affect Bcl-2 promoter activity in an AR-independent manner and in the presence of AR, DHT may suppress Bcl-2 expression via AR. Further study will be needed to determine DHT effect in the absence of AR on Bcl-2 expression. We also co-transfected TR4 expression vector into COS-1 cells with different amounts of AR expression vectors and assayed AR effects on TR4-mediated induction of Bcl-2 promoter activity. As shown in Fig. 4b, AR suppressed TR4-induced-Bcl2 promoter activity in a dose-dependent manner under DHT treatment. This is consistent with the previous report and suggests that AR might regulate Bcl-2 expression through suppression of TR4-mediated transcription activity.
FIG. 4. Modulation of TR4-mediated transcriptional activity of Bcl-2 promoter by coregulators.

a, COS-1 cells were co-transfected with 2 μg pBcl-2-CAT and 1.5 μg pCMX-TR4 with increasing amounts of RIP140 (0, 0.25, 0.5, and 1 μg) and appropriate amount of empty vector (1, 0.75, 0.5, 0 μg) as a balance. The relative CAT activities were compared with the CAT activities with empty vector alone (set as 1-fold). b, Suppressive effect of AR on TR4-mediated induction of Bcl-2 promoter activity. COS-1 cells were co-transfected with 2 μg pBcl-2-CAT and 1.5 μg pCMX-TR4 with increasing amounts of pCMV-AR (0, 1.5, 3, and 4.5 μg) and appropriate amount of empty vector (4.5, 3, 1.5 μg) as a balance. After 24 h transfection, the cells were treated with 10 nM DHT. After another 24 h, the cells were harvested for CAT assay. The relative CAT activities were compared with the CAT activities with empty vector alone in the absence of DHT (set as 1-fold). All the promoter CAT assays were from 3 individual sets of experiments and the difference were reached statistical significance using T-test (p<0.05).
DISCUSSION
Loss of TR4 in MEF cells resulted in delayed adhesion, growth retardation, and significant sensitization to apoptosis-inducing agents. Interestingly, the Bcl-2 protein expression is nearly absent in slowly adhering cells[17]. This indicates that TR4 may participate in cell survival via regulation of Bcl-2 level. Previous reports have shown that UV-irradiation causes decline of Bcl-2 protein level and this decline enhances caspase-3 activity leading to cell death[11]. The expression of Bcl-2 was down-regulated in TR4−/−, further, UV treatment decreased Bcl-2 to a even lower level. Recently, enhanced caspase-3 activity was shown to contribute to Bcl-2 degradation TR4−/− as well, which further facilitates cell apoptosis. TR4−/− MEF cells could increase caspase-3 activity after UV-irradiation, suggesting the other mechanism might contribute to down-regulation of Bcl-2.
Global loss of TR4 caused aberrant cerebellar developmental with increased apoptosis in the granule cells[18]. However, Zhang et al. reported that TR4 deficiency resulted in impaired myelination of forebrain neurons with no obvious increase in oligodendrocyte apoptosis[19], indicating that increased apoptosis due to TR4 deficiency may not be a universal phenomena. Thus, TR4 may modulate cell apoptosis in a complex network in different cells although further study is necessary to determine the mechanisms that control TR4 regulation of apoptosis in a cell-specific manner.
It is now well established that nuclear receptors may coordinate with coregulators for the proper and maximal functions[20–22]. Few coregulators were identified as corepressors of nuclear receptor function. Here we show that both RIP140 and AR can suppress TR4 function in Bcl-2 gene expression. Recently, it was reported that RIP140 could be recruited to AF1 domain of TR4 and this interaction is dependent on MAPK-mediated phosphorylation of TR4[23], suggesting that RIP140 may suppress TR4-mediated Bcl-2 expression by inhibiting recruitment of other coactivators via interaction with TR4 AF1 domain. We have demonstrated previously that AR interacts with TR4 to down-regulate TR4-mediated transactivation in the presence or absence of DHT[14]. These two findings support that AR suppresses TR4-mediated regulation of Bcl-2 gene.
Previous studies suggest that the androgen-independent prostate cancer may be the result of de-repression of Bcl-2 expression. This de-repression might be a cause of blocking of apoptosis induced by androgen withdrawal. Despite the suppression role of androgen in Bcl-2 expression, the involvement of AR in Bcl-2 expression has not yet been defined. In contrast to PC-3 cells, LNCaP cells are androgen-responsive and have a higher amount of endogenous AR. PC-3 cells show higher survivability than LNCaP cells in the absence of trophic factors and this higher survivability may be correlated with Bcl-2 expression level[24]. Recently, we compared endogenous TR4 expression levels in prostate cancer cell lines as well as other cancer cell lines[25]. LNCaP and PC-3 cells have high level of endogenous TR4 compared to DU145 cells. This may explain why PC-3 cells have higher endogenous Bcl-2 than LNCaP cells even though there is a similar level of endogenous TR4, suggesting that AR play a suppressive role in TR4-mediated up-regulation of Bcl-2 gene expression.
In summary, our report demonstrated that TR4 modulates cell apoptosis via regulation of Bcl-2 gene expression and caspase-3 activity. Our discovery of the new regulation pathway in apoptosis by TR4 might provide a new foothold toward the clarification of the regulation mechanisms of orphan nuclear receptors in apoptosis.
Supplementary Material
Acknowledgments
We thank Drs. M. Parker, R.H. Goodman, and J.C. Reed for kindly providing plasmids. This work was supported by the National Institutes of Health Grant and a George Whipple Professorship Endowment. The TR4−/− mice were generated in collaboration with Lexicon Genetics, Inc. We also thank K. Wolf for help in manuscript preparation.
Footnotes
Authors’ Disclosure Summary: All authors have nothing to declare
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83:841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 2.Lee HJ, Lee Y, Burbach JP, Chang C. Suppression of gene expression on the simian virus 40 major late promoter by human TR4 orphan receptor. A member of the steroid receptor superfamily. J Biol Chem. 1995;270:30129–30133. doi: 10.1074/jbc.270.50.30129. [DOI] [PubMed] [Google Scholar]
- 3.Lee YF, Pan HJ, Burbach JP, Morkin E, Chang C. Identification of direct repeat 4 as a positive regulatory element for the human TR4 orphan receptor. A modulator for the thyroid hormone target genes. J Biol Chem. 1997;272:12215–12220. doi: 10.1074/jbc.272.18.12215. [DOI] [PubMed] [Google Scholar]
- 4.Lee YF, Young WJ, Burbach JP, Chang C. Negative feedback control of the retinoid-retinoic acid/retinoid × receptor pathway by the human TR4 orphan receptor, a member of the steroid receptor superfamily. J Biol Chem. 1998;273:13437–13443. doi: 10.1074/jbc.273.22.13437. [DOI] [PubMed] [Google Scholar]
- 5.Lee YF, Young WJ, Lin WJ, Shyr CR, Chang C. Differential regulation of direct repeat 3 vitamin D3 and direct repeat 4 thyroid hormone signaling pathways by the human TR4 orphan receptor. J Biol Chem. 1999;274:16198–16205. doi: 10.1074/jbc.274.23.16198. [DOI] [PubMed] [Google Scholar]
- 6.Lin TM, Young WJ, Chang C. Multiple functions of the TR2-11 orphan receptor in modulating activation of two key cis-acting elements involved in the retinoic acid signal transduction system. J Biol Chem. 1995;270:30121–30128. [PubMed] [Google Scholar]
- 7.Tsujimoto Y, Gorham J, Cossman J, Jaffe E, Croce CM. The t(14;18) chromosome translocations involved in B-cell neoplasms result from mistakes in VDJ joining. Science. 1985;229:1390–1393. doi: 10.1126/science.3929382. [DOI] [PubMed] [Google Scholar]
- 8.Hockenbery D, Nunez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- 9.Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med. 1997;3:614–620. doi: 10.1038/nm0697-614. [DOI] [PubMed] [Google Scholar]
- 10.Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 11.Dunkern TR, Fritz G, Kaina B. Ultraviolet light-induced DNA damage triggers apoptosis in nucleotide excision repair-deficient cells via Bcl-2 decline and caspase-3/-8 activation. Oncogene. 2001;20:6026–6038. doi: 10.1038/sj.onc.1204754. [DOI] [PubMed] [Google Scholar]
- 12.Kurland JF, Kodym R, Story MD, Spurgers KB, McDonnell TJ, Meyn RE. NF-kappaB1 (p50) homodimers contribute to transcription of the bcl-2 oncogene. J Biol Chem. 2001;276:45380–45386. doi: 10.1074/jbc.M108294200. [DOI] [PubMed] [Google Scholar]
- 13.Collins LL, Lee YF, Heinlein CA, Liu NC, Chen YT, Shyr CR, Meshul CK, Uno H, Platt KA, Chang C. Growth retardation and abnormal maternal behavior in mice lacking testicular orphan nuclear receptor 4. Proc Natl Acad Sci U S A. 2004;101:15058–15063. doi: 10.1073/pnas.0405700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee YF, Shyr CR, Thin TH, Lin WJ, Chang C. Convergence of two repressors through heterodimer formation of androgen receptor and testicular orphan receptor-4: a unique signaling pathway in the steroid receptor superfamily. Proc Natl Acad Sci U S A. 1999;96:14724–14729. doi: 10.1073/pnas.96.26.14724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cavailles V, Dauvois S, L’Horset F, Lopez G, Hoare S, Kushner PJ, Parker MG. Nuclear factor RIP140 modulates transcriptional activation by the estrogen receptor. Embo J. 1995;14:3741–3751. doi: 10.1002/j.1460-2075.1995.tb00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Treuter E, Albrektsen T, Johansson L, Leers J, Gustafsson JA. A regulatory role for RIP140 in nuclear receptor activation. Mol Endocrinol. 1998;12:864–881. doi: 10.1210/mend.12.6.0123. [DOI] [PubMed] [Google Scholar]
- 17.Tiberio R, Marconi A, Fila C, Fumelli C, Pignatti M, Krajewski S, Giannetti A, Reed JC, Pincelli C. Keratinocytes enriched for stem cells are protected from anoikis via an integrin signaling pathway in a Bcl-2 dependent manner. FEBS Lett. 2002;524:139–144. doi: 10.1016/s0014-5793(02)03040-5. [DOI] [PubMed] [Google Scholar]
- 18.Chen YT, Collins LL, Uno H, Chang C. Deficits in motor coordination with aberrant cerebellar development in mice lacking testicular orphan nuclear receptor 4. Mol Cell Biol. 2005;25:2722–2732. doi: 10.1128/MCB.25.7.2722-2732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Chen YT, Xie S, Wang L, Lee YF, Chang SS, Chang C. Loss of testicular orphan receptor 4 impairs normal myelination in mouse forebrain. Mol Endocrinol. 2007;21:908–920. doi: 10.1210/me.2006-0219. [DOI] [PubMed] [Google Scholar]
- 20.Chen JD, Evans RM. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature. 1995;377:454–457. doi: 10.1038/377454a0. [DOI] [PubMed] [Google Scholar]
- 21.Onate SA, Tsai SY, Tsai MJ, O’Malley BW. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995;270:1354–1357. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- 22.Heinlein CA, Chang C. Androgen receptor (AR) coregulators: an overview. Endocr Rev. 2002;23:175–200. doi: 10.1210/edrv.23.2.0460. [DOI] [PubMed] [Google Scholar]
- 23.Huq MD, Gupta P, Tsai NP, Wei LN. Modulation of testicular receptor 4 activity by mitogen-activated protein kinase-mediated phosphorylation. Mol Cell Proteomics. 2006;5:2072–2082. doi: 10.1074/mcp.M600180-MCP200. [DOI] [PubMed] [Google Scholar]
- 24.Rokhlin OW, Bishop GA, Hostager BS, Waldschmidt TJ, Sidorenko SP, Pavloff N, Kiefer MC, Umansky SR, Glover RA, Cohen MB. Fas-mediated apoptosis in human prostatic carcinoma cell lines. Cancer Res. 1997;57:1758–1768. [PubMed] [Google Scholar]
- 25.Yang Y, Wang X, Dong T, Kim E, Lin WJ, Chang C. Identification of a novel testicular orphan receptor-4 (TR4)-associated protein as repressor for the selective suppression of TR4-mediated transactivation. J Biol Chem. 2003;278:7709–7717. doi: 10.1074/jbc.M207116200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


