Abstract
Background
Previous studies have documented racial disparities in treatment for acute myocardial infarction (AMI) among Medicare beneficiaries. However, the extent to which unobserved differences between hospitals explain some of these differences is unknown.
Objective
To determine whether the observed racial treatment disparities for AMI narrow when analyses account for differences in where blacks and whites are hospitalized.
Research Design
Retrospective observational cohort study using Medicare claims and medical record review.
Subjects
130,709 white and 8,286 black Medicare patients treated in 4,690 hospitals in 50 U.S. states for confirmed AMI in 1994 and 1995.
Measures
Receipt of reperfusion, aspirin, and smoking cessation counseling during hospitalization; prescription of aspirin, angiotensin-converting enzyme inhibitor, and beta-blocker at hospital discharge; receipt of cardiac catheterization, percutaneous coronary intervention (PCI), or bypass surgery (CABG) within 30 days of AMI; 30-day, and 1-year mortality.
Results
Within-hospital analyses narrowed or erased black-white disparities for medical treatments received during the acute hospitalization, widened black-white disparities for follow-up surgical treatments, and augmented the survival advantage among blacks. These findings indicate that, on average, blacks went to hospitals that had lower rates of evidence-based medical treatments, higher rates of cardiac procedures, and worse risk-adjusted mortality after AMI.
Conclusions
Incorporating the hospital effect altered the findings of racial disparity analyses in AMI and explained more of the disparities than race. A policy of targeted hospital-level interventions may be required for success of national efforts to reduce disparities.
Keywords: race & ethnicity, disparities, myocardial infarction, mortality, hospital quality
Inequalities in the treatment of black patients after acute myocardial infarction (AMI) have generated a great deal of clinical, research, and policy interest in recent years. Studies in various clinical populations using both administrative and clinically-detailed registry data indicate that black patients are less likely than white patients to receive angiography, percutaneous coronary interventions (PCI), and coronary artery bypass graft (CABG) 1–9. Blacks also are less likely to receive thrombolytic therapy after AMI 10–12. Fewer studies have explored racial variations in low-intensity treatments such as aspirin and beta-blockers after AMI. Black-white differences in receipt of these treatments vary from large to absent altogether 11, 13–15.
These nationally aggregated findings do not account for the fact that blacks and whites tend to live in segregated regions and use different hospitals. If these hospitals differ in treatment patterns, some of the observed racial disparities may be mediated by a hospital effect rather than by race. Several studies have explored the contribution of differential access and racial disparities. Blacks live in regions with different local practice patterns 16 and use managed care plans 17, hospitals 18–20, cardiac surgeons 21, and primary care providers 22 that differ systematically in quality, practice patterns, and resources.
Yet most of the studies of racial disparities in care after AMI cited above that attempt to control for a hospital effect only go as far as to include hospital-level characteristics in patient-level regressions. This approach seeks to answer the question: if two patients with AMI who differed only in race (one black and one white, but otherwise with the same measured clinical characteristics) went to two different hospitals with the same measured characteristics (e.g., teaching status, AMI volume), would they experience the same care and outcomes? Since blacks and whites may go to different hospitals that vary in a number of unmeasured domains, such as the average time to thrombolytic therapy or angioplasty in minutes, physician quality, and nurse staffing, even rich controls, such as those for teaching status, AMI volume, or presence of a catheterization laboratory, may not account for these omitted variables. Furthermore, utilization of a particular hospital is likely correlated with important omitted socioeconomic variables that are associated with AMI care and survival, and controlling for hospital effect may control for some of these important omitted variables 23. Finally, including hospital-level characteristics in patient-level regressions can incorrectly attribute sources of variance between correlated variables such as a hospital and race and is no longer considered an appropriate modeling technique for hierarchically-organized data 24. Currently advocated approaches include multi-level (hierarchical) modeling or the use of individual hospital fixed effects in patient-level regressions.
We reanalyzed treatment and survival outcomes among black and white patients in the Cooperative Cardiovascular Project (CCP) using both advocated methods to explore whether observed racial disparities after AMI are mediated by race or by provider. Specifically, we sought to answer the question: if a black and white patient with similar clinical characteristics went to the same hospital, were their care and outcomes different?
Methods
Data Collection
The CCP used bills submitted by acute care hospitals (UB-92 claims form data) and contained in the Medicare National Claims History File to identify all Medicare discharges with an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) principal diagnosis of 410 (myocardial infarction), excluding those with a fifth digit of 2, which designates a subsequent episode of care. The study consecutively sampled all Medicare beneficiaries with acute myocardial infarction during a 4–8 month window (depending on the state) between 1994 and 1995 25. The Claims History File does not reliably include bills for all of the approximately 12% of Medicare beneficiaries insured through managed care risk contracts, but the sample was representative of the Medicare fee-for-service (FFS) patient population in the United States in the mid-1990s. After sampling, the CCP collected hospital charts for each patient and sent these to a study center where trained chart abstracters abstracted clinical data. Abstracted information included elements of the medical history, physical examination, and data from laboratory and diagnostic testing, in addition to documentation of administered treatments. The CCP monitored the reliability of the data by monthly random reabstractions. Details of data collection and quality control have been reported previously 26. We supplemented the abstracted clinical data with diagnosis and procedure codes extracted from Medicare billing records and dates of death from the Medicare Enrollment Database.
For our analyses, we selected only those patients in the CCP sample with confirmed AMI and who were black or white, excluding those with other or unknown race. We transformed continuous physiologic variables into categorical variables (e.g., systolic BP < 100 mm Hg or ≥ 100 mm Hg, creatinine <1.5, 1.5–2.0 or >2.0 mg/dL). We used date of death to identify patients who did or did not survive through 30 days and 1 year after the AMI. We used two different mortality measures because 30-day mortality may reflect the effectiveness of acute treatments during the AMI hospitalization and 30-day procedure-associated mortality, whereas 1-year mortality may reflect the effectiveness of procedures and compliance with follow-up medical treatments. For all patients, we identified whether they received each of 6 treatments during the index admission: reperfusion (defined as either thrombolysis or PCI within 12 hours of arrival at the hospital), aspirin during hospitalization, aspirin at discharge, angiotensin-converting enzyme (ACE) inhibitors at discharge, beta-blockers at discharge, smoking cessation counseling, and each of 3 treatments that occurred within 30-days of the AMI: cardiac catheterization, PCI, or CABG. We conceptualized reperfusion, aspirin, beta-blockers, ACE inhibitors, and smoking cessation counseling as “medical” treatments (88% of reperfusions were thrombolysis) and 30-day catheterization, PCI, and CABG as “surgical.” These also differ in that the medical treatments are lower intensity and under the direct purview of the admitting hospital whereas the surgical treatments are higher intensity and may rely upon follow-up or referral and thus may not be strictly controlled by the admitting hospital.
We used CCP quality criteria to identify patients for whom reperfusion, aspirin, ACE inhibitors, beta-blockers, and smoking cessation counseling were “ideal” (e.g., best practice) 26, 27, and we used the ACC/AHA guidelines 28 for coronary angiography to identify patients who were ideal (Class I), uncertain (Class II), or inappropriate (Class III) for angiography. Because angiography is a prerequisite for PCI or CABG, we assumed that if patients were Class III for angiography, then they were inappropriate candidates for these downstream procedures.
Statistical analysis
We categorized patients into four subgroups according to race and sex: white men, white women, black men, and black women, and compared their demographic and clinical characteristics. We explored the distribution of black and white AMI among the 4,690 hospitals in our sample. We tested the hypothesis that hospital may mediate the observed relationship between race and treatment and outcomes after AMI using elements of Baron and Kenny’s three-stage regression procedure 29. We then performed two types of statistical analyses for each of the 9 treatments and 2 mortality measures: logistic regression models for the computation of odds ratios and ordinary least squares linear probability models (corrected for heteroscedasticity of unknown form) for computation of the adjusted marginal probability of each outcome if a patient was black instead of white 30. We performed separate analyses for men and for women because we found an interaction between race and sex. For all analyses of treatment, only patients who were eligible for that treatment were included in the regression (e.g., only current smokers are eligible for cessation counseling). For catheterization, PCI, and CABG, we restricted the analysis to ACC/AHA Class I and Class II patients, excluding Class III patients for whom catheterization and downstream interventions would have been considered inappropriate. We retained all patients in our analyses for reperfusion, aspirin, ACE inhibitor, and beta-blocker treatment because patients for whom some treatments were once believed controversial or contraindicated may be reasonable candidates for treatment 31, but included whether the patient was ideal for the treatment as a control variable.
We used three different specifications for both the logistic and linear probability models, age- and race-adjustment (age/race), age-, race-, and clinical condition-adjustment (age/race/clinical), and age-, race-, clinical-, and specific hospital-adjustment (age/race/clinical/hospital). The characteristics used for clinical adjustment are those listed in Table 1. For the logistic regressions in the age/race and age/race/clinical models, we used generalized estimating equations (GEE) 32 with hospital entered as the clustering variable. GEE allows for unobserved patient-level or hospital-level factors that were omitted from the model and which systematically raise or lower utilization or mortality of all patients in that hospital, and thereby corrects the standard errors for any resulting within-hospital correlation (clustering) in patient outcomes.
Table 1.
Demographic, Clinical, Treatment and Outcomes of the Patients, by Sex and Race
| Characteristic | Men | Women | ||||
|---|---|---|---|---|---|---|
|
White
(N=66,762) |
Black
(N=3,411) |
P-value |
White
(N=63,947) |
Black
(N=4,875) |
P-value | |
| Demographics and clinical history | ||||||
| Mean (± se) age (yr) | 75.3 (± 0.03) | 74.6 (± 0.12) | < 0.0001 | 78.2 (± 0.03) | 76.3 (± 0.12) | < 0.0001 |
| Diabetes (%) | 27.6 | 33.8 | < 0.0001 | 31.8 | 47.9 | < 0.0001 |
| Hypertension (%) | 54.7 | 73.2 | < 0.0001 | 66.6 | 84.0 | < 0.0001 |
| Current smoking (%) | 15.4 | 25.2 | < 0.0001 | 13.3 | 12.7 | 0.31 |
| Prior myocardial infarction (%) | 33.3 | 31.0 | 0.005 | 25.4 | 27.7 | 0.0007 |
| Prior heart failure (%) | 18.4 | 23.6 | < 0.0001 | 24.6 | 29.7 | < 0.0001 |
| Prior revascularization† (%) | 22.5 | 11.6 | < 0.0001 | 12.2 | 8.8 | < 0.0001 |
| Prior peripheral vascular disease | 11.4 | 12.2 | 0.12 | 9.2 | 12.8 | < 0.0001 |
| Dementia (%) | 4.7 | 7.2 | < 0.0001 | 7.5 | 9.1 | < 0.0001 |
| Limited mobility (%) | 13.9 | 21.0 | < 0.0001 | 23.7 | 29.8 | < 0.0001 |
| Admitted from nursing facility (%) | 3.3 | 5.2 | < 0.0001 | 8.4 | 6.8 | 0.0002 |
| Systolic blood pressure < 100 (%) | 3.6 | 3.8 | 0.38 | 4.1 | 3.5 | 0.02 |
| Cardiogenic shock (%) | 2.2 | 2.0 | 0.23 | 2.4 | 2.0 | 0.08 |
| Received CPR (%) | 3.9 | 4.0 | 0.72 | 3.0 | 4.3 | < 0.0001 |
| Complete heart block (%) | 17.2 | 14.4 | < 0.0001 | 14.6 | 13.8 | 0.14 |
| Congestive heart failure (%) | 25.7 | 30.4 | < 0.0001 | 31.3 | 35.1 | < 0.0001 |
| MI location | ||||||
| Anterior (%) | 30.0 | 32.2 | 0.005 | 31.6 | 30.0 | 0.03 |
| Inferior (%) | 21.0 | 17.6 | < 0.0001 | 18.2 | 14.7 | < 0.0001 |
| Creatinine > 2.0 mg/dL (%) | 12.2 | 21.0 | < 0.0001 | 8.2 | 18.2 | < 0.0001 |
| Albumin < 3.0 mg/dL (%) | 3.8 | 5.8 | < 0.0001 | 5.1 | 7.6 | < 0.0001 |
| Hematocrit < 0.30 (%) | 3.8 | 7.3 | < 0.0001 | 5.3 | 10.3 | < 0.0001 |
| Treatment (eligible* population) | ||||||
| Reperfusion (N=44,921), % | 42.8 | 36.0 | <0.0001 | 35.8 | 30.2 | <0.0001 |
| Aspirin during hospitalization (N=138,629), % | 80.2 | 79.6 | 0.856 | 76.3 | 76.1 | 0.416 |
| Aspirin upon discharge (N=87,365), % | 70.4 | 69.8 | 0.543 | 65.7 | 67.1 | 0.084 |
| ACE upon discharge (N=21,794), % | 56.0 | 59.9 | 0.075 | 60.3 | 63.4 | 0.004 |
| β-blocker upon discharge (N=87,365), % | 39.5 | 36.4 | 0.03 | 37.5 | 35.6 | 0.002 |
| Smoking cessation counseling (N=20,264), % | 33.0 | 27.0 | 0.007 | 32.1 | 27.1 | <0.0001 |
| Catheterization within 30 days (N=121,476), % | 57.4 | 45.8 | <0.0001 | 45.7 | 40.6 | <0.0001 |
| PCI within 30 days (N=121,476), % | 21.4 | 14.3 | <0.0001 | 18.7 | 12.7 | <0.0001 |
| Bypass surgery within 30 days (N=121,476), % | 18.5 | 11.5 | <0.0001 | 11.9 | 8.7 | <0.0001 |
| Outcome | ||||||
| 30-day mortality, % | 17.2 | 15.3 | 0.003 | 20.7 | 18.3 | < 0.0001 |
| 1-year mortality, % | 30.4 | 31.0 | 0.423 | 34.9 | 34.7 | 0.722 |
History of percutaneous coronary intervention or coronary artery bypass.
See Appendix Table 1 for eligibility criteria for CCP quality measures; for catheterization, PCI, and CABG, eligible patients were those patients in ACC/AHA Class I and Class II.
The GEE approach for statistical adjustment for patient clustering used in the age/race/clinical models assumes all unobserved hospital-level factors affecting treatment are unrelated to patient characteristics such as race. This assumption would not be true, however, if blacks were systematically admitted to hospitals that provided different rates of treatment. For example, if blacks were cared for at hospitals that under-provide treatment to all patients (independent of race), then the GEE method would inadvertently attribute lower rates of treatment to the race of the patient rather than to the hospital providing care. The age/race/clinical/hospital models addressed this limitation by including all of the same risk-adjusters in a logistic model with a fixed effect for hospital, allowing a separate intercept for all 4,690 hospitals in our sample 33. These fixed-effect models control for any hospital-level factor that affects the treatment of all patients, so that any remaining estimated racial disparity reflects within-hospital differences in the treatment of black and white patients.
We performed all computations with Stata statistical software (version 8.2, Stata Corporation, College Station, TX). The Committee for the Protection of Human Subjects at Dartmouth College approved the study. We had complete independence from the funding agency in the design, conduct, and reporting of this study.
Results
Demographic and Clinical Characteristics
The study cohort included 130,709 white and 8,286 black Medicare patients treated for confirmed AMI in 4,690 hospitals. Among both men and women, black patients generally were younger, yet had a higher prevalence of chronic disease, smoking, functional impairment, and nursing home residence (Table 1). Fewer black than white patients had undergone prior revascularization. Upon admission to the hospital, black and white men had comparable rates of hypotension, cardiogenic shock, and receipt of cardiopulmonary resuscitation (CPR), but white men had a higher rate of complete heart block and black men had a higher rate of heart failure (Table 1). White women were more likely than black women to have been hypotensive or in cardiogenic shock, although more black women had received CPR upon admission and had a higher rate of heart failure. Both black men and black women had higher rates of renal insufficiency, low serum albumin, and anemia than whites. All statistically significant differences also are clinically meaningful differences.
Relationship of Race to Hospital
Eighty five percent of all black AMI patients were admitted to 1,000 hospitals; only 40% of all white AMI patients were treated at these same hospitals. Most hospitals (n=2,691) treated no black AMI patients but treated 40% of all white AMI patients (Figure 1). Thus, race affects which hospital treats an AMI patient. Furthermore, the coefficient estimate of race is lessened in models that include hospital for all medical treatments under the direct purview of the admitting hospital, suggesting that hospital is a mediator of the observed racial disparity in these treatments (discussed below) 29.
Figure 1. Cumulative proportion of black and white AMI patients treated by all of the hospitals in the CCP sample.
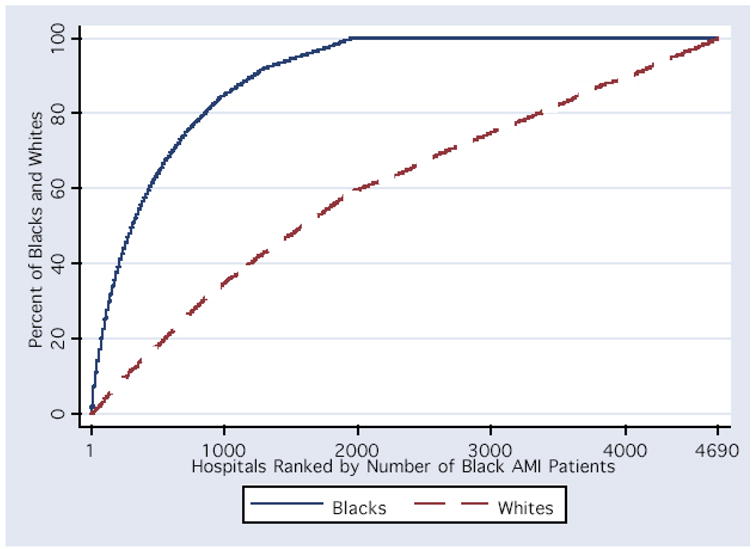
The graph depicts the cumulative distribution function for black and white AMI patients among hospitals 1-4690. Each of the hospitals in the CCP sample are arrayed on the x-axis from highest to lowest by the proportion of all black AMI patients treated at the hospital.
Treatment
Black patients had lower crude treatment rates than whites for all measures except aspirin and ACE inhibitors (Table 1). We present the odds ratios (ORs) for the medical (Figures 2 and 3) and surgical (Figure 4) treatments among black patients compared to whites for each of the three model specifications. The confidence intervals for each of the three models overlap for all treatments; however, for the purposes of this analysis, we focus on the pattern of change in the point estimate as we move from the model with the least to the most sophisticated statistical adjustment. For both the medical and surgical treatments, clinical risk-adjustment narrowed the apparent differences between black and white treatment rates, consistent with our understanding that some of the observed treatment differences are confounded by illness severity and appropriateness for treatment.
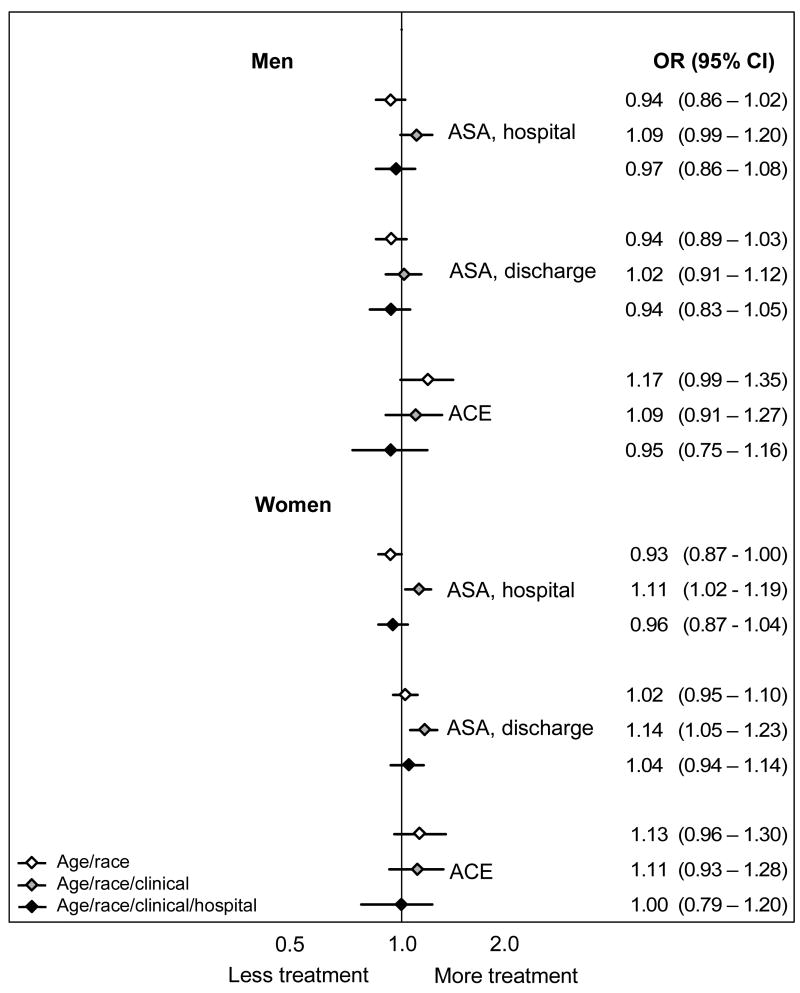
Figure 2. Odds ratios for receipt of medical treatments by black patients during the initial hospitalization.
For each treatment the odds ratios and 95% confidence intervals reflect estimates for treatment receipt among blacks compared to whites using models that sequentially add groups of independent variables. The first model adjusts for age, the second model adjusts for age and clinical conditions (condition upon AMI admission, co-morbid conditions, and whether the patient was an ideal candidate for the treatment), and the third model adjusts for age, clinical conditions, and the specific admitting hospital. ASA=aspirin; ACE=angiotensin converting enzyme inhibitor
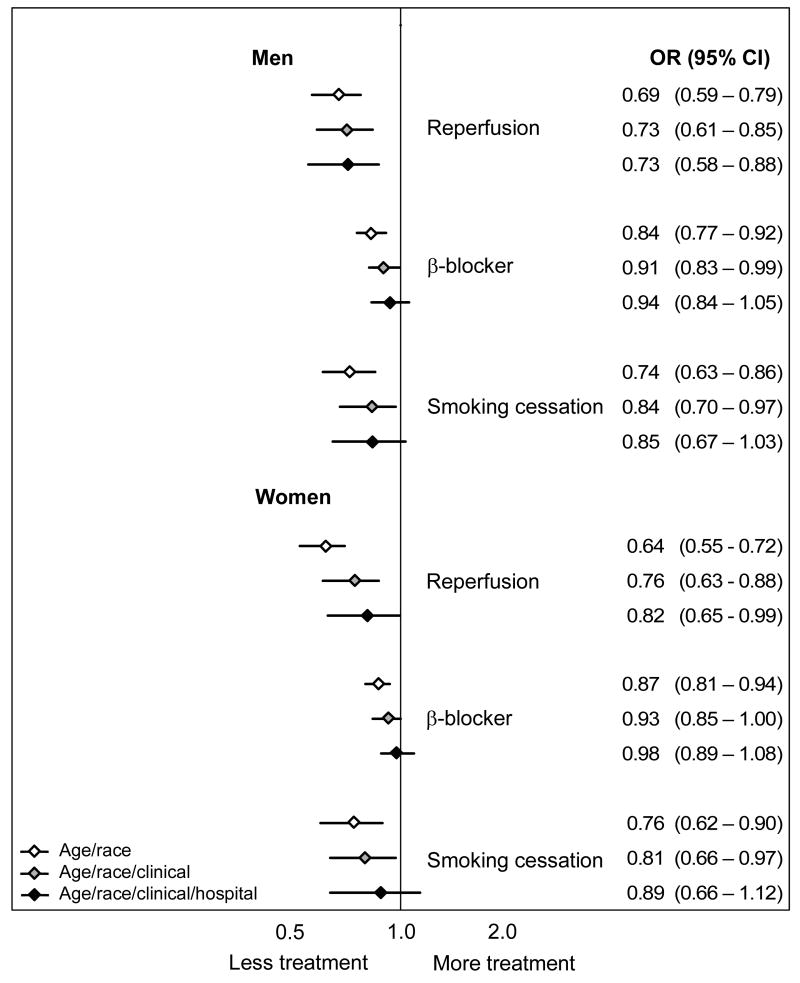
Figure 3. Odds ratios for receipt of medical treatments by black patients during the initial hospitalization.
For each treatment the odds ratios and 95% confidence intervals reflect estimates for treatment receipt among blacks compared to whites using models that sequentially add groups of independent variables. The first model adjusts for age, the second model adjusts for age and clinical conditions (condition upon AMI admission, co-morbid conditions, and whether the patient was an ideal candidate for the treatment), and the third model adjusts for age, clinical conditions, and the specific admitting hospital.
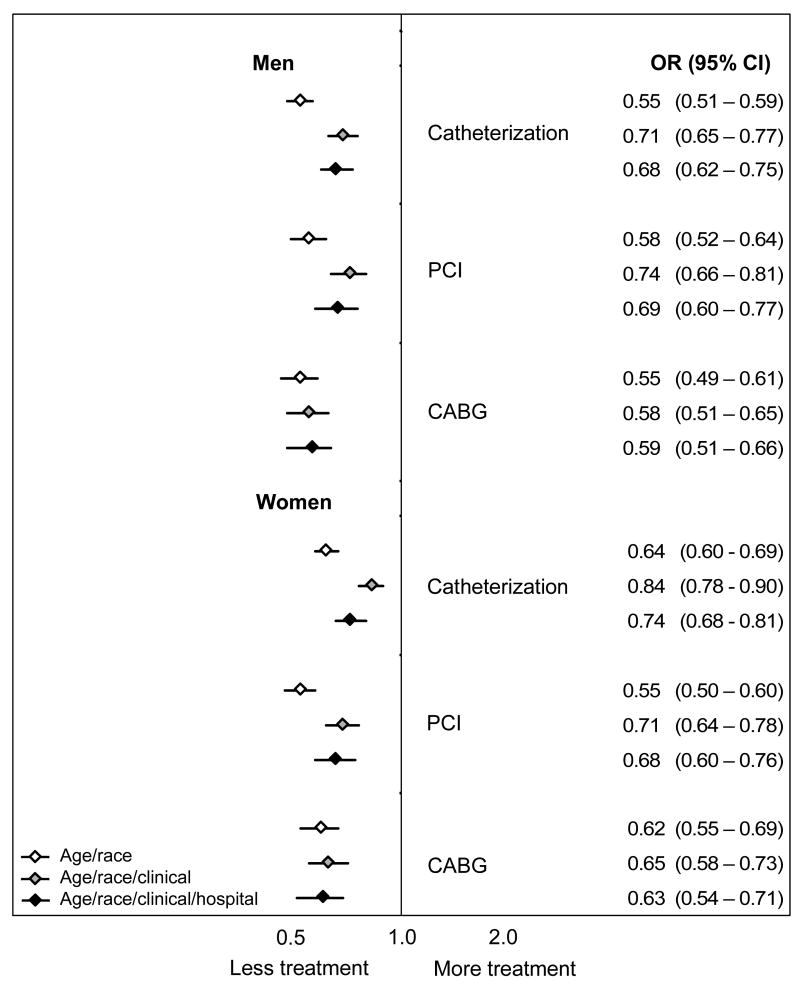
Figure 4. Odds ratios for receipt of surgical treatments by black patients during the 30 days after AMI.
For each treatment the odds ratios and 95% confidence intervals reflect estimates for treatment receipt among blacks compared to whites using models that sequentially add groups of independent variables. The first model adjusts for age, the second model adjusts for age and clinical conditions (condition upon AMI admission and co-morbid conditions) and the third model adjusts for age, clinical conditions, and the specific admitting hospital.
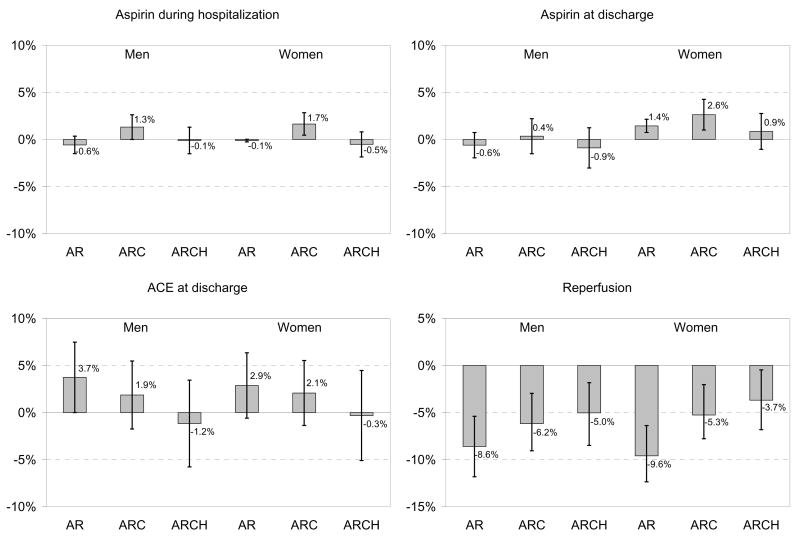
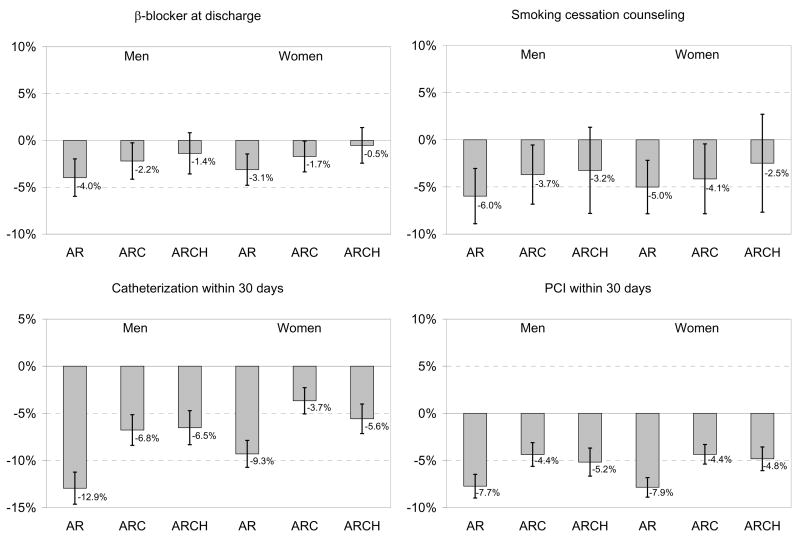
For the medical treatments for which risk-adjusted disparities persisted (Figure 3), the addition of the hospital fixed effect further narrowed the treatment gap, and in the case of beta-blockers and smoking cessation counseling, erased it altogether. This indicates that for these measures blacks and whites in the same hospital were treated more similarly than would have been assumed based on aggregate risk-adjusted data. For example, based on age-adjusted estimates, black women had an absolute 9.6% lower rate of reperfusion after AMI (95% CI: −12.4 to −6.8%) than white women. Risk-adjustment reduced this disparity to −5.3% (95% CI: −7.8 to −2.7%), and hospital-adjustment further reduced it to −3.7% (95% CI: −6.8 to −0.5%). To reconcile the fact that we observe a marked disparity in reperfusion in the aggregate risk-adjusted data with the finding of smaller disparities for similar patients treated in the same hospital, we must conclude that black women, on average, went to hospitals with lower rates of reperfusion among both blacks and whites.
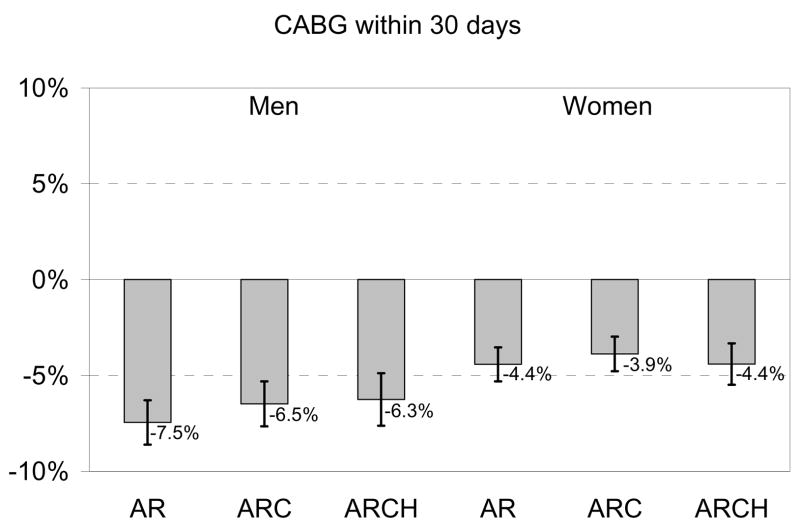
In contrast, the addition of the hospital effect either did not change or widened the gap for surgical treatments. This indicates that blacks received fewer surgical treatments than whites admitted to the same hospital, and this disparity was even larger than would have been assumed based on aggregate risk-adjusted data. For example, based on age-adjusted estimates, black women had an absolute 9.3% lower 30-day rate of catheterization (95% CI: −10.7 to −7.9%). Risk-adjustment reduced this disparity to -3.7% (95% CI: −5.1 to −2.3%), but the addition of the hospital effect widened the disparity; among women treated at the same hospital, blacks had a 5.6% absolute lower 30-day rate of catheterization (95% CI: −7.1 to −4.0). Thus, we must conclude that black women, on average, went to hospitals with higher rates of 30-day catheterization among both blacks and whites.
For detailed figures regarding absolute rate differences, see Appendix Figure 1.
Appendix Figure 1. Black-White Treatment Disparity.
Treatment Disparity. For each treatment, we present the marginal probability of receiving the treatment if black compared to white, all other things being equal. This is the absolute percentage point difference, with 95% confidence intervals, in treatment receipt among whites compared to blacks. AR = models including only age and race; ARC = models including age, race, and clinical condition, including being an ideal candidate for therapy (CCP quality indicators only); ARCH = models including age, race, clinical condition, being an ideal candidate, and hospital.
Mortality
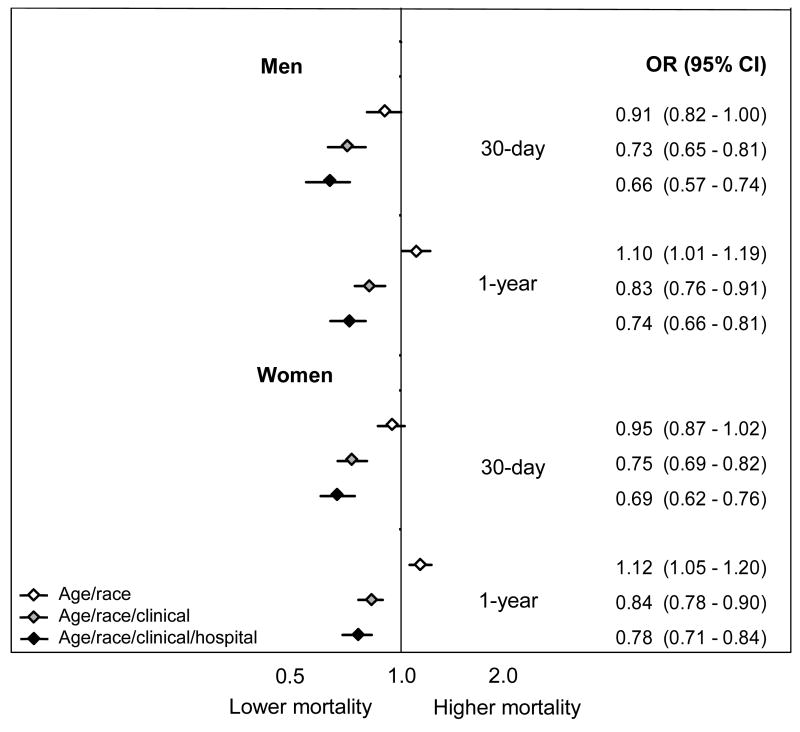
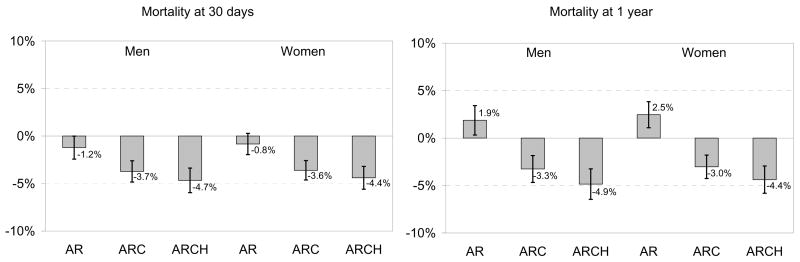
Black patients had equivalent or lower crude mortality rates than whites after AMI (Table 1). We present ORs for death among black patients for each of the three models (Figure 5). Risk-adjustment augmented the survival benefit among blacks; that is, blacks had lower mortality than would be expected by their illness severity. The addition of the hospital fixed effect further augmented the survival benefit, suggesting that black patients had a lower death rate after AMI than equivalent white patients treated at the same hospital. For example, based on age-adjusted estimates, black women have the same 30-day mortality as white women. Based on risk-adjusted estimates, however, black women had an absolute 3.5% lower 30-day mortality rate (95% CI: −4.6 to −2.6%), and among women admitted to the same hospital for their AMI, black women had an absolute 4.4% lower 30-day mortality rate (95% CI: -6.6 to -3.2%). Both 30-day and 1-year mortality follow identical patterns. Thus, we must conclude that black women, on average, went to hospitals with worse risk-adjusted survival after AMI among both blacks and whites.
Figure 5. Odds ratios for death among black patients.
For each treatment the odds ratios and 95% confidence intervals reflect estimates for the odds of death among blacks compared to whites using models that sequentially add groups of independent variables. The first model adjusts for age, the second model adjusts for age and clinical conditions (condition upon AMI admission and co-morbid conditions), and the third model adjusts for age, clinical conditions, and the specific admitting hospital.
For detailed figures regarding absolute mortality rate differences, see Appendix Figure 2.
Appendix Figure 2. Black-White Mortality.
Mortality Disparity. We present the marginal probability of death at 1 day, 30-days, and 1 year if a patient is black compared to white, all other things being equal. This is the absolute percentage point difference, with 95% confidence intervals, in mortality among whites compared to blacks; thus a negative difference suggests that blacks have lower mortality than whites. AR = models including only age and race; ARC = models including age, race, and clinical condition, including being an ideal candidate for therapy (CCP quality indicators only); ARCH = models including age, race, clinical condition, being an ideal candidate, and hospital.
Discussion
The initiatives to reduce racial disparities in health care utilization and outcomes have been motivated by observed treatment differences from nationally-aggregated data. Current policies, fueled by highly publicized evidence of physician discrimination in referral patterns 34, call upon providers to treat blacks and whites equally. However, there has been less attention paid to the way that systematic differences in environments, access, and provider quality might influence these disparities 17-20. In this paper we show that because blacks and whites tend to go to different hospitals for AMI care, unobserved differences across hospitals may play a role in observed racial disparities. These differences may be hospital practice or “quality” effects, proxies for local socioeconomic effects, or both. By using a method that allowed us to compare the treatment of black and white patients admitted to the same hospital, we demonstrated that for lower intensity medical treatments under the immediate purview of the admitting hospital, within-hospital racial disparities were smaller than aggregated estimates or absent altogether. In contrast, for 30-day high-intensity cardiac procedures that require follow-up and may not be under the direct control of the admitting hospital, within-hospital disparities were in some cases even larger than aggregate risk-adjusted estimates. Blacks’ within-hospital risk-adjusted survival advantage after AMI was larger than aggregate risk-adjusted estimates.
Our analysis of the same data used by previous researchers to explore racial disparities in AMI treatment took the analysis two steps further than most 6, 8, 12-15, 35 and one step further than more recent work 36 by adjusting for unobserved similarities that may exist among patients treated by the same hospital and for individual hospital effects that may actually correlate with race. First, our risk-adjusted model followed recent trends in health services research methods encouraging adjustments for provider-level clustering 24, 37–39. Specifically, this model adjusted the standard errors on patient-level regression coefficients to account for the fact that the patients treated by particular hospitals may be more similar in measured and unmeasured characteristics than patients treated at different hospitals. This approach is superior to simple logistic regression models that do not include any information about provider or that enter summary hospital characteristics in patient-level regressions. However, the model relies upon the assumption that the distribution of similar patients into one hospital versus another is independent of hospital characteristics. This assumption would be violated if patient characteristics systematically varied by hospital type. We hypothesized that blacks may systematically be admitted to lower quality hospitals. To address this hypothesis, our risk- and hospital-adjusted fixed-effects model allowed for patient characteristics to be correlated with hospital. Furthermore, unlike specifications that assume the systematic differences in hospitals that blacks and whites use are a linear function of the percent of white patients in the hospital 20, 40, our hospital fixed-effect model is not so constrained. This is important if all observable and unobservable measures of hospital quality are not summarized by percent white. For example, one can imagine that rural community hospitals in Appalachia that see mostly white AMI patients may have quality and resource limitations that are similar to rural community hospitals in the South that see mostly black AMI patients.
Although our analyses provide different quantitative conclusions regarding disparities by focusing at the hospital level, where actual treatment decisions are made, we emphasize that we are not making an effort to “explain away” racial treatment disparities for AMI or other conditions. The crude national figures prove that they exist. Rather, we are making an effort to better understand these disparities, and, in so doing, focus potential interventions. For example, because the disparity between blacks and whites decreased for lower intensity medical treatments with hospital adjustment, we can conclude that blacks, on average, went to hospitals that provided less of this evidence-based care. Because the disparity for 30-day cardiac procedures increased, this means that blacks, on average, went to hospitals that provided more of these services. Using hospitals with lower compliance with evidence-based medical treatments may increase mortality risk, but using hospitals with higher rates of invasive cardiac procedures (if they are used for patients with appropriate indications for treatment) may be protective. Thus, these indicators of AMI treatment suggest that blacks on average went to hospitals with lower quality medical treatment but higher quality surgical treatment; a more complex picture than we initially hypothesized. Regardless, though, if risk-adjusted mortality is the ultimate quality measure of interest, our data suggest that blacks went to lower-quality hospitals because blacks’ survival advantage would be even larger if they went to the same hospitals as whites. Indeed, if the 8,286 black patients in this cohort had been treated at the same hospitals in the same proportions as their white counterparts, 55 fewer men and 68 fewer women would have died by 1 year after their AMI. Initiatives targeted at hospitals that disproportionately serve black patients could simultaneously address quality deficiencies for all patients in the hospital and potentially decrease national health care disparities. Furthermore, by focusing at the hospital level, researchers might explore mediators of decreased surgical treatment rates among blacks and try to explain the paradoxical risk-adjusted medium-term survival advantage among blacks.
The current study has strengths as well as limitations. The primary strength is that it is based upon the CCP database, which offers a nationally-representative sample with rich clinical data for use in risk-adjustment models and information regarding prescription drugs. Limitations include the age of the data, which prohibits generalization to current practice, although it does allow direct comparison to other studies using the same data, and the fact that younger patients are not included in the cohort. Also, we cannot discern whether the hospital effect is a “quality” effect, a socioeconomic effect, or both. Our analysis only distinguishes whether a given provider appeared to treat blacks and whites differently (potential provider discrimination), but it does not address larger issues of cultural discrimination that lead to residential segregation and differential access to high quality hospitals. Importantly, our analysis doesn’t explain why some within-hospital differences by race do exist, particularly for “invasive” and expensive procedures. Fundamentally different mechanisms may play a role in lower rates of reperfusion among blacks than in lower rates of cardiac procedures such as catheterization, PCI, and CABG that rely on more complex processes of referral and follow-up. Finally, our findings may not extend to racial disparities in treatments and outcomes observed for other conditions or in other settings.
In summary, utilization of different hospitals by blacks and whites contributed substantially to observed treatment disparities. Policy interventions aimed at reducing treatment disparities should consider focused, provider-level efforts in addition to current national initiatives. Future research should focus on the mediators of these hospital-level effects, better understanding why within-hospital differences persist for invasive procedures (particularly for treatments requiring follow-up after initial hospitalization), and on explaining the paradoxical medium-term survival advantage of black patients despite their use of lower quality hospitals.
Acknowledgments
Funding was provided by National Institute on Aging (NIA) grants PO1 AG19783. Dr. Barnato was supported by NIA career-development grant and K08 AG021921. The NIA had no role in the in design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, and approval of the manuscript.
References
- 1.Maynard C, Fisher LD, Passamani ER, Pullum T. Blacks in the coronary artery surgery study (CASS): race and clinical decision making. Am J Public Health. 1986 Dec;76(12):1446–1448. doi: 10.2105/ajph.76.12.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whittle J, Conigliaro J, Good CB, Lofgren RP. Racial differences in the use of invasive cardiovascular procedures in the Department of Veterans Affairs medical system. N Engl J Med. 1993 Aug 26;329(9):621–627. doi: 10.1056/NEJM199308263290907. [DOI] [PubMed] [Google Scholar]
- 3.Ayanian JZ, Udvarhelyi IS, Gatsonis CA, Pashos CL, Epstein AM. Racial differences in the use of revascularization procedures after coronary angiography. JAMA. 1993;269(20):2642–2646. [PubMed] [Google Scholar]
- 4.Peterson ED, Wright SM, Daley J, Thibault GE. Racial variation in cardiac procedure use and survival following acute myocardial infarction in the Department of Veterans Affairs. [see comments] JAMA. 1994;271(15):1175–1180. [PubMed] [Google Scholar]
- 5.Peterson ED, Shaw LK, DeLong ER, Pryor DB, Califf RM, Mark DB. Racial variation in the use of coronary-revascularization procedures. Are the differences real? Do they matter? [see comments] New England Journal of Medicine. 1997;336(7):480–486. doi: 10.1056/NEJM199702133360706. [DOI] [PubMed] [Google Scholar]
- 6.Ford E, Newman J, Deosaransingh K. Racial and ethnic differences in the use of cardiovascular procedures: findings from the California Cooperative Cardiovascular Project. Am J Public Health. 2000 Jul;90(7):1128–1134. doi: 10.2105/ajph.90.7.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conigliaro J, Whittle J, Good CB, et al. Understanding racial variation in the use of coronary revascularization procedures: the role of clinical factors. Arch Intern Med. 2000 May 8;160(9):1329–1335. doi: 10.1001/archinte.160.9.1329. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Rathore SS, Radford MJ, Wang Y, Krumholz HM. Racial differences in the use of cardiac catheterization after acute myocardial infarction. [see comments] New England Journal of Medicine. 2001;344(19):1443–1449. doi: 10.1056/NEJM200105103441906. [DOI] [PubMed] [Google Scholar]
- 9.Kressin NR, Petersen LA. Racial differences in the use of invasive cardiovascular procedures: review of the literature and prescription for future research. Ann Intern Med. 2001 Sep 4;135(5):352–366. doi: 10.7326/0003-4819-135-5-200109040-00012. [DOI] [PubMed] [Google Scholar]
- 10.Maynard C, Litwin PE, Martin JS, et al. Characteristics of black patients admitted to coronary care units in metropolitan Seattle: results from the Myocardial Infarction Triage and Intervention Registry (MITI) Am J Cardiol. 1991 Jan 1;67(1):18–23. doi: 10.1016/0002-9149(91)90092-y. [DOI] [PubMed] [Google Scholar]
- 11.Pashos CL, Normand SL, Garfinkle JB, Newhouse JP, Epstein AM, McNeil BJ. Trends in the use of drug therapies in patients with acute myocardial infarction: 1988 to 1992. J Am Coll Cardiol. 1994 Apr;23(5):1023–1030. doi: 10.1016/0735-1097(94)90585-1. [DOI] [PubMed] [Google Scholar]
- 12.Canto JG, Allison JJ, Kiefe CI, et al. Relation of race and sex to the use of reperfusion therapy in Medicare beneficiaries with acute myocardial infarction. N Engl J Med. 2000 Apr 13;342(15):1094–1100. doi: 10.1056/NEJM200004133421505. [DOI] [PubMed] [Google Scholar]
- 13.Rathore SS, Berger AK, Weinfurt KP, et al. Race, sex, poverty, and the medical treatment of acute myocardial infarction in the elderly. [see comments] Circulation. 2000;102(6):642–648. doi: 10.1161/01.cir.102.6.642. [DOI] [PubMed] [Google Scholar]
- 14.Allison JJ, Kiefe CI, Centor RM, Box JB, Farmer RM. Racial differences in the medical treatment of elderly Medicare patients with acute myocardial infarction. J Gen Intern Med. 1996 Dec;11(12):736–743. doi: 10.1007/BF02598987. [DOI] [PubMed] [Google Scholar]
- 15.Krumholz HM, Radford MJ, Wang Y, Chen J, Heiat A, Marciniak TA. National use and effectiveness of beta-blockers for the treatment of elderly patients after acute myocardial infarction: National Cooperative Cardiovascular Project. JAMA. 1998 Aug 19;280(7):623–629. doi: 10.1001/jama.280.7.623. [DOI] [PubMed] [Google Scholar]
- 16.Skinner J, Weinstein JN, Sporer SM, Wennberg JE. Racial, ethnic, and geographic disparities in rates of knee arthroplasty among Medicare patients. N Engl J Med. 2003 Oct 2;349(14):1350–1359. doi: 10.1056/NEJMsa021569. [DOI] [PubMed] [Google Scholar]
- 17.Schneider EC, Zaslavsky AM, Epstein AM. Racial disparities in the quality of care for enrollees in medicare managed care. JAMA. 2002 Mar 13;287(10):1288–1294. doi: 10.1001/jama.287.10.1288. [DOI] [PubMed] [Google Scholar]
- 18.Kahn KL, Pearson ML, Harrison ER, et al. Health care for black and poor hospitalized Medicare patients. JAMA. 1994 Apr 20;271(15):1169–1174. [PubMed] [Google Scholar]
- 19.Diehr P, Yergan J, Chu J, et al. Treatment modality and quality differences for black and white breast-cancer patients treated in community hospitals. Med Care. 1989 Oct;27(10):942–958. doi: 10.1097/00005650-198910000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Schneider EC, Leape LL, Weissman JS, Piana RN, Gatsonis C, Epstein AM. Racial differences in cardiac revascularization rates: does “overuse” explain higher rates among white patients? Ann Intern Med. 2001 Sep 4;135(5):328–337. doi: 10.7326/0003-4819-135-5-200109040-00009. [DOI] [PubMed] [Google Scholar]
- 21.Rothenberg BM, Pearson T, Zwanziger J, Mukamel D. Explaining disparities in access to high-quality cardiac surgeons. Ann Thorac Surg. 2004 Jul;78(1):18–24. doi: 10.1016/j.athoracsur.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 22.Bach PB, Pham HH, Schrag D, Tate RC, Hargraves JL. Primary care physicians who treat blacks and whites. N Engl J Med. 2004 Aug 5;351(6):575–584. doi: 10.1056/NEJMsa040609. [DOI] [PubMed] [Google Scholar]
- 23.Alter DA, Naylor CD, Austin P, Tu JV. Effects of socioeconomic status on access to invasive cardiac procedures and on mortality after acute myocardial infarction. New England Journal of Medicine. 1999;341(18):1359–1367. doi: 10.1056/NEJM199910283411806. [DOI] [PubMed] [Google Scholar]
- 24.Austin PC, Tu JV, Alter DA. Comparing hierarchical modeling with traditional logistic regression analysis among patients hospitalized with acute myocardial infarction: should we be analyzing cardiovascular outcomes data differently? Am Heart J. 2003 Jan;145(1):27–35. doi: 10.1067/mhj.2003.23. [DOI] [PubMed] [Google Scholar]
- 25.O’Connor GT, Quinton HB, Traven ND, et al. Geographic variation in the treatment of acute myocardial infarction: the Cooperative Cardiovascular Project. JAMA. 1999;281(7):627–633. doi: 10.1001/jama.281.7.627. [DOI] [PubMed] [Google Scholar]
- 26.Marciniak TA, Ellerbeck EF, Radford MJ, et al. Improving the quality of care for Medicare patients with acute myocardial infarction: results from the Cooperative Cardiovascular Project. JAMA. 1998 May 6;279(17):1351–1357. doi: 10.1001/jama.279.17.1351. [DOI] [PubMed] [Google Scholar]
- 27.Ellerbeck EF, Jencks SF, Radford MJ, et al. Quality of care for Medicare patients with acute myocardial infarction. A four-state pilot study from the Cooperative Cardiovascular Project. JAMA. 1995 May 17;273(19):1509–1514. [PubMed] [Google Scholar]
- 28.Scanlon PJ, Faxon DP, Audet AM, et al. ACC/AHA guidelines for coronary angiography. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on Coronary Angiography). Developed in collaboration with the Society for Cardiac Angiography and Interventions. J Am Coll Cardiol. 1999 May;33(6):1756–1824. doi: 10.1016/s0735-1097(99)00126-6. [DOI] [PubMed] [Google Scholar]
- 29.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986 Dec;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 30.Wooldridge JM. Econometric Analysis of Cross-section and Panel Data. Cambridge, MA: MIT Press; 2002. [Google Scholar]
- 31.Gottlieb SS, McCarter RJ, Vogel RA. Effect of beta-blockade on mortality among high-risk and low-risk patients after myocardial infarction. N Engl J Med. 1998 Aug 20;339(8):489–497. doi: 10.1056/NEJM199808203390801. [DOI] [PubMed] [Google Scholar]
- 32.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986 Mar;42(1):121–130. [PubMed] [Google Scholar]
- 33.Hosmer DW, Lemeshow S. Applied Logistic Regression. 2. New York, NY: John Wiley and Sons; 2000. [Google Scholar]
- 34.Schulman KA, Berlin JA, Harless W, et al. The effect of race and sex on physicians’ recommendations for cardiac catheterization. [see comments]. [erratum appears in N Engl J Med 1999 Apr 8;340(14):1130] New England Journal of Medicine. 1999;340(8):618–626. doi: 10.1056/NEJM199902253400806. [DOI] [PubMed] [Google Scholar]
- 35.Sheifer SE, Rathore SS, Gersh BJ, et al. Time to presentation with acute myocardial infarction in the elderly: associations with race, sex, and socioeconomic characteristics. Circulation. 2000 Oct 3;102(14):1651–1656. doi: 10.1161/01.cir.102.14.1651. [DOI] [PubMed] [Google Scholar]
- 36.Garg PP, Landrum MB, Normand SL, et al. Understanding individual and small area variation in the underuse of coronary angiography following acute myocardial infarction Use of cholesterol-lowering therapy by elderly adults after myocardial infarction. Medical Care. 2002;40(7):614–626. doi: 10.1097/00005650-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Localio AR, Berlin JA, Ten Have TR, Kimmel SE. Adjustments for center in multicenter studies: an overview. Ann Intern Med. 2001 Jul 17;135(2):112–123. doi: 10.7326/0003-4819-135-2-200107170-00012. [DOI] [PubMed] [Google Scholar]
- 38.Greenfield S, Kaplan SH, Kahn R, Ninomiya J, Griffith JL. Profiling care provided by different groups of physicians: effects of patient case-mix (bias) and physician-level clustering on quality assessment results. Ann Intern Med. 2002;136(2):111–121. doi: 10.7326/0003-4819-136-2-200201150-00008. [DOI] [PubMed] [Google Scholar]
- 39.Panageas KS, Schrag D, Riedel E, Bach PB, Begg CB. The effect of clustering of outcomes on the association of procedure volume and surgical outcomes. Ann Intern Med. 2003 Oct 21;139(8):658–665. doi: 10.7326/0003-4819-139-8-200310210-00009. [DOI] [PubMed] [Google Scholar]
- 40.Berlin JA, Kimmel SE, Ten Have TR, Sammel MD. An empirical comparison of several clustered data approaches under confounding due to cluster effects in the analysis of complications of coronary angioplasty. Biometrics. 1999 Jun;55(2):470–476. doi: 10.1111/j.0006-341x.1999.00470.x. [DOI] [PubMed] [Google Scholar]