Abstract
Chronic ethanol consumption causes increased production of reactive oxygen species in hepatic mitochondria accompanied by elevations in products of lipid peroxidation such as 4-hydroxynonenal (4-HNE). In the current study we investigated the effects of chronic ethanol consumption on a prominent protein-4-HNE adduct in liver mitochondria. Male Sprague-Dawley rats were fed a liquid diet for 31 days in which ethanol constituted 36% of total calories. Immunoblot analyses of liver mitochondria from ethanol-fed and control animals, using an antibody to a 4-HNE-protein adduct, demonstrated elevated 4-HNE binding (+50%) to a mitochondrial protein of ~55 kDa due to chronic ethanol consumption. Analysis of this protein using AspN digestion and tandem mass spectrometry identified it as the mitochondrial form of 3-hydroxy-3-methylglutaryl CoA (HMG-CoA) synthase. Activity of activated form of this enzyme was unchanged in livers from ethanol-fed animals, but the protein level was elevated by 36%, which suggests a compensatory mechanism to maintain constant levels of synthase activity in the mitochondrion in the face of continuous inactivation by 4-HNE. Treatment of isolated mitochondria with 4-HNE demonstrated that the enzyme activity decreased as a function of 4-HNE concentration and with time of exposure. This study demonstrates that ethanol consumption increases the formation of a 4-HNE adduct with mitochondrial HMG-CoA synthase, which has the potential to inactivate the enzyme in situ.
Keywords: HMG CoA synthase, 4-HNE, mitochondria, chronic ethanol
The reactive aldehyde, 4-hydroxy-2-trans-nonenal (4-HNE)3 is a prominent lipid peroxidation product resulting from the elevations in ROS, which are generated in higher amounts in several disease states. It is an oxidation product of the ω–6 polyunsaturated fatty acids [1] and contains a double bond at the C3 position which reacts readily with thiol groups via a Michael addition and a C1 aldehyde group that can form Schiff’s bases with His and Lys residues. These reactive groups render 4-HNE capable of forming adducts with proteins, thereby altering their properties and/or denaturing them [1]. In addition to the direct effects of 4-HNE in disrupting enzyme catalyzed processes, aldehyde-protein adducts are also believed to act as neo-antigens promoting tissue inflammation and disease severity [2]. These capabilities have implicated 4-HNE in a number of pathologies, such as atherosclerosis, cancer, kidney disease and neurological disorders, where increased levels of 4-HNE-protein adducts have been detected [3].
There is increasing evidence that 4-HNE may be an etiological factor in the development of alcoholic liver disease (ALD), as increased levels are detected in livers from patients suffering with ethanol-related pathology [2] and in animal models of ALD [4]. In animal models for fetal alcohol syndrome and ALD, acute exposure to ethanol resulted in increased 4-HNE concentrations, particularly in the mitochondrion [5,6]. These elevations are likely due to increases in hepatic mitochondrial ROS production, which has been demonstrated in both rat liver mitochondria [7,8] and hepatocytes [9,10] following acute and chronic ethanol administration. The increase in mitochondrial 4-HNE is also attributed to decreases in activities of glutathione S-transferase and aldehyde dehydrogenase, two enzymes involved in clearance of lipid peroxides [6,11] and to the reduced recycling of GSH [12]. In more recent studies 4-HNE adducts of particular liver proteins have been identified following chronic ethanol administration in rat models of ALD. Higher amounts of 4-HNE adducts have been detected against cytosolic heat shock proteins HSP72 and HSP90 in livers from ethanol-fed animals, which were correlated with decreases in folding activities [13,14]. 4-HNE also forms an adduct with the ATPase RPT4 in the 26S proteosome and is implicated in loss of proteasomal activity as a consequence of chronic ethanol consumption [15].
Elevations in the mitochondrial concentrations of 4-HNE might be expected to result in damage to matrix and inner membrane proteins. In liver, using an immunochemical analysis, an HNE adduct was detected with subunit 4 of cytochrome oxidase [16], which illustrated the potential for in situ formation in this tissue. In the current investigation another prominent mitochondrial 4-HNE-protein adduct was identified in an effort to provide additional evidence for lipid peroxidation contributing to ethanol-related alterations in mitochondrial metabolism. Increased levels of a 4-HNE adduct were observed as a consequence of chronic ethanol consumption. Using mass spectrometry, this protein adduct was identified as HMG CoA synthase, the rate limiting enzyme for ketone body formation. In ethanol consumers a compensatory increase in the protein concentration of this enzyme was observed, presumably to maintain the HMG CoA synthase activity constant under conditions where it was being inactivated due to adduct formation with 4-HNE. The observations made in this study emphasize the essential role of the mitochondrial ketogenic system in dealing with increased levels of acetyl CoA that result when chronic ethanol consumption is combined with ingestion of a high fat diet [17,18].
MATERIALS AND METHODS
Reagents
The 4-HNE was obtained from Cayman Chemical Company, Ann Arbor, MI. The 4-HNE-ovalbumin and antibody for detecting 4-HNE-protein adducts, produced against a 4-HNE-keyhole lymphet hemocyanin (KLH) adduct in rabbits, were purchased from Alpha Diagnostic International, San Diego, TX. Since the HNE was coupled to KLH protein, presumably to multiple amino acid residues, the polyclonal antibody raised against this preparation has no known specificity toward a particular amino acid-HNE adduct (personal communication, Alpha Diagnostics International). The antibody against HMG CoA synthase was a generous gift from Dr. P. Quant, Oxford University, UK. The secondary antibody, goat anti-rabbit IgG horseradish peroxidase conjugate, was purchased from Sigma-Aldrich, St. Louis, MO. Supersignal West Pico Chemiluminescent Substrate (ECL reagent) was obtained from Pierce, Rockford, IL. Digitonin and ultrapure sucrose were obtained from ICN Biomedicals, Aurora, OH. Lieber-DeCarli liquid diets were obtained from Bio-Serve, Frenchtown, NJ. All other chemicals were analytical reagent grade.
Feeding regimen
Male Sprague Dawley rats weighing 150 to 175 g were obtained from Charles River Laboratories, Wilmington, MA. Upon attaining 200 g body weight, rats were fed the Lieber-DeCarli liquid diet for a minimum of 31 days [19]. Ethanol and fat comprised 36% and 35% of total calories, respectively. Controls were isocalorically pair-fed the same diet with maltose-dextrin substituted for ethanol. Body weights at the end of the study were 329 ± 13 g (mean ± SEM, n=8) for controls and 316 ± 14 g for ethanol-fed animals. The rate of ethanol consumption was 11 g/kg body weight/day for the last 3 weeks of the feeding regimen.
Mitochondria isolation
Mitochondria were prepared from rat liver by differential centrifugation as previously described [20] following 31 days of ethanol feeding. Mitochondria were also made from hepatocyte preparations that exhibited a viability >90% [21]. Aliquots of hepatocytes (2 × 108 cells) were sedimented, resuspended in isolation buffer (0.25 M sucrose, 5 mM Tris-HCl, 1 mM EDTA, pH 7.5) and then sonicated on ice with a Branson Model 250 sonicator, using a power output setting of 1 and 50% duty cycle. Suspensions were sonicated for 1 min followed by a 40 sec burst, allowing a 1 min interval between sonication steps. The broken cell suspensions were then fractionated by differential centrifugation identically to whole liver homogenates [20]. Mitochondria prepared by differential centrifugation were further purified utilizing a 30% Percoll cushion. Following centrifugation for 40 min at 95,000 g in a Beckman 50.2 Ti rotor the upper two thirds of the Percoll gradient (containing microsomes and peroxisomes) was discarded. The mitochondrial band was collected and washed two times by centrifuging for 10 min at 8,600 g. The protein concentration was determined by the Lowry method [22], which was also used for subsequent protein determinations. The respiration rates and respiratory control ratios were determined as previously described [20]. Mitochondria were used immediately for enzyme assays, with the remainder stored at −80°C.
Mitochondria fractionation
The mitochondrion was also fractionated into its compartments [outer membrane plus inter membrane space, SMP (inner membrane) and matrix]. The outer membrane and inter membrane space fraction was obtained by treating mitochondria (20 mg/mL) with digitonin (0.11 mg/mg mitochondrial protein) for 15 min on ice. The mixture was diluted 5 fold with isolation buffer and centrifuged for 10 min at 8,600 g. The supernatant, which contained the outer membrane and inter membrane space, was collected and stored at −80°C. The pellet was used to obtain the SMP and matrix fractions. It was resuspended in 25 mL of sonication buffer (10 mM HEPES, 1 mM ATP, 5 mM succinate, 0.2 mM NADH, 1 mg/mL BSA, 1 mM MgCl2, pH 7.1) and centrifuged at 8,600 g for 10 min. The pellet was resuspended in 10 mL of sonication buffer, but with a final MgCl2 concentration of 0.1 M. Aliquots (2.5 mL) were sonicated on ice for 1 min using a Branson Model 250 sonicator at a power output setting of 2.5 and 50% duty cycle. Following sonication the suspension was diluted 3 fold with sonication buffer and centrifuged for 10 min at 29,000 g. The pellet was discarded and the supernatant centrifuged at 145,000 g for 1 h. The supernatant (matrix fraction) was collected and the pellet (SMP) was resuspended in 0.25 M sucrose (pH 7.4). The fractions were stored at −80°C until used.
Electrophoresis & Immunoblotting
Mitochondrial proteins (20 μg/lane) were analyzed by one dimensional SDS-PAGE using 10% resolving gels. Proteins were transferred to nitrocellulose membranes and probed for aldehyde-adducts using the 4-HNE-adduct antibody. For detection of 4-HNE-protein adducts, blots were incubated for 1–2 h with rabbit antiserum at a dilution of 1:2000 and then with goat antirabbit IgG diluted 1:100,000 for 1 h. Bands were visualized using the ECL detection kit and densitometric quantification of bands was carried out using the NIH Image 1.62 software. The intensity of bands was linear up to 30 μg of mitochondrial protein. 4-HNE-ovalbumin (approx. 45 kDa) was utilized as a positive control. In experiments where densitometry intensities were compared, all samples were analyzed on the same gel. When blots of 1D gels were analyzed for the presence of HMG CoA synthase they were probed overnight with rabbit antiserum against mitochondrial HMG-CoA synthase at a dilution of 1:500. The remainder of the analysis was as described above for 4-HNE-protein adducts. The intensity of the band was linear up to 1 μg of mitochondrial protein.
To detect and identify individual protein-aldehyde adducts mitochondrial proteins were separated by NEPHGE as described according to O’Farrell et al. [23]. The first dimension tube gels (11 cm in length) produced a pH range of 6.5–8.5. Mitochondrial proteins (100 μg) were loaded at the anode end and run for 4000 volt hours. The remainder of the procedure was described by Cahill et al. [24]. Identical gels were also transferred overnight at 115 mA/gel constant current, followed by blocking and immunodetection of 4-HNE-protein adducts as described above. PVDF membranes were stained with 0.025% Coomassie Brilliant Blue R-250 followed by destaining. Blots of 2D gels, prepared as described above, were also probed with anti-HMG CoA synthase antibody at a dilution of 1:500.
Mass spectrometry
The protein spot identified showing immunoreactivity towards 4-HNE was cut from the 2 dimension gel and provided to Harvard Microchemistry, Harvard University, where it was subjected to in-gel digestion with the protease, AspN. Sequence analysis was performed at the Harvard Microchemistry facility by μLC/MS/MS on a Finnigan LCQ DECA XP quadrupole ion trap mass spectrometer. The fragmentation spectra were correlated with known sequences using the algorithm Sequest [25] and additional programs [26] to identify the protein.
Enzyme assays for mitochondrial HMG-CoA synthase
Mitochondrial HMG-CoA synthase activity is regulated in situ by its degree of succinylation. Succinyl-CoA inactivates the enzyme by preventing acetoacetyl-CoA from reacting with the succinyl-enzyme intermediate [27,28]. Enzyme activity is therefore defined as active, which is the activity measured before the enzyme is chemically desuccinylated, and total, which is the activity after chemical desuccinylation [29]. Mitochondrial HMG-CoA synthase activity (active and total) was determined by following the rate of loss of absorbance at 303 nm as acetoacetyl CoA is converted to HMG CoA. The assay procedure is described in detail by Lascelles and Quant [29]. Enzyme activities were obtained in the standard assay system, which was modified slightly by increasing the concentration of acetyl CoA to 100 μM and decreasing the amount of phosphotransacetylase (PTA) to 10 units. To measure active HMG CoA synthase activity freshly prepared mitochondria (100 μg protein) were first lysed by incubating at 30°C for 4 min with Triton-X-100 added to a final concentration of 1.4 % (v/v). The preparation was then assayed as previously described [29]. Total mitochondrial HMG-CoA synthase activity was determined after incubating prewarmed mitochondria (240 μg protein) in the desuccinylation medium (50 mM Tris-HCl, pH 7.4, 5mM MgCl2, 15% glycerol, 0.2mM DTT, 1% Triton X-100, 5 mM acetyl phosphate, 0.2 mM acetyl CoA and 10 units of PTA) for 3 min at 30°C [29]. The percentage of succinylated enzyme was calculated from the total and active HMG CoA synthase activities, as previously described [29].
In vitro effect of 4-HNE on HMG-CoA synthase activity
In experiments to compare the effect of 4-HNE on active and total (desuccinylated) activities of the enzyme three mitochondrial lysates were utilized: active-untreated, active-4-HNE treated and total (desuccinylated)-4-HNE treated. Since it was necessary to remove the desuccinylation medium before 4-HNE treatment all mitochondrial samples were subjected to the same incubation procedure, with or without the desuccinylation medium, and then passed through a 30 kD Apollo High-Performance Centrifugal Concentrator (Orbital Biosciences, Topsfield, MA). This additional step had no effect on the activity of the enzyme. The protein concentrations in all preparations were determined again following recovery from the concentrator. The preparations were then incubated, with or without 5 μM 4-HNE for 0, 30, 60, 90, or 120 min. Free 4-HNE remaining after the incubation periods was scavenged by bringing the incubation mixture to 5 mM cysteine and incubating for 3 min. Aliquots containing 100 μg of mitochondrial protein were then assayed for HMG CoA synthase activity as described above. An aliquot from each incubation mixture was also taken for immunoblot analyses.
Statistics
Unless otherwise stated, results from all experiments are expressed as the mean ± SEM from five to eight mitochondrial preparations. Statistical analyses between pair-fed control and ethanol-fed rats were analyzed by Student’s paired t-test. Regression analyses of HMG CoA synthase activity loss vs 4-HNE enzyme adduct intensity were conducted using a statistical package within the Microsoft Excel program. Significance was set at p<0.05.
RESULTS
Immunoblot analyses for 4-HNE-protein adducts
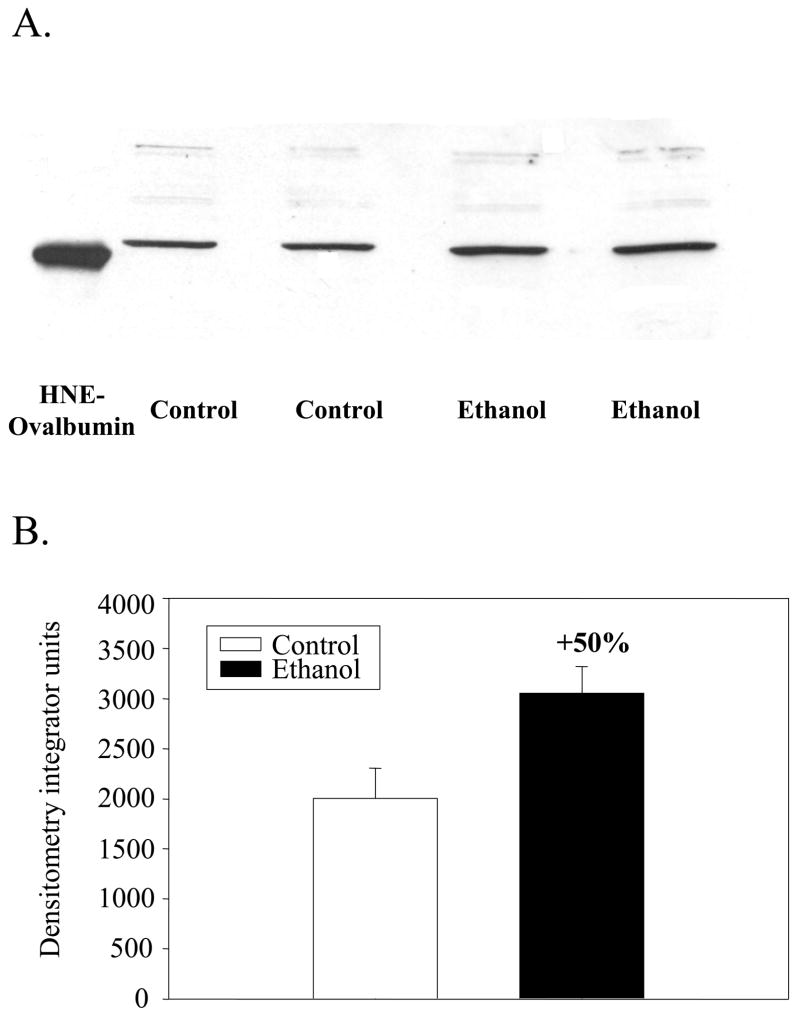
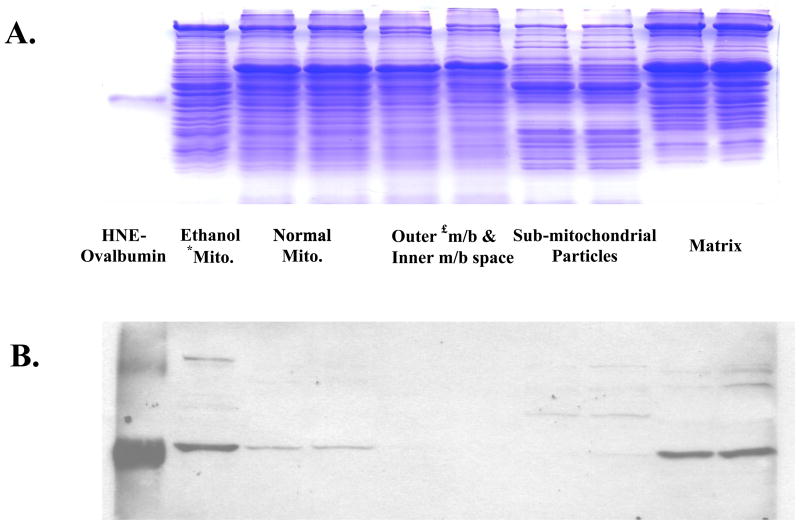
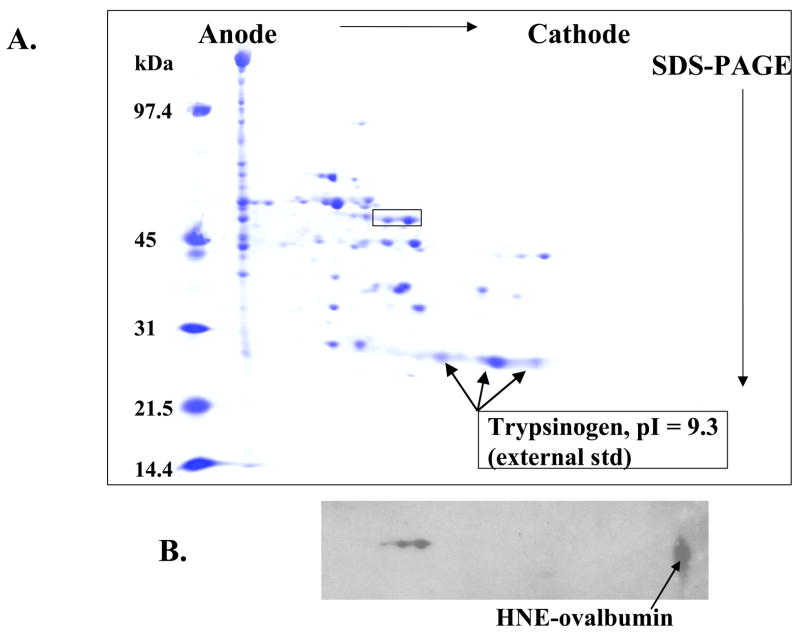
The respiratory control ratio was determined, using succinate as the oxidizable substrate, for liver mitochondria prepared from ethanol and control pair-fed animals. It was significantly depressed in mitochondria from ethanol fed rats (3.1 ± 0.40) when compared to controls (5.0 ± 0.26; p=0.02) with succinate as the oxidizable substrate, an observation made in previous studies [20,30] Aliquots of these mitochondria were analyzed for 4-HNE-protein adducts by immunoblot analyses. In immunoblots of 1D gels it was observed that a mitochondrial protein of approximately 55 kDa exhibited elevated 4-HNE binding in ethanol mitochondria (Fig. 1A). The 4-HNE reactivity toward this protein was increased by 50% (p=0.002) in ethanol mitochondria when compared with control mitochondria (Fig. 1B). A protein at approximately 100 kDa also exhibited a faint band for 4-HNE binding in both control and ethanol mitochondria. To identify the mitochondrial compartment of the 55 kDa protein, mitochondria from chow fed rats were fractionated into outer membrane plus inter membrane space, SMP and matrix. Immunoblot analyses revealed that the protein adduct was located in the matrix (Fig. 2). Proteins showing faint signals for reactivity with 4-HNE were also observed around 90 kDa in the matrix fraction and around 60–65 kDa in the SMP fraction (Fig. 2). Two dimensional NEPHGE was utilized to identify the particular matrix protein from ethanol fed rats showing increased reactivity with 4-HNE. The electrophoresis pattern of matrix proteins is shown in Fig. 3A and immunoblot analyses demonstrated two protein spots again with a molecular weight around 50–55 kDa with very close isoelectric points (Fig. 3B). The protein spot showing the highest immunoreactivity with the anti-4-HNE antibody was excised from the gel and analyzed using mass spectrometry. Western blots using 2D NEPHGE revealed that the less intense spot observed in Fig. 3B also had immunoreactivity with anti-HMG CoA synthase antibody, which suggests it is a more negatively charged form of HMG CoA synthase (data not shown).
Fig. 1. Immunoblot analysis of mitochondrial proteins from control and ethanol fed rats following chronic ethanol consumption.
Mitochondria were prepared as described in the Materials and Methods. A. Mitochondrial proteins (20 μg) were separated by SDS-PAGE and subjected to immunoblot analyses using a primary antibody against 4-HNE. 3 μg of HNE-ovalbumin (50 kDa) was utilized as a positive control. B. Densitometric analysis of mitochondrial HNE-protein adducts from control and ethanol fed rats.
Fig. 2. Identification of HNE-protein adducts in different submitochondrial compartments.
Mitochondria from normal chow fed rats were fractionated into submitochondrial compartments, loaded (20 μg) onto 10% resolving gels and and analyzed by an immunoblot. A. Coomassie stained SDS-PAGE gel. B. Immunoblot of the gel shown in A probed using the anti-HNE antibody. *mito., mitochondria; £m/b, membrane.
Fig. 3. Two dimensional electrophoresis (NEPHGE) of mitochondrial matrix proteins from ethanol fed rats.
The matrix fraction from ethanol fed rats was prepared as described in the Materials and Methods. Matrix protein (100 μg) was loaded onto tube gels (pH 6.5–8.5) and electrofocused for 4000 volt hours under non-equilibrium conditions in the first dimension and SDS-PAGE (10% resolving gels) in the second dimension. A. Coomassie stained gel. B. Immunoblot of identical gel shown in A probed using the anti-HNE antibody. 4 μg trypsinogen (pI 9.3) was added as an external marker.
Protein Identification by Mass Spectrometry
The protein was digested using AspN and analyzed by μLC/MS/MS as outlined in the Methods section. Table 1 reports sequences of peptides that provide 96% coverage of the sequence of the rat form of the mitochondrial HMG-CoA synthase. Other than gaps between G166 and P170 and between F373 and S385, the complete sequence was confirmed. The protein identity was also independently confirmed, again with high confidence, employing a Bruker Esquire ion trap mass spectrometer (Center for Structural Biology, Wake Forest University). The mature form of the protein contains 466 amino acids and has a molecular mass of 52714 daltons with a calculated pI of 8.24 [28]. Mitochondrial HMG-CoA synthase has a 37 amino acid precursor sequence which is cleaved upon entry into the mitochondrion, but before import its mass is 56912 daltons [28].
Table 1. Sequence of aspN peptides.
This series of sequenced peptides covers 96% of the mitochondrial HMG CoA synthase primary sequence and represents 20% (23/113) of the sequences obtained. It does not contain the presequence required for import.
| Peptide | Amino acid position |
|---|---|
| TIPPAPLAKT | 1-10 |
| DTWPKDVGILAL | 11-22 |
| EVYFPAQYVDQT | 23-34 |
| DLEKFNNVEAGKYTVGLGQTRM*GFC#SVQE | 32-63 |
| DISNLC^LTVVQRLMERTKLPW | 64-84 |
| DAVGRLEVGTETIIDKSKAVKTVLM*ELFQ | 85-113 |
| DSGNTDIEGIDTTNAC^YGGTASLFNAANWM | 114-143 |
| DTTNAC^YGGTASLFNAANWMESSYW | 124-148 |
| DGRYALVVC^G | 149-158 |
| DIAVYPSG | 159-166 |
| PTGGAGAVAM*LIGPKAPLVLEQGLRGTHM*ENAY | 170-202 |
| DFYKPNLASEYPLVDGKLSIQC^Y | 203-225 |
| LRALDRC^YAAY | 226-236 |
| DRC^YAAYRRKIQNQWKQAG | 230-248 |
| NNQPFTLDDVQYM*IFHTPFC#KM*VQKSLARLM*FN | 249-281 |
| NDFLSSSSDKQNNLY | 281-295 |
| KGLEAFKGLKLEETY | 296-310 |
| TNKDVDKALLKASLDM*FNKKTKASLY | 311-336 |
| LSTNNGNMYTSSLYGC^LASLLSHHSAQELAGSRIGAF | 337-373 |
| SFRVSKDASPGSPLEKLVSSVSDLPKRL | 385-412 |
| DSRRRMSPEEFTEIM*NQREQF | 413-433 |
| EQFYHKVNFSPPGDTSNLFPGTWYLERV | 431-458 |
| YLERVDEMHRRKY | 454-466 |
M*: Met sulfoxide (low pH artifact)
C#: carboxyamidomethyl Cys
C^: proprionamido Cys (gel artifact)
Effect of ethanol consumption on mitochondrial HMG CoA synthase activity
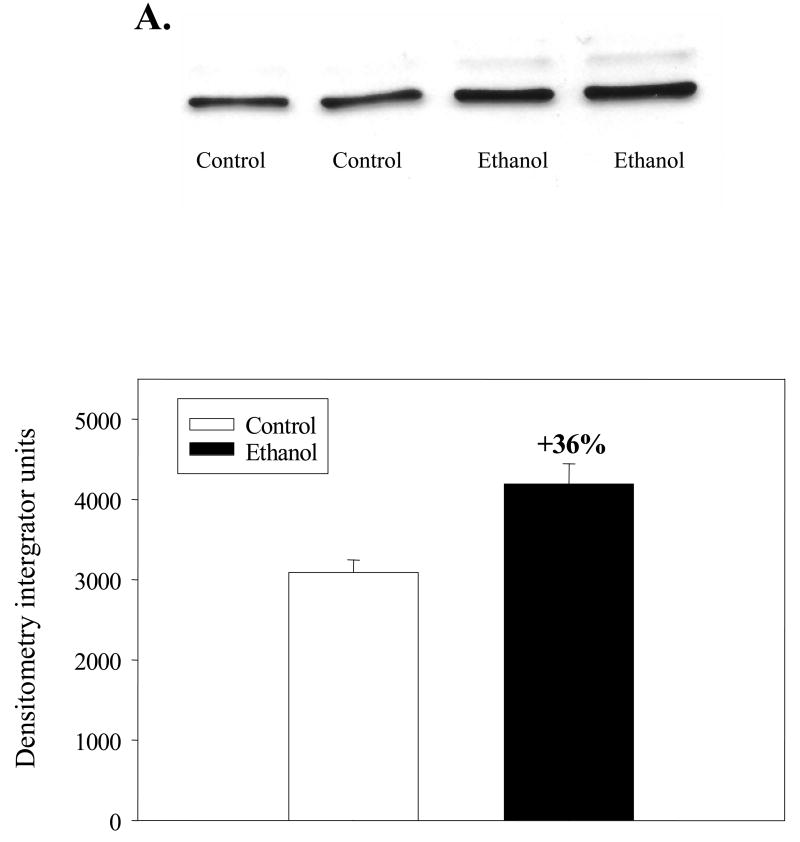
Mitochondrial HMG-CoA synthase (EC 4.1.3.5) is the rate limiting enzyme for ketone body formation [28]. The enzyme initially forms a covalent intermediate with acetyl-CoA and condensation occurs with acetoacetyl-CoA to form HMG-CoA, which is released subsequent to a hydrolysis step [28]. HMG CoA synthase activities were determined in lysates of ethanol and control mitochondria before and after desuccinylation of the enzyme (Table 2). The activity measured in ethanol mitochondria before chemical desuccinylation (active) was 16% higher (p<0.05) than in control mitochondria. However, when the enzyme was chemically desuccinylated, no increase in activity was observed in ethanol mitochondria, whereas control mitochondrial enzyme activity increased by 19% (p<0.01). To determine the relative protein levels of mitochondrial HMG-CoA synthase immunoblot analyses were carried out (Fig. 4A). Densitometric analyses indicated that the protein level of mitochondrial HMG-CoA synthase increased by 36% (p<0.005) following chronic ethanol consumption (Fig. 4B).
Table 2.
Active (succinylated) and Total (Desuccinylated) Mitochondrial HMG-CoA synthase activity following chronic ethanol consumption.
| Enzyme Activity | Control (mU/mg protein) | Ethanol-fed (mU/mg protein) |
|---|---|---|
| Active HMG-CoA synthase | 31.9 ± 3.0 | 37.1 ± 4.7a |
| Total (Desuccinylated) HMG-CoA synthase | 38.1 ± 4.3b | 36.1 ± 3.6 |
| % Succinylatedc | 16.1 % | −2.8 % |
p<0.05;
p<0.01 (versus control active HMG-CoA synthase)
Fig. 4. Immunoblot analysis of mitochondrial HMG-CoA synthase from control and ethanol fed rats following chronic ethanol consumption.
Mitochondria were prepared as described in the Materials and Methods. A. Mitochondrial proteins (0.5 μg) were separated by SDS-PAGE and subjected to immunoblot analyses using a primary antibody against mitochondrial HMG-CoA synthase. B. Densitometric analysis of mitochondrial HMG-CoA synthase from control and ethanol fed rats.
In vitro treatment of mitochondria with 4-HNE
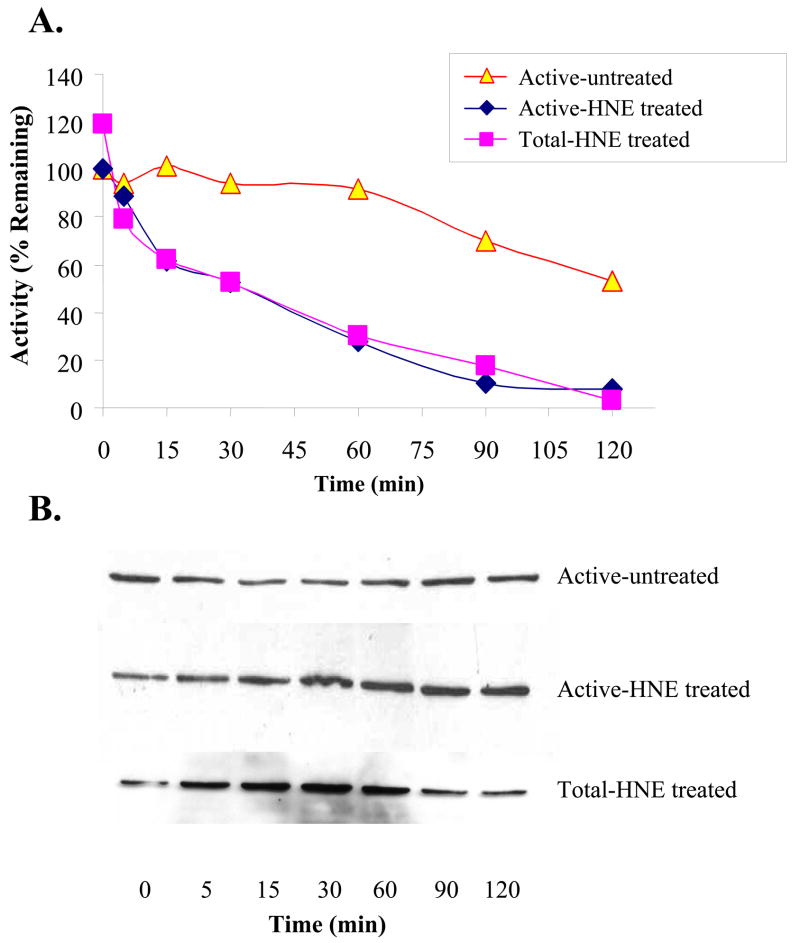
As shown in Fig. 5A, inhibition of both the “active” and total (desuccinylated) preparations of the enzyme increases with time of exposure to 5μM 4-HNE. A similar pattern occurred with the active and total preparations of the enzyme, with activities decreasing for both with identical kinetic profiles. The immunoblots of samples of the “active” preparation, taken to correspond with the times for activity measurements, displayed increased 4-HNE binding over the exposure period (Fig. 5B). With the total (desuccinylated) preparation, the corresponding immunoblots also demonstrated elevated 4-HNE binding initially, which then decreased with longer incubation times (Fig. 5B). Activity loss correlated positively with the initial increases in 4-HNE binding to both the “active” (r2 = 0.94; p = 0.019; 0 – 60 min) and total (r2 = 0.96; p = 0.019; 0 – 30 min) forms of the treated enzyme. In contrast, the untreated enzyme activity decreased to a lesser degree and the 4-HNE-adduct level was unchanged (Fig. 5B). Moreover, there was no relationship between activity loss and intensity of immunoblots in the enzyme preparation untreated with 4-HNE (r2 = 0.004; p = 0.92; 0–60 min).
Fig. 5. Time course for inhibition of and adduct formation with HMG-CoA synthase.
Active (succinylated) and total (desuccinylated) preparations of the enzyme were incubated with 5 μM HNE as detailed in the Methods section. At the times specified aliquots were taken for activity measurements (A) and immunoblot analyses (B). 100 μg of HNE treated mitochondrial lysate protein was assayed for HMG-CoA synthase activity and 20 μg was subjected to immunoblot analyses.
DISCUSSION
The formation of highly reactive lipid aldehydes, malondialdehyde and 4-HNE, are early events in the development of alcoholic liver disease [4] and may contribute to pathology based on their capacity to form adducts with proteins, thereby denaturing them or modifying their normal function [31–34]. The presence of 4-HNE-protein adducts has been detected in the livers of patients suffering from chronic ethanol abuse [35],[36][37] and in animal models for alcoholic liver disease [38–40] and they appear to increase in intensity with the severity of the disease [2]. The presence of these adducts has been detected primarily by immunohistochemistry, but more recently the combination of immunochemical techniques and mass spectrometry has been used to identify particular proteins such as the proteosomal ATPase RPT4 [15] and cytosolic HSPs 72 and 90 [13,14] that form 4-HNE adducts in higher amounts in animal models for ALD.
A liver mitochondrial protein previously identified as being modified by 4-HNE in situ, is cytochrome oxidase subunit 4 [16]. Furthermore, evidence has been provided suggesting that mitochondrial aldehyde dehydrogenase [41]and the adenine nucleotide transporter [42] may also form adducts with 4-HNE in situ in the liver. In the present study we identified a 4-HNE adduct of HMG CoA synthase in liver mitochondria, which is increased in concentration 50% as a result of chronic ethanol consumption [19] for a one month period. It is notable that an adduct of similar molecular weight was detected in the analysis of the HNE-cytochrome oxidase adduct [16]. The 4-HNE adduct of HMG-CoA synthase was localized to the matrix, the compartment of the mitochondrion where this enzyme participates in ketone body formation [27]. The identification was accomplished using tandem mass spectrometry, which resulted in 96% coverage of the protein.
Even though HMG CoA synthase protein levels were elevated in ethanol mitochondria (Fig. 4B) there was no increase in total enzyme activity measured after the enzyme was desuccinylated (Table 2). This demonstrates that a significant portion of the mitochondrial content of HMG-CoA synthase protein was inactive in ethanol consumers. It is likely that activity loss was related to reaction with HNE because adduct levels were elevated in ethanol consumers. Indeed, direct impairment of functional activities following treatment with HNE has been demonstrated with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) [43], the α-ketoglutarate dehydrogenase complex [44], isocitrate dehydrogenase [45], cytochrome oxidase [46] and hepatic HSPs [13,14].
In the present study further evidence for 4-HNE forming an adduct with residues involved in catalysis was derived by comparing the inhibition profiles of both the succinylated and desuccinylated forms of the enzyme. The enzyme exists in the succinylated form, the level of which is controlled by the ratio of succinyl CoA and oxaloacetate [28]. Succinylation occurs at the active site Cys (Cys 129), but when the enzyme is desuccinylated it is fully active, with the catalytic site Cys available for reaction with substrate or, alternatively, with 4-HNE. When the succinylated and desuccinylated forms of the enzyme were incubated with 4-HNE the inhibition profiles were exactly the same (Fig. 5), thus demonstrating that increased activity of the enzyme is readily inhibited by 4-HNE. This would have not been the case if 4-HNE were interacting exclusively with residues not involved with catalysis. Candidate residues implicated in catalysis are Cys 129, His 264 and Lys 46, -269 and -273 [28,47,48], which are residues known to form adducts with 4-HNE [49]. Other candidate residues are Cys 224 and 268, which are felt to be located close to the catalytic site [47].
With GAPDH, EFABP, cyt c and the hepatic HSPs, mass spectrometry has been utilized to identify amino acid residues modified after incubation with added 4-HNE [13,14,43,50,51]. An adduct was formed with Cys in GAPDH, EFABP and the HSPs, whereas His and Lys adducts were observed in GAPDH and cyt c. In the present study MS analyses were carried out on the enzyme recovered from 2D electrophoresis gels after being detected both with the 4-HNE adduct and the HMG CoA synthase antibodies. MS/MS failed to detect the presence of an adduct, which would have been evident in fragmentation patterns or if the mass of a peptide were increased by 156.2 mass units. This lack of success in detecting an adduct by MS analyses in HMG CoA synthase recovered from an electrophoresis gel corresponds with the inability in other studies to detect adducts by MS analyses in samples obtained from tissues untreated with 4-HNE. For example, adducts of EFABP and the HSPs could not be detected unless the purified proteins were first treated with 4-HNE [13,14,50] and in the present study a source of purified HMG CoA synthase was not available. Furthermore, no adduct was detected in MS analyses of the enzyme after treatment of fragmented mitochondrial suspensions with 4-HNE at concentrations up to 25 μM and subsequent recovery of the enzyme from electrophoresis gels.
It is possible that 4-HNE adducts with particular residues could have been detected with supra pharmacological concentrations of added 4-HNE, but it is not clear that this would designate the adduct detected by immunoblots of mitochondrial matrix from control and ethanol-fed animals. As an example, when GAPDH, which has a Cys involved in catalysis, was incubated with 1 mM 4-HNE, the active site Cys residue was not modified, whereas amino acids located primarily on the surface of the enzyme formed adducts [43]. At concentrations 10 fold lower (100 μM) significant inhibition of activity was observed [52], which suggested modification of an amino acid residue involved in catalysis that could not be demonstrated subsequently by MS analyses [43]. Similarly, modified amino acid residues were identified in the EFABP when it was incubated with 100 μM 4-HNE, but attempts to determine the amino acid residue(s) modified in vivo were unsuccessful using MS/MS[50]. In contrast, evidence was provided that the 4-HNE-Cys adducts identified in the HSPs were, indeed, those involved in the protein folding mechanisms employed by these proteins [13,14].
The total (desuccinylated) HMG CoA synthase was not decreased as a result of chronic ethanol consumption (Table 2). This observation might suggest that formation of 4-HNE adducts with the enzyme did not interfere with its catalytic capacity. However, this activity measurement was expressed in terms of total mitochondrial protein and not in terms of the concentration of HMG CoA synthase protein. When the protein levels of the enzyme were estimated in immunoblots with anti HMG CoA synthase antibody, the band intensities indicated there was a 36% increase in the concentration of the enzyme protein in mitochondria. An increase in enzyme protein, but no increase in total (desuccinylated) activity is consistent with the possibility that a portion of the total pool of enzyme in the mitochondria was being deactivated, presumably by 4-HNE. In turn, additional enzyme was being produced and imported into the matrix as a compensatory response to maintain mitochondrial activity levels constant.
A compensatory response to maintain normal levels of active enzyme under conditions where inactivation is being increased is understandable when lipid metabolism in the alcohol consumer is considered. Ketone body production is elevated as a consequence of ethanol consumption [53], particularly in individuals ingesting diets relatively high in lipid [17,18], as was the case in this study. This reflects, at least in part, the increased acetyl CoA load in the liver, which results from lipid beta oxidation and ethanol oxidation [17,53]. Ketone body production results when acetyl CoA concentrations exceed the capacity of the liver to oxidize it through the Kreb’s cycle. Mitochondrial HMG CoA synthase is the rate limiting enzyme in ketone body production in liver [28] and as mentioned above, its activity is controlled by the degree of its succinylation, being less active in the succinylated form. In mitochondria from ethanol-fed rats the enzyme activity does not increase after the desuccinylation procedure (Table 2), which implies that HMG CoA synthase is completely desuccinylated, likely due to a requirement for maximal ketogenic activity. In contrast, the enzyme in mitochondria from control animals is partially succinylated, which also suggests that ketogenic activity is not as great due to the absence of ethanol.
As mentioned above, HMG CoA synthase activity is not increased by the desuccinylation procedure. This also suggests that the other mechanism which influences the level of active enzyme, control of expression of the gene for HMG CoA synthase [28], is up-regulated to maximize the rate of ketogenesis. If the enzyme were being inactivated by forming an adduct with HMG CoA synthase, it can be expected that inactive enzyme would be replaced by an increase in its expression and import. If there is a compensatory up-regulation of expression of this enzyme, it would provide an example of the capacity of ethanol to alter and/or maintain the level of enzymes involved in metabolism of ethanol and its metabolites. Another notable example of this phenomenon is the elevation of cytochrome P-450 2E1 activity in the livers of chronic ethanol consumers, which increases the capacity of the liver to oxidize ethanol, particularly when its concentration is elevated [54].
Acknowledgments
This study was supported by Grants 02887, 13610 and 00279 from the National Institute on Alcohol Abuse and Alcoholism.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 2.Niemelä O. Distribution of ethanol-induced protein adducts in vivo: Relationship to tissue injury. Free Radic Biol Med. 2001;31:1533–8. doi: 10.1016/s0891-5849(01)00744-4. [DOI] [PubMed] [Google Scholar]
- 3.Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog Lipid Res. 2003;42:318–43. doi: 10.1016/s0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- 4.Sampey BP, Korourian S, Ronis MJ, Badger TM, Petersen DR. Immunohistochemical characterization of hepatic malondialdehyde and 4-hydroxynonenal modified proteins during early stages of ethanol-induced liver injury. Alcohol Clin Exp Res. 2003;27:1015–22. doi: 10.1097/01.ALC.0000071928.16732.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen JJ, Schenker S, Henderson GI. 4-hydroxynonenal levels are enhanced in fetal liver mitochondria by in utero ethanol exposure. Hepatology. 1997;25:142–7. doi: 10.1002/hep.510250126. [DOI] [PubMed] [Google Scholar]
- 6.Chen JJ, Schenker S, Henderson GI. 4-hydroxynonenal detoxification by mitochondrial glutathione S-transferase is compromised by short-term ethanol consumption in rats. Alcohol Clin Exp Res. 2002;26:1252–8. doi: 10.1097/01.ALC.0000024081.89523.81. [DOI] [PubMed] [Google Scholar]
- 7.Boveris A, Fraga CG, Varsavsky AI, Koch OR. Increased chemiluminescence and superoxide production in the liver of chronically ethanol-treated rats. Arch Biochem Biophys. 1983;227:534–41. doi: 10.1016/0003-9861(83)90482-4. [DOI] [PubMed] [Google Scholar]
- 8.Kukielka E, Dicker E, Cederbaum AI. Increased production of reactive oxygen species by rat liver mitochondria after chronic ethanol treatment. Arch Biochem Biophys. 1994;309:377–86. doi: 10.1006/abbi.1994.1127. [DOI] [PubMed] [Google Scholar]
- 9.Bailey SM, Cunningham CC. Acute and chronic ethanol increases reactive oxygen species generation and decreases viability in fresh, isolated rat hepatocytes. Hepatology. 1998;28:1318–26. doi: 10.1002/hep.510280521. [DOI] [PubMed] [Google Scholar]
- 10.Bailey SM, Cunningham CC. Effect of dietary fat on chronic ethanol-induced oxidative stress in hepatocytes. Alcoholism: Clin Exp Res. 1999;23:1210–8. [PubMed] [Google Scholar]
- 11.Mitchell DY, Petersen DR. Inhibition of rat hepatic mitochondrial aldehyde dehydrogenase-mediated acetaldehyde oxidation by trans-4-hydroxy-2-nonenal. Hepatology. 1991;13:728–34. doi: 10.1016/0270-9139(91)92572-p. [DOI] [PubMed] [Google Scholar]
- 12.Bailey SM, Patel VB, Young TA, Asayama K, Cunningham CC. Chronic ethanol consumption alters the glutathione/glutathione peroxidase-1 system and protein oxidation status in rat liver. Alcoholism: Clin Exp Res. 2001;25:726–33. [PubMed] [Google Scholar]
- 13.Carbone DL, Doorn JA, Kiebler Z, Sampey BP, Petersen DR. Inhibition of Hsp72-mediated protein refolding by 4-hydroxy-2-nonenal. Chem Res Toxicol. 2004;17:1459–67. doi: 10.1021/tx049838g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carbone DL, Doorn JA, Kiebler Z, Ickes BR, Petersen DR. Modification of Heat Shock Protein 90 by 4-Hydroxynonenal in a Rat Model of Chronic Alcoholic Liver Disease. J Pharmacol Exp Ther. 2005;315:8–15. doi: 10.1124/jpet.105.088088. [DOI] [PubMed] [Google Scholar]
- 15.Bardag-Gorce F, Li J, French BA, French SW. The effect of ethanol-induced CYP2E1 on proteasome activity: the role of 4-hydroxynonenal. Exp Mol Pathol. 2005;78:109–15. doi: 10.1016/j.yexmp.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Chen JJ, Robinson NC, Schenker S, Frosto TA, Henderson GI. Formation of 4-hydroxynonenal adducts with cytochrome c oxidase in rats following short-term ethanol intake. Hepatology. 1999;29:1792–8. doi: 10.1002/hep.510290611. [DOI] [PubMed] [Google Scholar]
- 17.Lefevre A, Adler H, Lieber CS. Effect of ethanol on ketone metabolism. J Clin Invest. 1970;49:1775–82. doi: 10.1172/JCI106395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lieber CS, Lasker JM, DeCarli LM, Saeli J, Wojtowicz T. Role of acetone, dietary fat and total energy intake in induction of hepatic microsomal ethanol oxidizing system. J Pharmacol Exp Ther. 1988;247:791–5. [PubMed] [Google Scholar]
- 19.Lieber CS, DeCarli LM. The feeding of alcohol in liquid diets: Two decades of applications and 1982 update. Alcohol Clin Exp Res. 1982;6:523–31. doi: 10.1111/j.1530-0277.1982.tb05017.x. [DOI] [PubMed] [Google Scholar]
- 20.Spach PI, Parce JW, Cunningham CC. Effect of chronic ethanol administration on energy metabolism and phospholipase A2 activity in rat liver. Biochem J. 1979;178:23–33. doi: 10.1042/bj1780023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young TA, Bailey SM, Van Horn CG, Cunningham CC. Chronic ethanol consumption decreases mitochondrial and glycolytic production of ATP in liver. Alcohol Alcohol. 2006;41:254–60. doi: 10.1093/alcalc/agl017. [DOI] [PubMed] [Google Scholar]
- 22.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein Measurement with the Folin Phenol Reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 23.O’Farrell PZ, Goodman HM, O’Farrell PH. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977;12:1133–42. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- 24.Cahill A, Baio DL, Cunningham CC. Isolation and characterization of rat liver mitochondrial ribosomes. Anal Biochem. 1995;232:47–55. doi: 10.1006/abio.1995.9962. [DOI] [PubMed] [Google Scholar]
- 25.Eng JK, McCormack AL, Yates I., JR An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J Am Soc Mass Spectrom. 1994;5:976–89. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 26.Chittum HS, Lane WS, Carlson BA, Roller PP, Lung FD, Lee BJ, Hatfield DL. Rabbit beta-globin is extended beyond its UGA stop codon by multiple suppressions and translational reading gaps. Biochemistry. 1998;37:10866–70. doi: 10.1021/bi981042r. [DOI] [PubMed] [Google Scholar]
- 27.Quant PA. The role of mitochondrial HMG-CoA synthase in regulation of ketogenesis. Essays Biochem. 1994;28:13–25. [PubMed] [Google Scholar]
- 28.Hegardt FG. Mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase: a control enzyme in ketogenesis. Biochem J. 1999;338(Pt 3):569–82. [PMC free article] [PubMed] [Google Scholar]
- 29.Lascelles CV, Quant PA. Investigation of human hepatic mitochondrial 3-hydroxy-3-methylglutaryl-coenzyme A synthase in postmortem or biopsy tissue. Clin Chim Acta. 1997;260:85–96. doi: 10.1016/s0009-8981(96)06507-2. [DOI] [PubMed] [Google Scholar]
- 30.Spach PI, Cunningham CC. Control of state 3 respiration in liver mitochondria from rats subjected to chronic ethanol consumption. Biochim Biophys Acta. 1987;894:460–7. doi: 10.1016/0005-2728(87)90125-3. [DOI] [PubMed] [Google Scholar]
- 31.Chen JJ, Schenker S, Frosto TA, Henderson GI. Inhibition of cytochrome c oxidase activity by 4-hydroxynonenal (HNE) - Role of HNE adduct formation with the enzyme subunits. Biochim Biophys Acta Gen Subj. 1998;1380:336–44. doi: 10.1016/s0304-4165(98)00002-6. [DOI] [PubMed] [Google Scholar]
- 32.Picklo MJ, Amarnath V, McIntyre JO, Graham DG, Montine TJ. 4-Hydroxy-2(E)-nonenal inhibits CNS mitochondrial respiration at multiple sites. J Neurochem. 1999;72:1617–24. doi: 10.1046/j.1471-4159.1999.721617.x. [DOI] [PubMed] [Google Scholar]
- 33.Vieira HLA, Belzacq AS, Haouzi D, Bernassola F, Cohen I, Jacotot E, Ferri KF, El Hamel C, Bartle LM, Melino G, Brenner C, Goldmacher V, Kroemer G. The adenine nucleotide translocator: a target of nitric oxide, peroxynitrite, and 4-hydroxynonenal. Oncogene. 2001;20:4305–16. doi: 10.1038/sj.onc.1204575. [DOI] [PubMed] [Google Scholar]
- 34.Echtay KS, Pakay JL, Esteves TC, Brand MD. Hydroxynonenal and uncoupling proteins: a model for protection against oxidative damage. Biofactors. 2005;24:119–30. doi: 10.1002/biof.5520240114. [DOI] [PubMed] [Google Scholar]
- 35.Paradis V, Kollinger M, Fabre M, Holstege A, Poynard T, Bedossa P. In situ detection of lipid peroxidation by-products in chronic liver diseases. Hepatology. 1997;26:135–42. doi: 10.1053/jhep.1997.v26.pm0009214462. [DOI] [PubMed] [Google Scholar]
- 36.Niemelä O, Parkkila S, Britton RS, Brunt E, Janney C, Bacon B. Hepatic lipid peroxidation in hereditary hemochromatosis and alcoholic liver injury. J Lab Clin Med. 1999;133:451–60. doi: 10.1016/s0022-2143(99)90022-7. [DOI] [PubMed] [Google Scholar]
- 37.Ohhira M, Ohtake T, Matsumoto A, Saito H, Ikuta K, Fujimoto Y, Ono M, Toyokuni S, Kohgo Y. Immunohistochemical detection of 4-hydroxy-2-nonenal-modified-protein adducts in human alcoholic liver diseases. Alcoholism: Clin Exp Res. 1998;22(Suppl):145S, 9S. doi: 10.1111/acer.1998.22.s3_part1.145s. [DOI] [PubMed] [Google Scholar]
- 38.Niemelä O, Parkkila S, Ylä-Herttuala S, Villanueva J, Ruebner B, Halsted CH. Sequential acetaldehyde production, lipid peroxidation, and fibrogenesis in micropig model of alcohol-induced liver disease. Hepatology. 1995;22:1208–14. [PubMed] [Google Scholar]
- 39.Niemelä O, Parkkila S, Pasanen M, Iimuro Y, Bradford B, Thurman RG. Early alcoholic liver injury: Formation of protein adducts with acetaldehyde and lipid peroxidation products, and expression of CYP2E1 and CYP3A. Alcoholism: Clin Exp Res. 1998;22:2118–24. doi: 10.1111/j.1530-0277.1998.tb05925.x. [DOI] [PubMed] [Google Scholar]
- 40.Ronis MJ, Butura A, Sampey BP, Shankar K, Prior RL, Korourian S, Albano E, Ingelman-Sundberg M, Petersen DR, Badger TM. Effects of N-acetylcysteine on ethanol-induced hepatotoxicity in rats fed via total enteral nutrition. Free Radic Biol Med. 2005;39:619–30. doi: 10.1016/j.freeradbiomed.2005.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hartley DP, Lindahl R, Petersen DR. Covalent modification of class 2 and class 3 aldehyde dehydrogenase by 4-hydroxynonenal. Adv Exp Med Biol. 1995;372:93–101. doi: 10.1007/978-1-4615-1965-2_13. [DOI] [PubMed] [Google Scholar]
- 42.Echtay KS, Esteves TC, Pakay JL, Jekabsons MB, Lambert AJ, Portero-Otin M, Pamplona R, Vidal-Puig AJ, Wang S, Roebuck SJ, Brand MD. A signalling role for 4-hydroxy-2-nonenal in regulation of mitochondrial uncoupling. Embo J. 2003;22:4103–10. doi: 10.1093/emboj/cdg412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishii T, Tatsuda E, Kumazawa S, Nakayama T, Uchida K. Molecular basis of enzyme inactivation by an endogenous electrophile 4-hydroxy-2-nonenal: identification of modification sites in glyceraldehyde-3-phosphate dehydrogenase. Biochemistry. 2003;42:3474–80. doi: 10.1021/bi027172o. [DOI] [PubMed] [Google Scholar]
- 44.Humphries KM, Szweda LI. Selective inactivation of alpha-ketoglutarate dehydrogenase and pyruvate dehydrogenase: reaction of lipoic acid with 4-hydroxy-2-nonenal. Biochemistry. 1998;37:15835–41. doi: 10.1021/bi981512h. [DOI] [PubMed] [Google Scholar]
- 45.Benderdour M, Charron G, DeBlois D, Comte B, Des Rosiers C. Cardiac mitochondrial NADP+-isocitrate dehydrogenase is inactivated through 4-hydroxynonenal adduct formation: an event that precedes hypertrophy development. J Biol Chem. 2003;278:45154–9. doi: 10.1074/jbc.M306285200. [DOI] [PubMed] [Google Scholar]
- 46.Chen JJ, Henderson GI, Freeman GL. Role of 4-hydroxynonenal in modification of cytochrome c oxidase in ischemia/reperfused rat heart. J Mol Cell Cardiol. 2001;33:1919–27. doi: 10.1006/jmcc.2001.1454. [DOI] [PubMed] [Google Scholar]
- 47.Miziorko HM, Behnke CE, Wang HH. Mapping of reactive sulfhydryls in avian liver 3-hydroxy-3-methylglutaryl coenzyme A synthase. Biochim Biophys Acta. 1990;1041:273–8. doi: 10.1016/0167-4838(90)90284-m. [DOI] [PubMed] [Google Scholar]
- 48.Misra I, Miziorko HM. Evidence for the interaction of avian 3-hydroxy-3-methylglutaryl-CoA synthase histidine 264 with acetoacetyl-CoA. Biochemistry. 1996;35:9610–6. doi: 10.1021/bi9605797. [DOI] [PubMed] [Google Scholar]
- 49.Doorn JA, Petersen DR. Covalent adduction of nucleophilic amino acids by 4-hydroxynonenal and 4-oxononenal. Chem Biol Interact. 2003;143–144:93–100. doi: 10.1016/s0009-2797(02)00178-3. [DOI] [PubMed] [Google Scholar]
- 50.Bennaars-Eiden A, Higgins L, Hertzel AV, Kapphahn RJ, Ferrington DA, Bernlohr DA. Covalent modification of epithelial fatty acid-binding protein by 4-hydroxynonenal in vitro and in vivo - Evidence for a role in antioxidant biology. J Biol Chem. 2002;277:50693–702. doi: 10.1074/jbc.M209493200. [DOI] [PubMed] [Google Scholar]
- 51.Isom AL, Barnes S, Wilson L, Kirk M, Coward L, Darley-Usmar V. Modification of Cytochrome c by 4-hydroxy- 2-nonenal: evidence for histidine, lysine, and arginine-aldehyde adducts. J Am Soc Mass Spectrom. 2004;15:1136–47. doi: 10.1016/j.jasms.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 52.Uchida K, Stadtman ER. Covalent attachment of 4-hydroxynonenal to glyceraldehyde-3-phosphate dehydrogenase. A possible involvement of intra- and intermolecular cross-linking reaction. J Biol Chem. 1993;268:6388–93. [PubMed] [Google Scholar]
- 53.Moser J, Bagchi D, Akubue PI, Stohs SJ. Excretion of malondialdehyde, formaldehyde, acetaldehyde and acetone in the urine of rats following acute and chronic administration of ethanol. Alcohol Alcohol. 1993;28:287–95. [PubMed] [Google Scholar]
- 54.Lieber CS. Hepatic, metabolic and toxic effects of ethanol: 1991 Update. Alcoholism: Clin Exp Res. 1991;15:573–92. doi: 10.1111/j.1530-0277.1991.tb00563.x. [DOI] [PubMed] [Google Scholar]