Abstract
Background
Optical mapping is a widely-used experimental tool providing high-resolution recordings of cardiac electrical activity. However, the technique is limited by signal distortion due to photon scattering in the tissue. Computational models of the fluorescence recording are capable of assessing these distortion effects, providing important insight to assist experimental data interpretation.
Methods
We present results from a new panoramic optical mapping model, which is used to assess distortion in ventricular optical mapping signals during pacing and arrhythmogenesis arising from three-dimensional photon scattering.
Results/Conclusions
We demonstrate that accurate consideration of wavefront propagation within the complex ventricular structure, along with accurate representation of photon scattering in three dimensions, is essential to faithfully assess distortion effects arising during optical mapping. In this paper, examined effects include: (i) the specific morphology of the optical action potential upstroke during pacing; and (ii) the shift in the location of epicardial phase singularities obtained from fluorescent maps.
Keywords: Optical mapping, computer simulations, cardiac electrophysiology, arrhythmogenesis, phase singularities
1 Introduction
Optical mapping is a widely used experimental technique capable of providing high spatio-temporal resolution recordings of electrical activity from the surface of the heart. The technique utilizes specialized membrane-bound fluorescent dyes, which, upon illumination at the correct wavelength, transduce local changes in transmembrane potentials as changes in fluorescent emission. However, an important limitation of the optical mapping technique is signal distortion due to scattering of fluorescent photons from excited tissue. Such distortion could compromise experimental data analysis and interpretation as well as the use of optical recordings to validate computer simulations of electrical activity.
Indeed, the optical mapping technique does not record electrical potentials from the epicardium only. Instead, the optical signal contains a form of depth-weighted average of transmembrane potential levels from within a scattering volume of tissue beneath the epicardial recording site, acting to blur the signal detected from the tissue surface. This photon scattering artifact has been suggested to underlie characteristics of experimentally recorded optical signals, which render them different from both epicardial microelectrode recordings. For example, differences include the prolongation of the paced optical action potential upstroke (1, 2) and the existence of dual-humped action potentials recorded from the vicinity of the scroll wave filament during arrhythmias (4, 5).
Recently, computational modeling studies have sought to simulate these distortion effects over simplified models of ventricular geometry, to gain a basic understanding of the underlying mechanisms of this photon scattering artifact. However, these preliminary models have proved insufficient in successfully simulating the interaction of 3D photon scattering with the complex ventricular geometry and heterogeneity present is experimental preparations. The complex 3D cardiac ventricular geometry and the presence of both structural and anatomical heterogeneity is known to play an important role in the dynamics of electrical activity, both during normal sinus rhythm and, more importantly, during arrhythmogenesis and defibrillation. Recently, the development of panoramic whole-heart optical mapping systems have demonstrated the ability of the optical imaging technique to track and measure complex activity over the entire epicardial surface (6). As a result, it has become necessary to develop computational models of photon scattering in the entire heart to simulate the generation of panoramic fluorescent signals over 3D realistic ventricular geometries and assess the distortion present in them.
In this article, we first summarize the development of models of optical recording in simplified geometries. We then proceed to present our recent work on simulating panoramic optical imaging, where we take into account fluorescent photon scattering in three dimensions. Specifically, we focus on the important role cardiac anatomy plays in distortion of the fluorescent signal during pacing and in episodes of arrhythmogenesis.
2 Simulation of Photon Scattering in Geometrically Simplistic Models
Early studies, which simulated the distortion effects of photon scattering in optical recordings, were performed over model preparations of simplistic, regular geometries. The study by Ding et al. investigated the origin, within the tissue depth, of recorded epicardial fluorescent signals, using a combination of Monte Carlo simulations and optical mapping experiments (6). The study demonstrated that the majority of the content of the fluorescent signal at a given epicardial site originated from an extended 3D volume of tissue beneath the recording site itself, extending ~ 1–2 mm both radially and in depth. The specific dimensions of this scattering volume were found to depend upon the optical properties of the tissue at both the excitation and emission wavelengths, as well as on the particulars of the optical mapping set-up.
Despite the knowledge that photon scattering in optical mapping experiments was an inherently 3D phenomenon, the first group of studies which attempted to simulate the effects of this distortion on the recorded fluorescent signals did so by applying simplified one-dimensional depth-weighted averaging techniques to simulated transmembrane signals from beneath the epicardial surface (2, 7, 8). This method considered only the distortional effects of photon scattering in the direction normal to the tissue surface. Using simple exponential weighting functions in depth, Baxter et al. simulated the attenuation of illumination light with depth as well as the fluorescent emission profile within the myocardial wall. The weighting functions were obtained by fitting experimental data from slabs of sheep myocardium for trans- and epi-illumination (2). The authors demonstrated that collection of fluorescent photons from depth, modeled by the weighting functions, could account for the experimentally-documented prolongation of optical action potential upstroke (1, 2) as compared to the more rapid upstroke obtained by microelectrode measurements.
Later studies by Janks & Roth (8) and Bray & Wikswo (9) applied these weighting functions to complex transmembrane potential distributions and wavefront dynamics, which resulted in intriguing scattering artifact phenomena. Averaging of transmembrane signals from depth (1D scattering) was suggested by Janks & Roth (8) to account for the under-estimation of optical transmembrane potential magnitude during stimulation through a unipolar electrode, relative to the calculated values from a bidomain model. This reduction was thought to result from additional contributions, to the optical signal, of weakly polarized mid-myocardial layers of tissue. However, the above studies included only the effects of scattering from depth, thus ignoring transduction of lateral changes in transmembrane potential in the epicardial plane. Using a similar method, Bray & Wikswo (9) demonstrated that transduction of information regarding entirely-intramural wavefronts propagating beneath an epicardial recording site during reentrant activity could be responsible for the existence of dual-humped action potentials. These dual-humped action potentials, whereby a second hump is recorded during the plateau phase following the initial depolarization, had morphologies similar to those obtained during experimental recordings of arrhythmogenesis (4, 5). However, the prevalence of dual-humped action potentials in experimental recordings for a wide variety of different reentrant arrhythmia scenarios, whereby wholly intramural wavefronts may or may not exist, suggested that 1D scattering from depth might not be the only cause of their formation, and therefore a full 3D representation of the distortion effects of scattering had to be considered.
The studies described above had limited applicability because they ignored lateral scattering in planes parallel to the epicardium, where both excitation light intensity and fluorescent emission are strongest and thus likely to contribute to signal distortion. The landmark study by Hyatt et al. was the first to analyze, for simplified model geometries, the optical signal distortion effects due to 3D photon scattering in cardiac tissue (9). The authors simulated the global movement and diffusion of fluorescent photons within a slab of ventricular tissue through an analytical solution to the 3D steady-state photon diffusion equation. Uniform epicardial illumination was modeled as a simple mono-exponential decay of light intensity into the tissue depth. The study demonstrated that, during pacing, the optical action potential upstroke associated with a uniform propagating wavefront was distorted and prolonged with respect to the “true” epicardial action potential upstroke, derived from a solution to the monodomain model of cardiac electrical activity, which also acted as an input to the optical scattering model. More importantly, the authors demonstrated that the morphology of the distorted optical upstroke was specific to the global intramural direction of wavefront propagation: propagation parallel to the epicardium resulted in a symmetrically distorted upstroke, whilst propagation towards or away from the epicardium resulted in an asymmetrically distorted upstroke. These results were later validated experimentally, confirming that in tissue preparations with regular geometries, global wavefront propagation direction could be deduced from surface optical action potential upstroke morphology (11).
3 Photon Scattering Effects in 3D: The Role of Organ Anatomy
The 3D interaction between scattered photons and complex wavefronts in the heart cannot be examined with tissue models of regular geometries. This is particularly relevant to whole-heart panoramic optical imaging, increasingly used to evaluate complex wavefront dynamics associated with cardiac arrhythmias. It has been the goal of our research to address this problem, utilizing an anatomically-realistic finite-element bidomain model of the rabbit ventricles, which incorporates accurate organ geometry and fiber orientation. Such a model can represent the highly complex wavefront dynamics associated with reentrant arrhythmias throughout the volume of the ventricles. When combined with a detailed 3D model of photon transport and diffusion within cardiac tissue, the role of cardiac anatomy in optical signal distortion can be readily assessed (12). Here we present the application of our model to evaluate the impact of 3D fluorescent photon scattering on the wavefront dynamics during pacing and in reentrant arrhythmia, underscoring the role of organ anatomy.
3.1 Simulation of Distortion Effects during Pacing
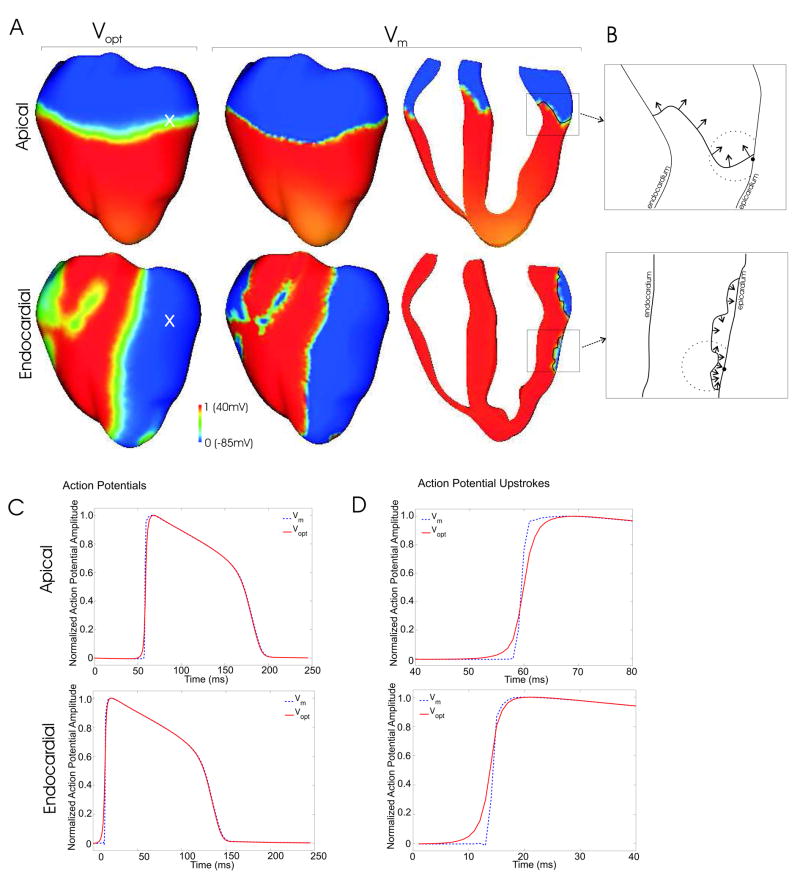
To model photon scattering in the anatomically-realistic ventricular model, and to accurately synthesize the optical mapping signals obtained in panoramic fluorescent recordings, photon scattering was simulated using the photon diffusion equation method, similar to that of Hyatt et al. (12). The model used a novel boundary condition, the partial current boundary condition, which successfully accounted for photon reflection at the epicardial surface due to a refractive index mismatch with the surrounding medium. Figure 1A presents a comparison of the epicardial distribution of the simulated optical signal (Vopt) with the input to the optical scattering model, the transmembrane potential distribution derived from bidomain simulations (Vm), for apical (50ms) and endocardial (6ms) pacing. Figure 1A demonstrates that, consistent with previous studies (9,10), there is a significant blurring in the optical activation wavefront, for both stimulation protocols, relative to that predicted by the bidomain simulations. Figure 1C & D shows that this blurring in the wavefront results from a prolonged optical action potential upstroke for Vopt (6.16ms for apical, 4.87ms for endocardial) as compared to that of Vm (1.59ms for apical, 0.97ms for endocardial). This distortion in Vopt upstroke depends sensitively upon the optical properties of the tissue (scattering and absorption coefficients), as well as on optical parameters associated with the particular experimental set-up (for example, the refractive index of the medium surrounding the heart). However, although Vopt action potential upstrokes were prolonged with respect to those of Vm, the specific dependence of Vopt upstroke morphology upon intramural wavefront propagation direction, as found by Hyatt et al (10, 11), was not documented in our study.
FIGURE 1.
Wavefront propagation in the rabbit ventricular bidomain model following apical and endocardial stimulation. A Epicardial optical Vopt (left) and transmembrane Vm (center) distributions as well as an apex-base cross section depicting intramural Vm distribution (right), for apical (top, 50ms after pacing stimulus) and endocardial (bottom, 6ms after pacing stimulus) stimulation. B Highlighted regions show local wavefront propagation direction, depicted by black arrows. The optical detection site on the epicardium is shown by the solid circle, with the approximate scattering volume depicted by the dashed partial-circle. C Action potential traces for Vm and Vopt from epicardial recording sites whose locations are shown by white crosses in the left-hand images of Figure 1A. Action potential upstrokes are presented in D.
In our simulations, the use of an anatomically-realistic model of electrical activity produces wavefront propagation with a high degree of complexity. The bidomain representation of the myocardium provides an accurate description of the interaction between tissue and surrounding volume conductor (blood or bath), which affects the nature of wavefront propagation beneath the epi- and endocardial surfaces. In addition, the use of anatomically-realistic geometry and fiber orientation, combined with realistic stimulation protocols, produces wavefronts that spread throughout the volume of the ventricles with a highly non-planar, almost jagged appearance. This is clearly documented in the apex-base cross-section Vm distributions shown in Figure 1A (right) and emphasized by the schematic diagrams in Figure 1B (dashed partial-circles represent the approximate scattering volume associated with a particular epicardial recording site, from which the majority of detected fluorescent photons originate).
For both stimulation protocols in Figure 1, although the global direction of wavefront propagation is fairly well defined (i.e. parallel to the epicardium for apical stimulation, and towards the epicardium for endocardial stimulation), the local direction of wavefront propagation within a particular scattering volume associated with an epicardial recording site is not (Figure 1B): the local angle of wavefront orientation with respect to the epicardium changes dramatically within the scattering volume (indicated by the solid circle), rotating by more than 90 degrees between different points along the wavefront. Furthermore, the wavefront itself is of intrinsically 3D nature, and thus, variations in local wavefront orientation, such as those shown in Figure 1B, are even more complex in three dimensions. In addition, the dynamic nature of excitation propagation results in a constant change in the shape of the wavefront as it passes through the scattering volume, and therefore the local orientation of the wavefront within the scattering volume changes over the course of the action potential upstroke. Consequently, the scattered photons, which are detected at the recording site, do not convey reliable and consistent information regarding the direction of wavefront propagation. Thus, although optical action potential upstrokes undoubtedly contain a wealth of useful information regarding intramural electrical dynamics, their utility in accurately determining intramural wavefront propagation patterns in whole-heart studies remains to be demonstrated.
3.2 Simulation of Distortion Effects During Arrhythmia
During pacing, due to the nature of global wavefront propagation, fluorescent optical signals are, except for prolonged upstrokes, very similar to the corresponding transmembane signals. However, the complex wavefront propagation during arrhythmia results in highly heterogeneous distributions of transmembrane potentials within a particular scattering volume associated with a surface fluorescent recording site, especially in regions close to scroll wave filaments, the 3D organizing centers of reentrant activity. These heterogeneous transmembrane potential distributions are conveyed by the scattered fluorescent photons originating from within this volume, and manifest themselves in the optical signal. As a result, important differences exist between optical and transmembrane potential signals during arrhythmia. Accurate assessment of the mechanisms giving rise to these differences necessitates the use of a 3D photon scattering model as well as simulations over a realistic representation of ventricular geometry and anatomy.
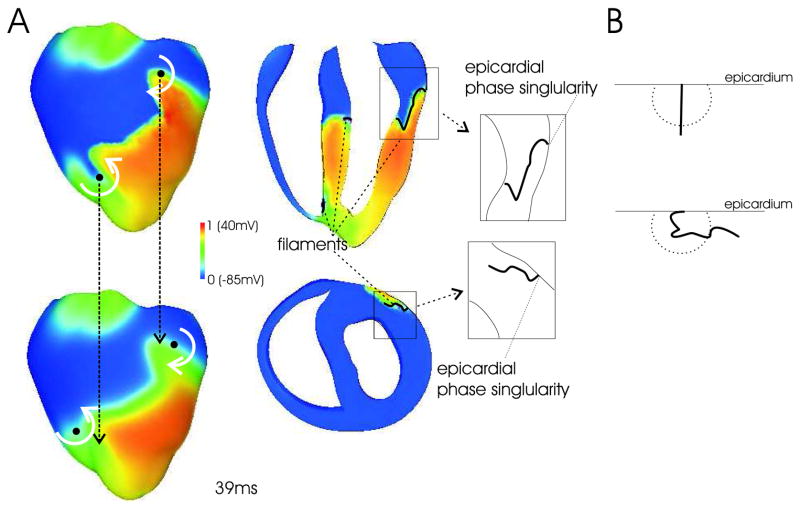
Figure 2A depicts epicardial Vm distributions (top) and corresponding epicardial Vopt maps (bottom) during an episode of shock-induced arrhythmogenesis; presented activity is at 39ms following the shock. Figure 2A (right) also depicts apex-base and anterior-posterior cross-sections through the 3D Vm distribution as well the locations of the scroll-wave filaments associated with the reentry; schematic diagrams highlight the specific orientation of the filament in the upper left ventricle (LV). Filaments were determined from the Vm distributions using the method of Larson et al (13); all signals were normalized with respect to the action potential amplitude during pacing, as in Figure 1.
FIGURE 2.
A (Left) Epicardial distribution of Vm (top) and Vopt (bottom) during an episode of arrhythmia induction with an electric shock (snap-shots at 39ms post-shock). White curved arrows indicate the direction of rotation in the figure-of-eight reentrant pattern. Solid black circles indicate the location of the epicardial phase singularity on the epicardial surface. Dashed black arrows show the relative shift in these locations between Vm and Vopt maps. (Right) Transmural apex-base (top) and anterior-posterior (bottom) cross-sections of Vm distribution depicting the location of the intramural filaments (shown in black). Highlighted in-sets show schematic diagrams of the complex orientation of the filament in the upper LV wall. B Examples of filaments with normal (top) and complex (bottom) orientations relative to the epicardial surface, and the scattering volume associated with the epicardial recording site.
As in previous studies, the dominant type of ensuing reentrant pattern for the shock protocol used here is a figure-of-eight reentry with one rotor on the anterior and another on the posterior (3), with rotation directions shown by curved white arrows in Figure 2A (left). The blurring in the width of the activation wavefront seen in the Vopt maps of Figure 1A is again evident here. However, the distortion in the optical signal recorded close to the core of epicardial reentrant activity leads to an error in the calculated position of the epicardial phase singularities (location of attachment of filament to epicardial surface, shown as black circles in Figure 2A). In the episode of shock-induced arrhythmogenesis shown, this shift can be up to 4.08mm in the plane of the epicardium. Since phase singularities (and scroll-wave filaments) are surrounded by different levels of transmembrane potential, distribution of Vm within a scattering volume associated with a recording site near the reentrant core is highly heterogeneous. This heterogeneity in transmembrane potential is transduced by photon scattering, resulting in fluorescent signal distortion.
Assessing correctly the magnitude of the distortion in the optical signal depends critically upon the inclusion of 3D photon scattering in the model. If photon scattering were assumed to occur only in depth (1D, i.e. in direction normal to the surface), then a shift in the phase singularity location would occur only if the filament is at an angle to the surface. Indeed, for a filament orientated normally to the epicardium (shown schematically in Figure 2B, top) potential values do not differ along the normal, and 1D depth averaging techniques would thus predict no shift. However, correctly accounting for photon scattering within a 3D scattering volume results in a shift in the phase singularity locations, even if the filament is normal to the surface, due to differences in transmembrane potential levels within this volume. This shift is amplified for arbitrary filament orientations (Figure 2B, bottom), and particularly by filament bending below the epicardium, as variations in transmembrane potential then occur closer to the surface, where fluorescence is stronger. Complex filament orientation and filament bending is present in Figure 2A, right (filament shown in black with the ventricles rotated relative to the left-hand images). Thus, the interaction of 3D, rather than 1D, photon scattering, and the complex filament orientations associated with the anatomy of the organ itself, explains the large shift in phase singularity location between the Vopt and Vm maps, which is significantly larger than shifts of just 0.57 ± 0.16mm found by Bray & Wikswo, in their 1D scattering study (9). The shift in the positions of optically-recorded phase singularities, as found here, could have important implications for protocols which use the optical mapping technique to accurately localize phase singularities (14,15).
4 Conclusions
The inquiry into distortional effects of fluorescent photon scattering in optical recordings of cardiac electrical activity has made important advances over the recent years. Such inquiry is important because it assists in the accurate interpretation of experimentally obtained fluorescent recordings by explaining unusual characteristics of the signals not observed in microelectrode recordings of similar phenomena. Modeling has been instrumental in achieving these goals. However, since photon movement and scattering is a truly 3D phenomenon, simple 1D depth-weighted averaging models are insufficient to fully evaluate the distortion effects in fluorescent signals. In this article, we demonstrate that the interaction of 3D photon scattering with the complex anatomy of the heart and the complex wavefront propagation is essential to accurately simulate the distortional scattering artifacts in experimental optical recordings of paced and reentrant activity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Girouard SD, Laurita KR, Rosenbaum DS. Unique properties of cardiac action potentials with voltage-sensitive dyes. J Cardiovasc Electrophysiol. 1996;7(11):1024–1038. doi: 10.1111/j.1540-8167.1996.tb00478.x. [DOI] [PubMed] [Google Scholar]
- 2.Baxter WT, Mironov SF, Zaitsev AV, Jalife J, Pertsoz AM. Visualizing excitation waves inside cardiac muscle using transillumination. Biophys J. 2001;80:516–530. doi: 10.1016/S0006-3495(01)76034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodriguez B, Li L, Eason JC, Efimov IR, Trayanova NA. Differences between left and right ventricular chamber geometry affect cardiac vulnerability to electric shocks. Circ Res. 2005;97:168–175. doi: 10.1161/01.RES.0000174429.00987.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Efimov IR, Aguel F, Cheng Y, Wollebzier B, Trayanova NA. Virtual electrode polarization in the far field: implications for external defibrilation. Am J Physiol Heart Circ Physiol. 2000;279:H1055–H1070. doi: 10.1152/ajpheart.2000.279.3.H1055. [DOI] [PubMed] [Google Scholar]
- 5.Efimov IR, Sidorov V, Cheng Y, Wollenzier B. Evidence of three-dimensional scroll waves with ribbon-shaped filaments as a mechanism of ventricular tachycardia in isolated rabbit heart. J Cardiovasc Electrophysiol. 1999;10:1451–1462. doi: 10.1111/j.1540-8167.1999.tb00204.x. [DOI] [PubMed] [Google Scholar]
- 6.Kay MW, Amison PM, Rogers JM. Three-Dimensional Surface Reconstruction and Panoramic Optical Mapping of Large Hearts. IEEE Trans Biomed Eng. 2005;(7):1219–29. doi: 10.1109/TBME.2004.827261. [DOI] [PubMed] [Google Scholar]
- 7.Ding L, Splinter R, Knisley S. Quantifying spatial localization of optical mapping using monte carlo simulations. IEEE Trans Biomed Eng. 2001;48(10):1098–1107. doi: 10.1109/10.951512. [DOI] [PubMed] [Google Scholar]
- 8.Janks DL, Roth BJ. Averaging over depth during optical mapping of unipolar simulation. IEEE Transactions on Biomedical Engineering. 2002;49:1051–1054. doi: 10.1109/TBME.2002.802057. [DOI] [PubMed] [Google Scholar]
- 9.Bray MA, Wikswo JP. Examination of optical depth effects on fluorescence imaging of cardiac propagation. Biophys J. 2003;85:4134–4145. doi: 10.1016/S0006-3495(03)74825-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hyatt CJ, Mironov SF, Wellner M, Berenfeld O, Popp AK, Weitz DA, Jalife J, Pertsov AM. Synthesis of voltage-sensitive fluorescence signals from three-dimensional myocardial activation patterns. Biophys J. 2003;85:2673–2683. doi: 10.1016/s0006-3495(03)74690-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hyatt CJ, Mironov SF, Vetter FJ, Zemlin CW, Pertsov AM. Optical action potential upstroke morphology reveals near-surface transmural propagation direction. Circ Res. 2005 Aug 5;97(3):277–84. doi: 10.1161/01.RES.0000176022.74579.47. [DOI] [PubMed] [Google Scholar]
- 12.Bishop MJ, Rodriguez B, Eason J, Whiteley JP, Trayanova NA, Gavaghan DJ. Synthesis of voltage-sensitive optical signals: Application to panoramic optical mapping. Biophys J. 2006;90:2938–2945. doi: 10.1529/biophysj.105.076505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larson C, Dragnev L, Trayanova NA. Analysis of electrically-induced reentrant circuits in a sheet of myocardium. Annals Biomed Eng. 2003;31:768–780. doi: 10.1114/1.1581289. [DOI] [PubMed] [Google Scholar]
- 14.Iyer AN, Gray RA. An Experimentalist’s approach to accurate localization of phase singularities during reentry. Annals Biomed Eng. 2001;29:47–59. doi: 10.1114/1.1335538. [DOI] [PubMed] [Google Scholar]
- 15.Ashihara T, Namba T, Ito M, Ikeda T, Nakazawa K, Trayanova NA. Spiral wave control by a localized stimulus: A bidomain model study. J Cardiovasc Electrophysiol. 2004;15(2):226–33. doi: 10.1046/j.1540-8167.2004.03381.x. [DOI] [PubMed] [Google Scholar]