Abstract
Background
Transmembrane activator and calcium modulator and cyclophilin ligand (CAML) interactor (TACI) is a receptor used by B-cell activating factor of the TNF family (BAFF) and a proliferation inducing ligand (APRIL) to induce isotype switching independently of CD40 and is mutated in patients with common variable immunodeficiency (CVID).
Objective
To determine whether TACI and CD40 cooperate in inducing class switch recombination (CSR) and immunoglobulin production.
Methods
Naïve mouse B cells were stimulated with suboptimal concentrations of anti-CD40+IL-4 in the presence or absence of APRIL or anti-TACI. IgG1 and IgE production was measured by ELISA. mRNA for Cγ1 and Cε germ line transcripts, activation-induced cytidine deaminase (AICDA) and mature γ1 and ε transcripts were measured by RT-PCR. Plasmablasts were enumerated by syndecan-1/CD138 staining. Interferon regulatory factor 4 (IRF4), B lymphocyte-induced maturation protein 1 (Blimp1) and IL-6 mRNA expression was measured by quantitative PCR.
Results
TACI ligation enhanced IgG1 and IgE secretion by naïve murine B cells stimulated by anti-CD40+IL-4, with little effect on B cell proliferation or CSR. In contrast, TACI ligation of anti-CD40+IL-4 stimulated B cells induced a significant increase in syndecan-1/CD138 positive cells. TACI ligation caused a modest, but significant increase in the expression of IRF4, with no detectable change in Blimp1 expression.
Conclusion
TACI and CD40 signaling converge to promote B cell differentiation into plasmabalsts.
Clinical implications
Our data suggest that TACI dysfunction could contribute to the impaired antibody response to T dependent antigens in CVID.
CAPSULE SUMMARY
This work shows that CD40 and TACI cooperate to promote B cell differentiation into plasma cells and increase immunoglobulin production.
Keywords: CD40, TACI, CVID, plasma cell, Immunoglobulin, B cells
INTRODUCTION
Humoral immune response to antigens is characterized by the production of high affinity antibodies by B lymphocytes, which terminally differentiate into plasma cells. B cells encounter antigens in the secondary lymphoid organs where they proliferate and undergo maturation and differentiation that includes CSR, affinity maturation and differentiation into memory B cells or antibody-secreting plasma cells. These processes are initiated and regulated by signals derived from specific ligand-receptor interactions such as between CD40 ligand (CD40L, CD154) and its receptor CD40, between APRIL (CD256, TNFSF13) and BAFF (CD257, TNFSF13B) and their receptor TACI (CD267, TNFRSF13B)1, 2 and between Toll-like receptor (TLR) ligands and their receptors expressed on B cells3.
Differentiation of activated B cells into plasma cells involves repression of genes typically expressed by germinal center (GC) B-cells, such as Pax5, AICDA and Bcl-6, and upregulation of plasma cell specific genes that include the transcription factors Prdm1/Blimp1, IRF4 and X-box binding protein 1 (XBP-1), and the proteoglycan syndecan-1/CD138. Both Blimp1 and IRF4 regulate the expression of XBP-14, and B cells deficient in Blimp1 or IRF4 are impaired in their ability to undergo plasma cell differentiation5, 6. It has been previously shown that Blimp1 expression is strongly induced by TLR4 ligation, but weakly by CD40 ligation in murine B cells7. CD40 ligation induces expression of IRF4 in human B cell lines8, but no data have been published on primary mouse B cells.
We and others9, 10 recently found that the TNFRSF13B gene, which encodes for TACI, is mutated in ~10% of patients with CVID 11, a disorder characterized by recurrent infections, hypogammaglobulinemia, defective antibody production and failure of B cells to differentiate into memory cells and plasma cells12. Naïve B cells from CVID patients with TACI mutations fail to secrete IgG and IgA in response to APRIL in vitro, but secrete normal amounts of IgG when stimulated by anti-CD40+IL-49. Similar results have been observed in B cells from TACI-/- mice1. These results suggest that CD40-induced Ig secretion in vitro is independent of TACI. Conversely, TACI induction of Ig secretion in vitro is independent of CD40 because it occurs normally in CD40 deficient B cells1. Taken together, these data suggest that the CD40 and TACI pathways may activate CSR and Ig secretion in B cells independently of each other. Nevertheless, despite intact CD40 signaling, patients with CVID are impaired in their ability to respond to T dependent antigens and to generate plasma cells12, raising the possibility that the CD40 and TACI pathways may intersect. In this study we demonstrate that these two pathways cooperate to promote B cell differentiation into plasma cells and increase immunoglobulin production.
MATERIALS AND METHODS
Mice
Balb/c mice were purchased from the Jackson Laboratory. B cell maturation antigen (BCMA)-/- mice and genetically matched (C57/Bl6-129Sv) wild type (WT) controls were previously described13. All mice were kept in a specific pathogen free animal facility. All procedures performed on the animals were in accordance with the Animal Care and Use Committee of the Children’s Hospital, Boston.
In vitro immunoglobulin production
Naïve B cells from Balb/c, WT and BCMA-/- mice were negatively sorted using a cocktail of biotin-conjugated mAbs to CD43, CD11b, Thy1.2, CD138, IgG1, IgG2a, IgG2b, IgG3, IgA and IgE and streptavidin magnetic beads (Dynal). B cells were cultured at 1×106/ml in RPMI containing 10% FCS, L-glutamine and 50 mM 2-ME (2-Mercaptoethanol) (complete medium). For Ig synthesis, B cells were cultured in one or more of the following: complete medium alone, IL-4 at 50 ng/ml (R&D Systems), suboptimal concentration of anti-CD40 mAb (75 ng/ml, BD-Pharmingen), APRIL at 1 μg/ml, plate bound polyclonal goat anti-TACI at 5 μg/ml or goat IgG at 5 μg/ml (all R&D Systems), and LPS at 10 μg/ml (Sigma). Cells were treated with 5 μg/ml polymyxin B (Sigma) except when LPS was added. After 6 days, supernatants were assayed for IgG1 and IgE by ELISA14.
RT-PCR for germ line transcripts (GLT), AICDA, and post switch (Iμ-CH) transcripts
RNA was extracted from 3-day cultured naïve B cells using TRIzol and was reverse transcribed by Supercript II RT (all Invitrogen). PCR primers used, Iμ , Iγ1, Cγ1, Iε, Cε, AICDA and β2-microglobulin (β2m), were previously described14, 15. All PCR reactions were performed on 1:1, 1:3 and 1:9 cDNA dilutions for semi-quantitative evaluation. Amplified products were separated on agarose gel and stained with ethidium bromide.
Staining and CFSE (carboxyfluoroscein succinimidyl ester) assay
Naïve B cells from Balb/c mice, stimulated with anti-CD40 for 72 hours, were stained with anti-TACI-PE (phycoeritrin, R&D) or anti-BCMA-FITC (fluorescein Isothiocynate, R&D), or were stimulated for six days as indicated and then stained with anti-CD138-PE and/or anti-IgG1 PE or biotin-conjugated (BD-Pharmingen) followed by streptavidin-APC (allophycocyanine, eBioscience). For CFSE assays, naïve B cells were loaded with 10 mM CFSE (Molecular Probes), then stimulated and stained as above. For survival assays B cells were stimulated as indicated and stained every day for six days with AnnexinV-FITC (BioVision). All staining were analyzed by FACS.
Quantitative PCR
cDNA obtained as described above was used for real time PCR reactions and run on an ABI Prism 7300 (Applied Biosystems). Taqman primers with 6-carboxyfluorescein-labeled probe for Blimp1, IRF4, IL-6 and the control β2m were obtained from Applied Biosystems. Relative gene expression among the different samples was calculated as described by Pfaffl16.
Statistics
p values were determined by the paired t test method using PRISM software.
RESULTS
APRIL increases immunoglobulin production induced by anti-CD40 and IL-4 by engaging TACI
To test the hypothesis that CD40-induced Ig production is increased by engagement of TACI, we first examined the effect of TACI ligation on Ig synthesis induced by a suboptimal concentration of anti-CD40 mAb in negatively sorted naïve splenic B cells from Balb/c mice. To determine the suboptimal concentration of anti-CD40 for our experiments, B cells were stimulated in vitro with concentrations of anti-CD40 ranging from 500 to 10 ng/ml and IL-4. Figure 1A shows that 100 ng/ml anti-CD40 consistently achieved optimal induction of IgG1 and IgE synthesis, while a concentration of 75 ng/ml of anti-CD40 resulted in just suboptimal synthesis of IgG1 and IgE. Subsequently, we used this concentration to examine the effect of TACI ligation on CD40 driven IgG1 and IgE synthesis. Fig. 1B shows that addition of APRIL caused a significant ~ 2.5 fold increase of IgG1 and IgE secretion by B cells treated with IL-4 and 75 ng/ml of anti-CD40. APRIL+IL-4 induced modest IgG1 and IgE synthesis as previously described1.
Figure 1. Effect of APRIL on IgG1 and IgE production induced by anti-CD40 and IL-4.

A) IgG1 and IgE production by naïve B cells in response to different concentrations of anti-CD40 and IL-4. B). Effect of APRIL on IgG1 and IgE production driven by a suboptimal concentration of anti-CD40 (75 ng/ml). Bars represent the mean±S.E. of three (A) and four (B) independent experiments.
APRIL binds to BCMA and heparan sulfate proteoglycans (HSPGs) in addition to TACI17, 18. Either of these receptors could potentially mediate the enhancing effect of APRIL on CD40 driven Ig production by naïve B cells. Figure 2 shows that naïve B cells express TACI, but no detectable BCMA. CD40 ligation upregulated TACI expression, but had no detectable effect on BCMA expression after 72 hrs. This finding suggests that the effect of APRIL on CD40 activation of B cells was mediated via TACI.
Figure 2. Effect of CD40 ligation on TACI expression.

FACS analysis of TACI and BCMA expression in naïve mouse B cells before and after stimulation with anti-CD40 for 72 hrs. Similar results were obtained in 3 independent experiments.
To ascertain the role of TACI in APRIL mediated enhancement of CD40 activation, we examined the effect of anti-TACI antibody. Figure 3A shows that addition of goat anti-TACI enhanced CD40+IL-4 driven IgG1 and IgE production by naïve B cells to a similar extent as APRIL. In contrast, control goat IgG had a negligible effect. Like APRIL, anti-TACI, in the presence of IL-4, induced IgG1 and IgE production (a net increase of 30.3 ng/ml for IgG1 and of 43 ng/ml of IgE), confirming our previous finding that engagement of TACI causes CSR in naive B cells.
Figure 3. Effect of TACI ligation on IgG1 and IgE production induced by anti-CD40 and IL-4.

Effect of anti-TACI on IgG1 or IgE production in B cells from (A) BALB/c mice and from (B) BCMA-/- mice and WT controls. Bars represent mean±S.E. of independent five experiments in A and four in B.
To exclude an autocrine mechanism by which engagement of TACI might induce expression of APRIL, which in turn might engage BCMA and HSPGs, we tested the ability of anti-TACI to increase Ig production induced by anti-CD40+IL-4 in B cells from BCMA-/- mice. In all experiments, B cells from WT control and BCMA-/- mice treated with anti-CD40+IL-4+ anti-TACI secreted higher amounts of IgG1 and IgE than B cells treated with anti-CD40+IL-4+control goat IgG. We observed variability between experiments in the amounts of IgG1 and IgE produced by B cells from both BCMA-/- and WT controls, possibly due to the mixed background of these mice (C57/Bl6-129Sv). For this reason we have expressed the data as fold increase in Ig secretion. There was no significant difference in the increase of IgG1 and IgE synthesis induced by TACI ligation between BCMA-/- mice and WT controls (Figure 3B). These results suggest that TACI mediates APRIL enhancement of Ig production induced by CD40. In addition, the use of anti-TACI eliminates potential contribution of BCMA or HSPGs that may occur with the use of APRIL.
TACI engagement does not increase B cell proliferation and survival induced by anti-CD40 and IL-4
CD40 driven CSR is thought to be division linked19. We tested the hypothesis that engagement of TACI increases the rate of division of CD40/IL-4 activated B cells. CFSE dye dilution analysis revealed that B cells stimulated with anti-CD40+IL-4 underwent eight division cycles in the presence of anti-TACI Ab versus nine cycles in the presence of control IgG (see Figure E1A in the Online Repository).
We also examined the effect of anti-TACI on the survival of B cells stimulated with anti-CD40. We used Annexin V staining to detect apoptotic cells at days 1, 2, 3, 4 and 6 after stimulation. As expected, the percentage of live cells was substantially higher in cells stimulated with anti-CD40+IL-4 compared to medium (see Figure E1B in the Online Repository). Addition of anti-TACI did not significantly affect the percentage or number of live cells. Altogether these data suggest that the increase in Ig production observed when both TACI and CD40 are engaged is neither due to clonal expansion nor to improved B cell survival.
TACI engagement has little effect on the induction of γ1 germ line transcript (GLT) εGLT, Iμ-Cγ1, Iμ-Cε and AID by anti-CD40 and IL-4
Molecular events involved in CSR include expression of GLTs and AICDA, followed by deletional switch recombination and expression of Iμ-CH mature transcripts20. We tested the possibility that TACI signaling cooperates with CD40 and IL-4 signaling in inducing molecular events leading to CSR. As expected, anti-CD40+IL-4 induced γ1GLT, εGLT and AICDA gene expression. There was only a slight increase in γ1GLT, εGLT, Iμ-Cγ1 Iμ-Cε, and AICDA mRNA expression upon addition of anti-TACI compared to goat IgG (Figure 4). Note that control goat IgG resulted in a non-specific increase in the γ1GLT and Iμ-Cγ1 signals.
Figure 4. Effect of TACI ligation on molecular events involved in CSR induced by anti-CD40+IL-4.

B cells were cultured for 3 days with the indicated stimuli. PCR was performed on cDNA samples diluted 1:1, 1:3, and 1:9. β2m was used as loading control. Similar results were obtained in three independent experiments.
TACI engagement increases the percentage of CD138+, but not of surface (s) , cells induced by anti-CD40 and IL-4
We next examined whether the increase in IgG1 secretion induced by TACI ligation of naïve B cells stimulated with anti-CD40+IL-4 was associated with increased induction of cells. Figure 5A (upper panels) and Figure 5B (left panel) show the results of a representative experiment and pooled results from 5 independent experiments in which unlabeled or CFSE labeled B cells were stained at day 6 for sIgG1 expression. Addition of anti-TACI to cultures of B cells stimulated with anti-CD40+IL-4 caused no significant increase in B cells compared to addition of control goat IgG. Anti-TACI+IL-4 caused no detectable increase in sIgG1 expression, possibly because TACI by itself is a relatively weak inducer of IgG1 switching. These results suggest that the addition of anti-TACI to anti-CD40 has a negligible effect on CSR to IgG1.
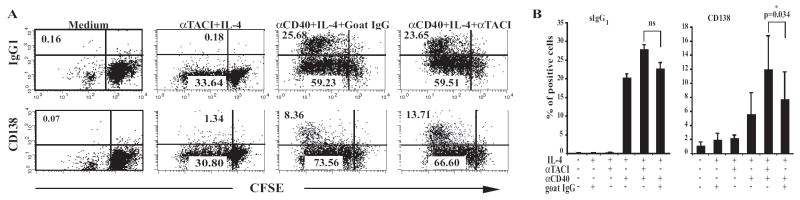
Figure 5. Effect of TACI ligation on IgG1 and CD138 surface expression induced by anti-CD40+IL-4.
(A) B cells were loaded with CFSE and cultured for 6 days then analyzed by FACS for IgG1 and CD138 surface expression. (B) Pooled results of 5 independent experiments. Bars represent mean±S.E.
In vitro stimulation with anti-CD40+IL-4 induces naïve B cells to differentiate into plasmablasts that secrete Igs in the culture supernatant and express on their surface syndecan-1/CD138. Figure 5A (lower panels) and Figure 5B (right panel) show the results of experiments in which the same B cells used to analyze sIgG1 expression were examined for CD138 expression 6 days after stimulation. There was robust induction of CD138 expression in cultures stimulated with anti-CD40+IL-4 but little induction of CD138 expression by anti-TACI+IL-4, again possibly because TACI is a relatively weak inducer of isotype switching. Addition of anti-TACI, but not control goat IgG, to cultures stimulated with anti-CD40+IL-4 caused a significant increase in the percentage of CD138+ cells compared to control goat IgG (13.71% versus 8.36%, p=0.034).
TACI engagement causes a modest increase in IRF4 expression in B cells stimulated with anti-CD40+IL-4
We examined the effect of anti-TACI on the expression of the plasma cell differentiation factors Blimp1 and IRF4 and on the expression of the cytokine IL-6 that delivers survival signals to bone marrow plasma cells21. Figure 6 shows that stimulation with anti-CD40+IL-4 and goat IgG for 3 days slightly increased IRF4 expression in naïve B cells and caused no detectable increase in Blimp1 expression. The same B cells strongly upregulated Blimp1 expression following LPS stimulation (43.9±27.2 fold induction, n=3). Addition of anti-TACI to anti-CD40+IL-4 caused a significant (p=0.028) increase in IRF4 mRNA expression compared to control goat IgG. In contrast, Blimp1 expression remained unaffected. Anti-TACI+IL-4 did not significantly upregulate either IRF4 or Blimp1 expression (data not shown). IL-6 mRNA expression was strongly upregulated by anti-CD40+IL-4. Addition of anti-TACI caused no further increase in IL-6 expression.
Figure 6. Effect of TACI ligation on the induction of IRF4, Blimp1 and IL-6 expression by anti-CD40+IL-4.

Real time PCR on RNA from B cells cultured for 3 days with anti-D40+IL-4 and either goat IgG or goat anti-TACI. Bars represent the mean±S.E. of four independent experiments.
DISCUSSION
Our data indicate that TACI enhances the differentiation of B cells activated under limiting conditions of CD40 ligation into Ig secreting plasmablasts. This suggests that, in addition to its key role in the antibody response to T-independent antigens, TACI may be important for the antibody response to T-dependent antigens (TD).
We demonstrated that APRIL and anti-TACI enhanced IgG1 and IgE synthesis by naïve B cells stimulated with suboptimal concentrations of anti-CD40 and IL-4. In turn, CD40 stimulation upregulated TACI expression on B cells. Thus, there is crosstalk between TACI and CD40, because TACI ligation enhances Igs production induced by CD40 and CD40 ligation induces increased expression of TACI on the surface of B cells. Recently, it was reported that engagement of HSPG is important for APRIL-induced B-cell proliferation and production of IgA22. It is possible that TACI induction of CSR to IgA may have different signaling requirements than TACI enhancement of CD40 driven B cell activation.
Among the functions that we analyzed TACI and CD40 cooperation is limited to the Igs secretion. There was only a modest increase in the induction of the molecular events underlying CSR and no significant increase in the generation of sIgG1+ switched B cells. Moreover, CD40-induced proliferation and viability of B cells was not affected by TACI engagement. In contrast, there was an increase in the percentage and number of CD138+ plasmablasts that correlated with the increase in Ig secretion. These results suggest that TACI ligation exerted its effect on CD40 stimulated B cells post switching by inducing more B cells to differentiate to plasmablasts and/or possibly by enhancing the survival of plasmablats. While TACI appears to promote the generation of plasmablasts, BCMA is thought to be important for the survival of long-lived bone marrow plasma cells, but is not implicated in the generation of short-lived plasma cells23. Thus, TACI and BCMA may cooperate in inducing optimal Ig secretion.
The transcription factors Blimp1 and IRF4 play a critical role in plasma cell differentiation4, 5, 24. Although TACI ligation caused a robust increase in the generation of plasmablasts following CD40/IL-4 stimulation, it caused a modest, albeit significant, increase in IRF4 expression and no detectable increase in Blimp1 expression. This suggests that TACI may activate pathways, in addition to IRF4 and other than Blimp1, that promote plasma cell generation and/or survival. Potential candidates include Mcl-1, which is induced by TACI ligation and promotes the survival of B cell blasts and plasmablasts25. Alternatively, the modest increase observed in the levels of IRF4 might be sufficient to promote plasma cell differentiation.
The synergy we have observed between TACI ligation and CD40 may involve signaling pathways common to both receptors. These include recruitment of TNF receptor associated factor (TRAF) molecules and activation of MAPKs and of the transcription factors NF-kB and AP-126, 27, which have been shown to be important for CD40-induced CSR14, 28. In particular, TRAF6, which is engaged by both CD40 and TACI, is thought to be important for plasma cell differentiation29. Alternatively, TACI may recruit specific signaling intermediates, such as CAML, that synergize with pathways activated by CD40 to promote B cell differentiation.
Sakurai et al. recently reported that agonistic anti-TACI mAb attenuates proliferation and IgG secretion by human B cells activated by a relatively high concentration of sCD40L (2 mg/ml)30. This suggests that TACI ligation may have opposite effects on CD40 activation of B cells depending on the strength of the CD40 signal. These opposite effects could be mediated by PGE2, the synthesis of which is induced by TACI ligation25. Low concentrations of PGE2 enhance B cell differentiation and Ig production, while high concentrations of PGE2 are inhibitory 25. CD40L expression has been reported to be decreased in CVID patients31. Our data suggest that impairment of TACI function could also contribute to the impaired antibody response to TD antigens in these patients. Although, it has been reported that TACI-/- mice mount a normal antibody response when immunized TD antigens using standard protocols32. Our data suggest that the response of these mice to suboptimal doses of TD antigen needs to be investigated.
Germinal centers (GCs) are a candidate location where B cells may receive signals via CD40, TACI and the B cell receptor (BCR). In GCs, B cells are in contact both with activated T cells that express CD40L and with dendritic cells which express APRIL and BAFF. Furthermore, ligation of the BCR, CD40 and TLR9 upregulates TACI expression on B cell33 (Figure 2). TACI upregulation would be expected to further promote co-operation between TACI and CD40 in the response to TD antigens.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health, AI031541 and AI031136 (RG), AI065762 (EC) and USIDNET, AI30070 (EC).
ABBREVATIONS
- APRIL
A proliferation inducing ligand
- AICDA
activation induced cytidine deaminase
- BAFF
B-cell activating factor of the TNF family
- BCMA
B cell maturation antigen
- Blimp1
B lymphocyte-induced maturation protein 1
- CSR
class switch recombination
- CVID
Common variable immunodeficiency
- GC
germinal center
- HSPGs
heparan sulfate proteoglycans
- IRF4
Interferon regulatory factor 4
- TACI
Transmembrane activator and calcium modulator and cyclophilin ligand (CAML) interactor
- TLR
Toll-like receptor
- XBP-1
X-box binding protein 1
Footnotes
Conflict of Interest Statement: None of the authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Castigli E, Wilson SA, Scott S, Dedeoglu F, Xu S, Lam KP, et al. TACI and BAFF-R mediate isotype switching in B cells. J Exp Med. 2005;201:35–9. doi: 10.1084/jem.20032000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3:822–9. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He B, Qiao X, Cerutti A. CpG DNA induces IgG class switch DNA recombination by activating human B cells through an innate pathway that requires TLR9 and cooperates with IL-10. J Immunol. 2004;173:4479–91. doi: 10.4049/jimmunol.173.7.4479. [DOI] [PubMed] [Google Scholar]
- 4.Klein U, Casola S, Cattoretti G, Shen Q, Lia M, Mo T, et al. Transcription factor IRF4 controls plasma cell differentiation and class-switch recombination. Nat Immunol. 2006;7:773–82. doi: 10.1038/ni1357. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro-Shelef M, Lin KI, McHeyzer-Williams LJ, Liao J, McHeyzer-Williams MG, Calame K. Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity. 2003;19:607–20. doi: 10.1016/s1074-7613(03)00267-x. [DOI] [PubMed] [Google Scholar]
- 6.Reimold AM, Iwakoshi NN, Manis J, Vallabhajosyula P, Szomolanyi-Tsuda E, Gravallese EM, et al. Plasma cell differentiation requires the transcription factor XBP-1. Nature. 2001;412:300–7. doi: 10.1038/35085509. [DOI] [PubMed] [Google Scholar]
- 7.Ohkubo Y, Arima M, Arguni E, Okada S, Yamashita K, Asari S, et al. A role for c-fos/activator protein 1 in B lymphocyte terminal differentiation. J Immunol. 2005;174:7703–10. doi: 10.4049/jimmunol.174.12.7703. [DOI] [PubMed] [Google Scholar]
- 8.Gupta S, Jiang M, Anthony A, Pernis AB. Lineage-specific modulation of interleukin 4 signaling by interferon regulatory factor 4. J Exp Med. 1999;190:1837–48. doi: 10.1084/jem.190.12.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castigli E, Wilson S, Garibyan L, Rachid R, Bonilla F, Schneider L, et al. TACI is mutant in common variable immunodeficiency and IgA deficiency. Nat Genet. 2005;37:829–34. doi: 10.1038/ng1601. [DOI] [PubMed] [Google Scholar]
- 10.Salzer U, Chapel HM, Webster AD, Pan-Hammarstrom Q, Schmitt-Graeff A, Schlesier M, et al. Mutations in TNFRSF13B encoding TACI are associated with common variable immunodeficiency in humans. Nat Genet. 2005;37:820–8. doi: 10.1038/ng1600. [DOI] [PubMed] [Google Scholar]
- 11.Castigli E, Wilson S, Garibyan L, Rachid R, Bonilla F, Schneider L, et al. Reexamining the role of TACI coding variants in common variable immunodeficiency and selective IgA. Nat Genet. 2007;39:430–1. doi: 10.1038/ng0407-430. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham-Rundles C, Ponda PP. Molecular defects in T- and B-cell primary immunodeficiency diseases. Nat Rev Immunol. 2005;5:880–92. doi: 10.1038/nri1713. [DOI] [PubMed] [Google Scholar]
- 13.Xu S, Lam KP. B-cell maturation protein, which binds the tumor necrosis factor family members BAFF and APRIL, is dispensable for humoral immune responses. Mol Cell Biol. 2001;21:4067–74. doi: 10.1128/MCB.21.12.4067-4074.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jabara H, Laouini D, Tsitsikov E, Mizoguchi E, Bhan A, Castigli E, et al. The binding site for TRAF2 and TRAF3 but not for TRAF6 is essential for CD40-mediated immunoglobulin class switching. Immunity. 2002;17:265–76. doi: 10.1016/s1074-7613(02)00394-1. [DOI] [PubMed] [Google Scholar]
- 15.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–63. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 16.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hendriks J, Planelles L, de Jong-Odding J, Hardenberg G, Pals S, Hahne M, et al. Heparan sulfate proteoglycan binding promotes APRIL-induced tumor cell proliferation. Cell Death Differ. 2005;12:637–48. doi: 10.1038/sj.cdd.4401647. [DOI] [PubMed] [Google Scholar]
- 18.Ingold K, Zumsteg A, Tardivel A, Huard B, Steiner Q, Cachero T, et al. Identification of proteoglycans as the APRIL-specific binding partners. J Exp Med. 2005;201:1375–83. doi: 10.1084/jem.20042309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasbold J, Corcoran LM, Tarlinton DM, Tangye SG, Hodgkin PD. Evidence from the generation of immunoglobulin G-secreting cells that stochastic mechanisms regulate lymphocyte differentiation. Nat Immunol. 2004;5:55–63. doi: 10.1038/ni1016. [DOI] [PubMed] [Google Scholar]
- 20.Manis JP, Tian M, Alt FW. Mechanism and control of class-switch recombination. Trends Immunol. 2002;23:31–9. doi: 10.1016/s1471-4906(01)02111-1. [DOI] [PubMed] [Google Scholar]
- 21.Minges Wols HA, Underhill GH, Kansas GS, Witte PL. The role of bone marrow-derived stromal cells in the maintenance of plasma cell longevity. J Immunol. 2002;169:4213–21. doi: 10.4049/jimmunol.169.8.4213. [DOI] [PubMed] [Google Scholar]
- 22.Sakurai D, Hase H, Kanno Y, Kojima H, Okumura K, Kobata T. TACI regulates IgA production by APRIL in collaboration with HSPG. Blood. 2007;109:2961–7. doi: 10.1182/blood-2006-08-041772. [DOI] [PubMed] [Google Scholar]
- 23.O’Connor BP, Raman VS, Erickson LD, Cook WJ, Weaver LK, Ahonen C, et al. BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med. 2004;199:91–8. doi: 10.1084/jem.20031330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sciammas R, Shaffer AL, Schatz JH, Zhao H, Staudt LM, Singh H. Graded expression of interferon regulatory factor-4 coordinates isotype switching with plasma cell differentiation. Immunity. 2006;25:225–36. doi: 10.1016/j.immuni.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 25.Mongini PK, Inman JK, Han H, Fattah RJ, Abramson SB, Attur M. APRIL and BAFF promote increased viability of replicating human B2 cells via mechanism involving cyclooxygenase 2. J Immunol. 2006;176:6736–51. doi: 10.4049/jimmunol.176.11.6736. [DOI] [PubMed] [Google Scholar]
- 26.Ni C, Oganesyan G, Welsh K, Zhu X, Reed J, Satterthwait A, et al. Key molecular contacts promote recognition of the BAFF receptor by TNF receptor-associated factor 3: implications for intracellular signaling regulation. J Immunol. 2004;173:7394–400. doi: 10.4049/jimmunol.173.12.7394. [DOI] [PubMed] [Google Scholar]
- 27.Xia XZ, Treanor J, Senaldi G, Khare SD, Boone T, Kelley M, et al. TACI is a TRAF-interacting receptor for TALL-1, a tumor necrosis factor family member involved in B cell regulation. J Exp Med. 2000;192:137–43. doi: 10.1084/jem.192.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jabara HH, Geha RS. Jun N-terminal kinase is essential for CD40-mediated IgE class switching in B cells. J Allergy Clin Immunol. 2005;115:856–63. doi: 10.1016/j.jaci.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 29.Ahonen C, Manning E, Erickson L, O’Connor B, Lind E, Pullen S, et al. The CD40-TRAF6 axis controls affinity maturation and the generation of long-lived plasma cells. Nat Immunol. 2002;3:451–6. doi: 10.1038/ni792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakurai D, Kanno Y, Hase H, Kojima H, Okumura K, Kobata T. TACI attenuates antibody production costimulated by BAFF-R and CD40. Eur J Immunol. 2007;37:110–8. doi: 10.1002/eji.200636623. [DOI] [PubMed] [Google Scholar]
- 31.Farrington M, Grosmaire L, Nonoyama S, Fischer S, Hollenbaugh D, Ledbetter J, et al. CD40 ligand expression is defective in a subset of patients with common variable immunodeficiency. Proc Natl Acad Sci U S A. 1994;91:1099–103. doi: 10.1073/pnas.91.3.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Bulow GU, van Deursen JM, Bram RJ. Regulation of the T-independent humoral response by TACI. Immunity. 2001;14:573–82. doi: 10.1016/s1074-7613(01)00130-3. [DOI] [PubMed] [Google Scholar]
- 33.Ng LG, Ng CH, Woehl B, Sutherland AP, Huo J, Xu S, et al. BAFF costimulation of Toll-like receptor-activated B-1 cells. Eur J Immunol. 2006;36:1837–46. doi: 10.1002/eji.200635956. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.