Abstract
L-selectin on leukocytes is critical in leukocyte tethering and adhesion to inflamed endothelium and lymphocyte homing to lymphoid organs. The spatial distribution of L-selectin on leukocytes controls cellular adhesive function in hydrodynamic shear. How L-selectin changes its position on the cell membrane remains an open question, but a possible candidate is shear stress encountered on the cell surface. Here we demonstrate shear-induced L-selectin polarization on the membrane during the process of centrifugation of resting neutrophils via immunofluorescent microscopy. It was found that randomly distributed L-selectin on neutrophils moves to a polar cap at one end of the cell after centrifugation (300× g for 2 minutes) without inflammatory stimuli. This L-selectin redistribution under shear was predicted by Monte Carlo simulations that show how convection dominates over diffusion, leading to L-selectin cap formation during centrifugation at 280× g or during leukocyte adhesion to the endothelial wall at 1 dyn/cm2. Those results point to a role for shear stress in the modulation of L-selectin distribution, and suggest a possible alternate mechanism and reinterpretation of previous in vitro studies of L-selectin mediated adhesion of neutrophils isolated via centrifugation.
Keywords: L-Selectin, Neutrophil, Shear, Receptor, Clustering
Introduction
Leukocyte trafficking to inflamed endothelia and lymphocyte homing to peripheral lymph nodes involve multistep sequential engagement of adhesion and signaling (Springer, 1994; Crockett-Torabi, 1998). In the initial step, leukocytes transiently tether and subsequently roll along the vascular wall under blood flow. L-, P- and E-selectin, which contain a single carbohydrate recognition domain, mainly mediate the initial process of inflammation by forming rapidly reversible bonds to sialylated and fucosylated oligosaccharides on the plasma membrane of leukocytes (Lawrence and Springer, 1991; Moore et al., 1992). In contrast to the upregulation of P- and E-selectin on endothelium in the presence of various inflammatory stimuli (Stenberg et al., 1985; Bevilacqua et al., 1989), L-selectin constitutively expressed on the tips of microvilli of leukocytes is downregulated during cell activation (Kishimoto et al., 1989; Kishimoto et al., 1990).
Fluid shear stress, a main driving force for leukocyte migration, modulates mechanical and biological properties of blood and vascular cells in the circulation. In response of shear force, adherent leukocytes retract pseudopods (Moazzam et al., 1997) or the expression of surface receptors on leukocytes (Fukuda and Schmid-Schonbein, 2003; Lee et al., 2007) or endothelia (Sheikh et al., 2003; Burns and Depaola, 2005) can be altered. Leukocyte recruitment can also be modulated by cell-cell hydrodynamic interactions (King and Hammer, 2001). Interestingly, L-selectin is localized to one end of the cell membrane in response to E-selectin engagement of neutrophils under shear flow in vitro (Green et al., 2004) and in vivo (Hidalgo et al., 2007) and is then expected to cause the alteration of cell rolling dynamics (King et al., 2005). In addition, Chiang et al. recently showed via in vivo imaging that PSGL-1 clusters on a migrating leukocyte were predominately located at the trailing edge of the cell membrane under blood flow (Chiang et al., 2007). Motivated by this rapid redistribution of the surface receptor and functional consequence, there is great interest in understanding how shear force applied to the leukocyte membrane may affect changes in the surface receptor local density or diffusivity.
Here we investigate altered L-selectin distribution that results from shear force applied to the leukocyte membrane, rather than L-selectin engagement with its ligands. We tested the hypothesis that L-selectin on the leukocyte membrane moves with the hydrodynamic shear flow encountering on the membrane. This is a reasonable hypothesis because the decrease in F-actin level on a resting neutrophil, which acts like a fluid body under low shear (< 150 s−1) (Chen et al., 2004) would favor the redistribution of L-selectin driven by extracellular shear force. Using immunofluorescent microscopy, we found that centrifugation of neutrophils induced the localization of L-selectin into a cap in the absence of inflammatory stimuli. Such cap formation was predicted by a mathematical model of the surface transport of L-selectin induced by applied shear force on the membrane during the process of centrifugation. The Monte Carlo simulation demonstrates that randomly distributed L-selectin on the spherical cell surface will move to a polar cap at one end of the cell after centrifugation at 280× g for 50 seconds. Taken together, the results suggest that shear acting on the leukocyte surface can regulate the distribution of L-selectin.
Materials and Methods
To exclude the effect of contamination by endotoxin on CXCR1 or L-selectin distribution, all collecting and centrifugal tubes, pipette tips and buffers were used under sterile conditions.
Neutrophil isolation
Human blood was obtained via venipuncture from healthy adult donors and collected into a tube containing sodium heparin (BD Biosciences). Neutrophils were then isolated by centrifugation (480× g for 50 minutes at 23°C) with 1-Step Polymorphs (Accurate Chemical & Scientific Co.) (Fig. S1A). After the initial centrifugation, isolated neutrophils, including the neutrophil separation solution and sparse erythrocytes, was diluted by sterile Plasma-Lyte A (pH 7.4) (Baxter healthcare Co.; 1:12 dilution) at 4°C. The cell suspension was gently mixed and then centrifuged at 193× g for 5 minutes at 4°C. The supernatant was removed and cell pellet resuspended and mixed in the same buffer. One more centrifugation step was then repeated. The hypotonic lysis of red blood cells was not performed to prevent possible neutrophil activation. The cells were diluted to a final concentration of 107/ml and immediately labeled with the fluorescent Abs described below.
Immunofluorescent microscopy
To observe the distribution of CXCR1 or L-selectin on the surface of suspended neutrophils, isolated cells (107/ml) were labeled with 5 μg/ml preconjugated Alexa Fluor 546-anti-CXCR1 Ab, 5 μg/ml preconjugated Alexa Fluor 488-anti-L-selectin Ab (Fig. S2) or 10 μl/ml pre-conjugated Qdot605-Leu-8/TQ1 anti-L-selectin Ab for 30 minutes at 4°C (anti-human CXCR1 Ab (R&D Systems), Leu-8/TQ1 anti-human L-selectin Ab (BD Biosciences), Alexa Fluor 488 and 546 mouse IgG2a Labeling Kits and Qdot605 Antibody Conjugation Kit (Invitrogen) were purchased; Pre-conjugated Abs were prepared according to the manufacturer’s instructions; The Qdot605-Leu-8/TQ1 Ab concentration was 0.4 μM which was estimated by spectrophotometry.). Note that the anti-CXCR1 or anti-L-selectin Ab was conjugated with Alexa Fluor 546 or Qdot605 respectively first and neutrophils labeled at 4°C to prevent possible L-selectin shedding (Palecanda et al., 1992) and minimize L-selectin clustering induced by physical cross-linking of L-selectin (Green et al., 2003). In addition, before and after labeling CXCR1 or L-selectin with the fluorescent Ab, neutrophils were neither fixed nor permeabilized for immunofluorescent microscopy, since the preparative procedures have been reported to significantly accelerate L-selectin shedding from the cell surface (McCarthy et al., 1994; Rebuck and Finn, 1994) and would prevent accurate measurement of L-selectin distribution. The cell suspension was centrifuged at 193× g for 2 minutes at 4°C three times to remove unlabeled Ab from the solution. The cell suspension was diluted to 1:10 with Plasma-Lyte A. The 100 μl labeled cell suspension was placed on a pre-cleaned and 1% BSA-coated (1 h with UV-irradiation) glass microslide and imaged using an Olympus IX81 fluorescent microscope (Olympus America Inc.) with an appropriate filter set. The images were taken immediately after labeling (0 hour) and after 1 hour under static conditions at 4°C without or with a centrifugation step at 300× g for 2 minutes at 4°C. The images were taken with a 40× (NA = 0.60; Type, Plan Fluorite; Olympus America Inc.) or 60× (NA = 0.70; Type, Plan Fluorite; Olympus America Inc.) objective using a Cooke SensiCam QE intensified CCD camera (The Cooke Corporation) mounted on the microscope and IPLab v3.9.4 r3 (BD Biosciences) image acquisition software and analyzed using ImageJ 1.37m (NIH) and Matlab 7.3.0.267 (R2006b) software (Mathworks).
Analysis of CXCR1 or L-selectin localization
For quantification of CXCR1 or L-selectin topography and localization, a method from the literature (Green et al., 2003; Green et al., 2004) was modified. A cluster was defined as a localized region of the membrane in which the pixel intensity is at least 1.5-fold greater than the average fluorescent intensity over the entire 2-D cell surface. Pixel intensity values are unitless and range from 0 to 255 on converted 8-bit grayscale images. After thresholding out background fluorescence, cluster surface area (μm2) and cluster arc angle from the cell centroid (expressed in degrees) were determined. All analyses of the fluorescence clusters were performed using lab-developed ImageJ macros to exclude any subjective measurements.
Model Formulation
A theoretical model of cell centrifugation as flow past a liquid drop was formulated, to determine whether the resulting shear forces are sufficient to result in mobile L-selectin receptor to be swept to the rear of the cell and lead to cap formation. Major model assumptions include: (i) The cell can be modeled as a high viscosity liquid drop (Evans and Yeung, 1989; Needham and Hochmuth, 1990); (ii) No slip between the extracellular and intracellular fluid phases, and the membrane; (iii) Membrane receptors move with the velocity of the interface. The translating drop problem for creeping flow (Re ≤ 0.004) can be solved via eigenfunction expansion, yielding the following solution for the stream function in the outer and inner phases, respectively (Leal, 1992):
| (1,2) |
where η = cos(θ), r is the radial spherical coordinate centered at the cell centroid, and λ is the ratio of inner-to-outer viscosity (for resting neutrophils, ≈ 5500). To model the surface transport of receptors, we are interested in the velocity uθ (r = 1), where u has been nondimensionalized with the (constant) sedimentation velocity and r has been scaled with the cell radius a:
| (3) |
Balancing buoyancy force with hydrodynamic drag yields the sedimentation velocity of the liquid drop (cell), in which the 2/9 factor in the well-known Stokes sedimentation velocity for solid spheres is replaced by a factor of (λ+1)/(2λ+2).
To determine the transport of surface L-selectin receptor the convention-diffusion equation was integrated in spherical coordinates as an initial value problem, which reduces to the following form, assuming axisymmetry:
| (4) |
where the L-selectin concentration C is nondimensionalized by the uniform initial receptor concentration, t has been scaled with a/U, and the nondimensional Peclet number has been defined as Pe = Ua/D, where D is the lateral diffusivity of L-selectin (recently measured on resting neutrophils to be D = 3.4 × 10−11 cm2/s (Gaborski et al., 2005)). The Peclet number gauges the relative importance of convection to diffusion. Note that 2(λ+1) ≈ 11,000, and the Peclet number at 1× g and 280× g respectively is 4000 or 1 × 106, so that the convective and diffusive terms are expected to balance each other at 1× g but convection is expected to dominate at 280× g (centrifugation conditions). Thus, from a simple scaling argument we expect that cap formation may occur during centrifugation, but molecular diffusivity will counteract any cap formation during sedimentation at 1× g. In a computer simulation developed in Matlab, 28,450 L-selectin surface receptors (Lee et al., 2007) were distributed randomly on the spherical cell surface, and the governing equations integrated for a 50 seconds sedimentation through a 5 cm tube length at 280× g, or for 3.9 hours for a 5 cm sedimentation at 1× g. At each discrete time step of the simulation, the 2-D random walk of each L-selectin molecule over the cell surface was calculated using a random number generator.
Results
Experiments
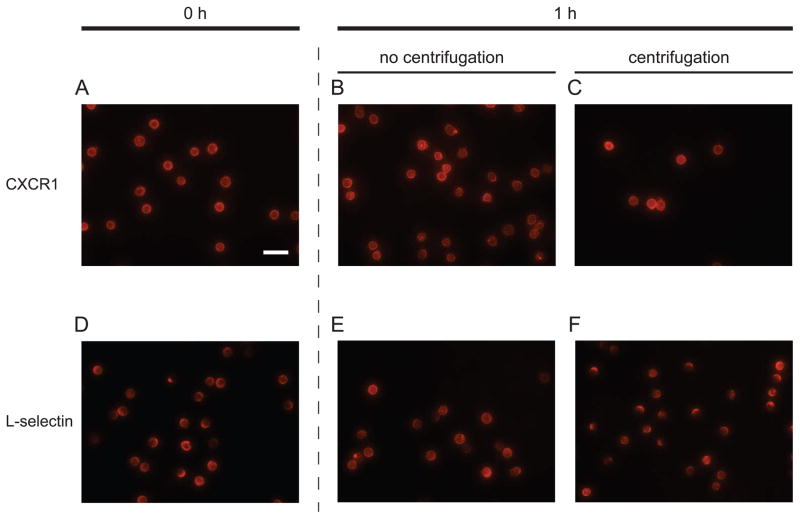
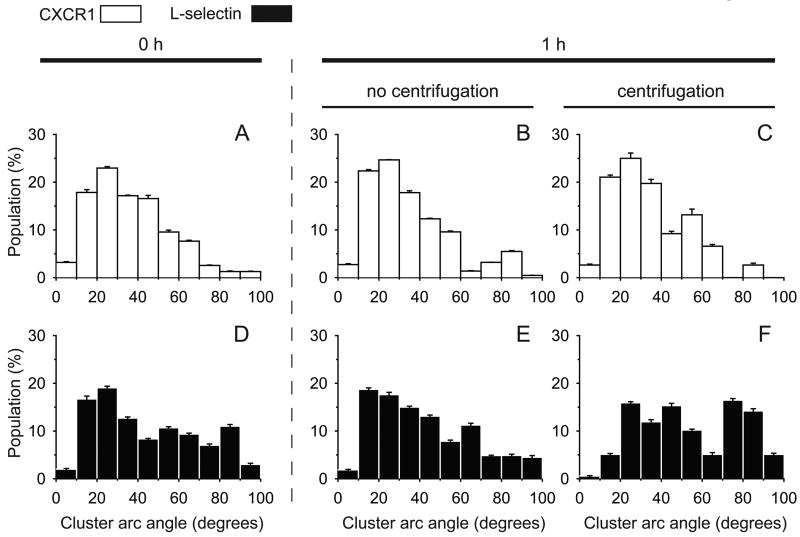
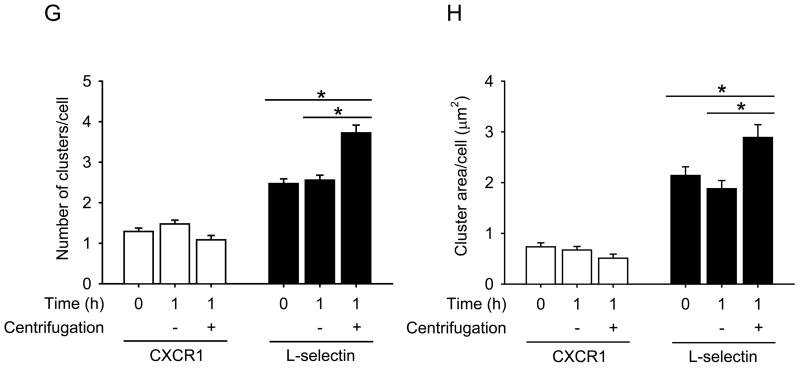
To test our hypothesis, we applied a centrifugation step on isolated neutrophils to determine whether it causes L-selectin localization on the surface. As a control, CXCR1 (IL-8 RA), a G protein-coupled receptor for chemokine CXCL8 (IL-8) was observed under the same condition. Directly conjugated fluorescent antibody (Ab) to CXCR1 or L-selectin did not exhibit significant localization on neutrophils left under static conditions for 1 h at 4°C (Fig. 1A,B,D,E). In contrast, with the addition of a centrifugation step at 300× g for 2 minutes at 4°C, most neutrophils exhibited large clusters along the cell periphery, indicating localization of fluorescent anti-L-selectin (Fig. 1F). However, the distribution of CXCR1 was not significantly altered after applying the same centrifugation step (Fig. 1C). Image analysis of populations of neutrophils showed that the distribution of neutrophil populations having at least one L-selectin cluster was shifted from positively skewed to distinctively bimodal, indicating an increase in the L-selectin “cluster arc angle” after centrifugation (Fig 2). Specifically, neutrophil populations exhibiting an L-selectin cluster arc angle greater than 50° increased from 33% to 51% after centrifugation (Fig. 2E,F). Additionally, the number of L-selectin clusters and the average L-selectin cluster area on a cell significantly increased compared to neutrophils that did not undergo centrifugation (Fig. 2A,B). As a control, neutrophil isolation from whole blood using centrifugation in dense medium (ρ=1.113 g/ml)with centrifugation steps interspersed with rest periods did not induce significant clustering of CXCR1 or L-selectin (Fig. 1A,D and 2A,D). We found that there was no contamination of isolated neutrophils with platelets (Fig. S1 B,C) indicating that P-selectin binding to PSGL-1 on neutrophils did not induce the observed L-selectin clustering on neutrophils. In addition, a different fluorophore did not affect the L-selectin localization induced by centrifugation (Fig. S2) and the localization of L-selectin seemed to depend on the centrifugation time applied (Fig. S3). Taken together, these results suggest that the cap formation of L-selectin induced by shear force on the surface does indeed occur during routine centrifugation, such as used by most researchers who isolate human neutrophils for in vitro flow experiments.
Figure 1. Representative micrographs of immunofluorescence-labeled CXCR1 or L-selectin on neutrophils.
Immunofluorescent micrographs were taken immediately after labeling (0 hour) with Alexa Fluor 546 conjugated anti-CXCR1 Ab or Qdot605-Leu-8/TQ1 anti-L-selectin Ab, respectively (A,D), after 1 hour under static conditions at 4°C without (B,E) or with (C,F) applying a centrifugation step at 300× g for 2 minutes. A total magnification of 400× was applied on all images and the scale bar on (A) indicates 25 μm.
Figure 2. Centrifugation induces L-selectin localization on neutrophils.
Maximal CXCR1 or L-selectin cluster arc angle on each neutrophil at each condition was measured and represented in a histogram. (A,D) tf = 0 h; (B,E) tf = 1 h static; (C,F) tf = 1 h with a centrifugation. Three (A-C) or four (D-F) independent experiments were performed and each bar is expressed with a weighted mean ± standard error of the weighted mean. The number of clusters (G) and the cluster area (H) on a cell were calculated and are expressed with mean ± s.e.m. *, P < 0.05 in unpaired and two-tailed student t-tests.
Theoretical Model
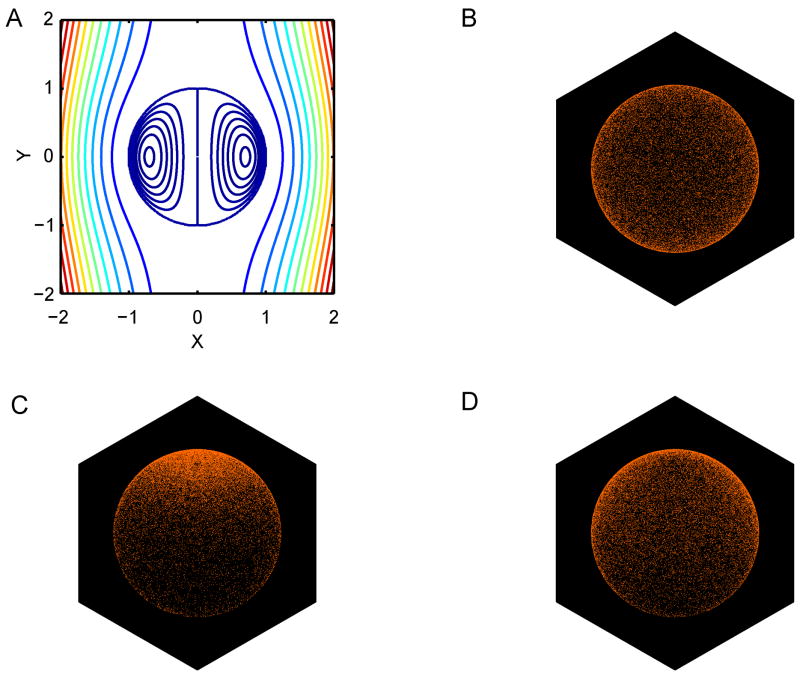
Fig. 3A shows the fluid streamlines inside and outside of a neutrophil sedimenting during centrifugation, according to the translating drop model. Note that there is little flow predicted in the central region of the cell, indicating that the surface flow solution used in the Monte Carlo simulations should be insensitive to the presence of the cell nucleus. Interestingly, the fluid streamlines are not a function of the viscosity ratio λ, although the magnitude of the surface velocity scales as 1/λ. Fig. 3C,D shows the final L-selectin surface distribution predicted from the Monte Carlo simulation after sedimentation at 280× g or 1× g, respectively. Note that a polar cap of L-selectin is clearly evident in the 280× g (centrifugation) simulation, but not in the simulation of sedimentation at 1× g. Thus, in the much slower flow field induced by sedimentation at 1× g, the random lateral diffusion of L-selectin molecule is enough to disperse and mostly eliminate any cap formation induced by the external flow. These simulation results are entirely consistent with the experimental measurements obtained in the current study, and while many simplifying assumptions have been made in formulating this approximate theoretical model, it nonetheless demonstrates that the two competing effects of convection and diffusion are of sufficient magnitude to explain the capping phenomenon occurring during centrifugation.
Figure 3. Monte Carlo simulation of L-selectin surface transport predicts cap formation during centrifugation.
(A) Fluid streamlines inside and outside of the neutrophil (modeled as a viscous fluid drop) during sedimentation. (B) 3-D plot showing the initial random distribution of L-selectin receptor in the Monte Carlo simulation of cell sedimentation. (C) Final surface distribution of L-selectin receptor after 50 seconds of sedimentation down 5 cm of test tube length at 280× g. (D) Final surface distribution of L-selectin receptor after 3.9 hours of sedimentation down 5 cm of test tube length at 1× g. Cell sedimentation is directed from top-to-bottom, producing an external flow directed from bottom-to-top when observed from a coordinate system moving with the cell center.
Discussion
In this study, we have presented experimental evidence and mathematical modeling of L-selectin localization to one end of the neutrophil surface in response to fluid shear stress during the process of centrifugation. Our analysis suggests that fluid shear on the leukocyte surface is an important mechanism facilitating L-selectin redistribution in the absence of inflammatory stimuli or cross-linking of the receptor.
The reversible adhesion via L-selectin is essential for initial steps of the leukocyte recruitment cascade. The topographic positioning of L-selectin (Li et al., 1998) on the cell membrane as well as the induced conformational change of individual L-selectin molecules (Lou et al., 2006; Phan et al., 2006) are reported to influence the cellular adhesiveness. However, how the leukocyte modulates the adhesion receptor location is not known. L-selectin on leukocytes is preferentially located at the tips of microvilli, and is almost entirely absent from the membrane of the cell body (Erlandsen et al., 1993). This spatial preference of L-selectin on the membrane folds is reported to be critical for effective leukocyte tethering in shear flow (von Andrian et al., 1995; Ivetic et al., 2004). Interestingly, on neutrophils treated with cytochalasin B (Finger et al., 1996) and lymphocytes with transfected L-selectin mutants defective for ezrin, radixin, moesin binding proteins (Ivetic et al., 2004), L-selectin is randomly redistributed on the planar cell membrane, suggesting that L-selectin diffusion strongly relies on cytoskeletal association. In addition, the fact that leukocytes modulate pseudopod formation in response to fluid shear stress (≈ 1 dyn/cm2) on their membrane (Moazzam et al., 1997; Coughlin and Schmid-Schonbein, 2004) suggests that L-selectin redistribution induced by shear stress may be associated with alteration of the cytoskeleton. The cytoplasmic tail of L-selectin constitutively associates with α-actinin, providing a link to the actin cytoskeleton (Pavalko et al., 1995). Interestingly, F-actin polymerization in inactivated neutrophils follows a biphasic response to shear, that is, F-actin located at the cell membrane boundary is initially broken down at low shear (< 150 s−1) and then increases its polymerization as shear on the cell membrane increases (Chen et al., 2004). Therefore, a possible mechanism is that the decrease in F-actin level induced by shear may cause cytoskeletal release of L-selectin to enable L-selectin to passively move with the external fluid shear. Another, less likely possibility, that the cortical actin cytoskeleton associated with L-selectin was partially redistributed during centrifugation, cannot be ruled out. Future work could address this issue by looking for variation in the expression of α-actinin and F-actin during shear-induced L-selectin capping in vitro. However, we have previously shown that shear stress on neutrophils did not induce L-selectin shedding (Lee et al., 2007), implying the L-selectin localization induced by shear did not simply result from local L-selectin dissociation on other regions of the cell surface.
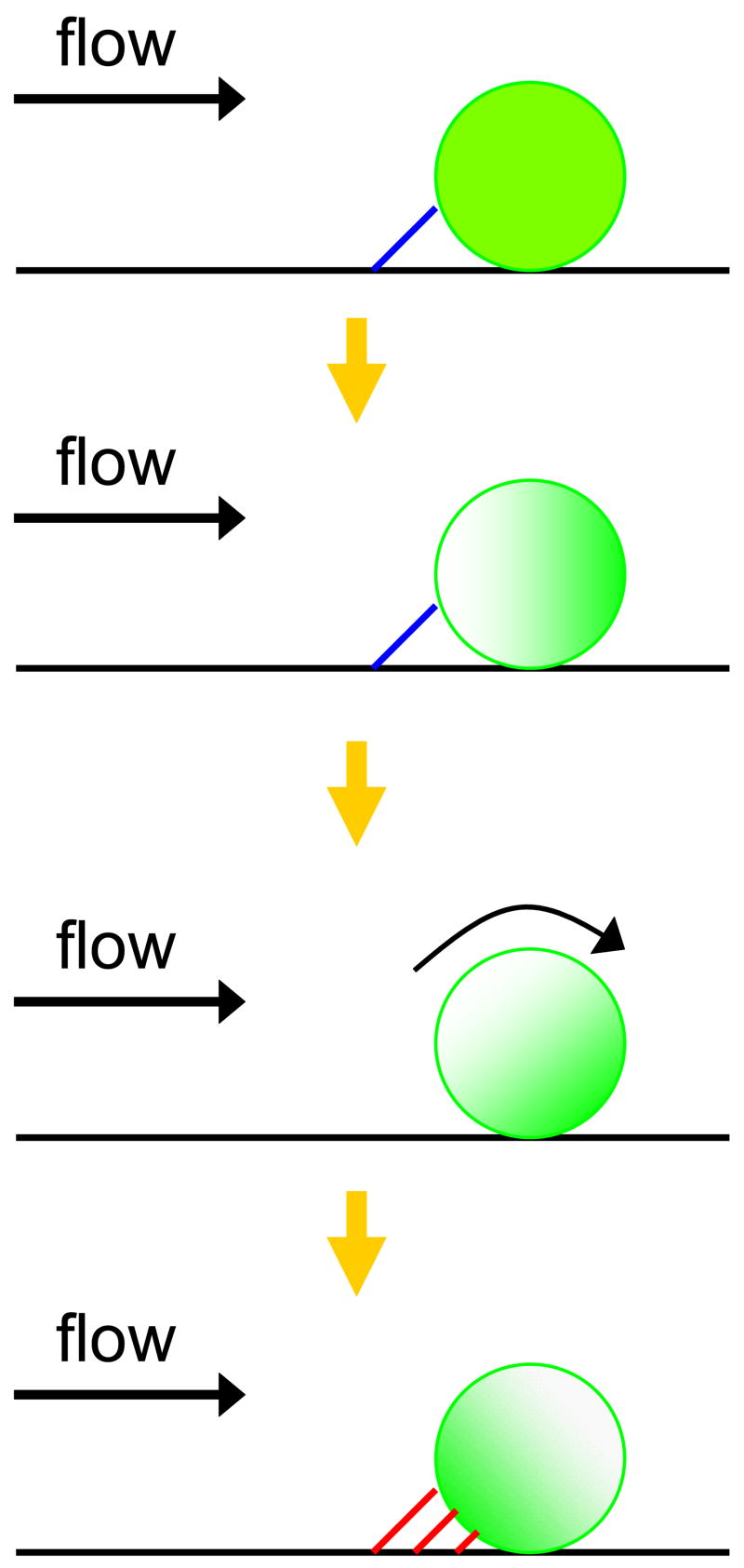
There are several possible mechanisms for the valency regulation of surface adhesion molecules on leukocytes, including “ligand-induced redistribution” which has been suggested by others (Carman and Springer, 2003). The ligand-induced redistribution of L-selectin can be categorized into several types: (i) L-selectin engagement (by binding its physiological ligands), (ii) ligation (by adding primary anti-L-selectin Ab alone), and (iii) cross-linking (by subsequently adding secondary Ab on ligated L-selectins or chemical cross-linkers). These induced physical contacts of L-selectin on neutrophils result in various cell responses including L-selectin clustering (Simon et al., 1999; Green et al., 2004; Chen et al., 2006) as mentioned above and then an increase in avidity and stability on its ligands (Li et al., 1998; Dwir et al., 2002), activation (Simon et al., 1995) and colocalization (Simon et al., 1999) of β2 integrins, L-selectin and PSGL-1 copolarization under shear (Green et al., 2004), rapid induction of F-actin polymerization (Simon et al., 1999), or colocalization F-actin and c-Abl, a nonreceptor tyrosine kinase, with L-selectin (Chen et al., 2006). However, as a control, these previous studies have applied the same procedures to label L-selectin on neutrophils using primary and fluorescent secondary Abs at 4°C instead of 37°C, showing evenly distributed L-selectin over the surface in resting neutrophils. This suggests that temperature change under physical cross-linking of L-selectin would be more important for inducing the L-selectin localization than cross-linking alone. To exclude this possible adverse effect, we have maintained the temperature at 4°C during the entire process including labeling procedures with pre-conjugated fluorescent anti-L-selectin Ab and the application of centrifugal force. A slight increase in temperature is unlikely to induce significant L-selectin localization after centrifugation since we applied the same procedures to all samples including controls. We also repeated the L-selectin labeling with a smaller sized fluorophore (Alexa Fluor 488; ~5 nm), which was found to not affect the L-selectin localization induced by centrifugation. This indicates that our observation of L-selectin capping through centrifugation is not an artifact of L-sele2ectin cross-linking by the relatively larger size of Quantum dot nanocrystals (Qdot605; 16 nm). However, we cannot exclude the possibility that homotypic cell-cell collision (or adhesion) during centrifugation could induce L-selectin redistribution partially. Fluid shear stress might also affect E-selectin engaged localization of L-selectin at the trailing edge of neutrophils during rolling studied as previously by others (Green et al., 2004). When flowing neutrophils are close enough to tether a reactive substrate, relatively long-lived adhesive interactions of E-selectin with PSGL-1 on a neutrophil may result in the cell pause (Fig. 4). As shear force is applied on the tethered cell, randomly distributed L-selectin on the cell membrane would be expected to shift toward the leading edge of the cell, as suggested by the current centrifugation modeling results. The E-selectin tether with PSGL-1 could then spontaneously dissociate due to hydrodynamic force, followed by rapid rotation of the cell. The rotating motion provides an opportunity for new multiple and then firm interactions between the concentrated L-selectin and E-selectin to replace those that were disrupted. This shear-induced L-selectin cap formation at the trailing edge of neutrophils is a possible explanation of the little known mechanism of L-selectin localization on neutrophils during rolling, as an alternative to the interpretation of Simon and colleagues (Green et al., 2004).
Figure 4. Proposed shear-induced L-selectin cap formation model for E-selectin mediated neutrophil rolling under flow.
The E-selectin tether with PSGL-1 is represented as a blue line and the E-selectin tether with L-selectin is represented as a red line. The green shades indicate L-selectin distribution on a cell and the yellow arrows indicate possible sequences of events. In the top diagram, a long-lived E-selectin:PSGL-1 tether results in a cell pause. During the cell pause, shear applied on the cell membrane induces L-selectin cap formation. As the applied shear force continues, the E-selectin:PSGL-1 tether dissociates, followed by rotation of the cell. Rotation allows new multiple adhesive interactions of E-selectin with the concentrated L-selectin cap to firmly arrest the cell. Eventually, localized L-selectin is observed upstream in neutrophil rolling experiments on E-selectin (bottom row).
In conclusion, we have shown by in vitro experiments and Monte Carlo simulations that shear stress applied by centrifugation induces L-selectin localization at one end of neutrophil membrane. Many previous in vitro studies of L-selectin-mediated neutrophil adhesion, with neutrophils normally isolated by centrifugation, may potentially contain experimental artifacts due to L-selectin cap formation, since L-selectin capping can significantly alter the dynamics of rolling adhesion (King et al., 2005).
Supplementary Material
Acknowledgments
The authors gratefully acknowledge data analysis work of William Mallia. This work was supported by a grant from the National Institutes of Health (HL018208) to M.R.K.
Footnotes
Authors’ contributions: D.L. designed and performed experiments, analyzed data and wrote the paper; M.R.K designed and performed computational simulations and wrote the paper; and both authors checked the final version of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bevilacqua MP, Stengelin S, Gimbrone MA, Jr, Seed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989;243:1160. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- Burns MP, Depaola N. Flow-conditioned HUVECs support clustered leukocyte adhesion by coexpressing ICAM-1 and E-selectin. Am J Physiol Heart Circ Physiol. 2005;288:H194. doi: 10.1152/ajpheart.01078.2003. [DOI] [PubMed] [Google Scholar]
- Carman CV, Springer TA. Integrin avidity regulation: are changes in affinity and conformation underemphasized? Curr Opin Cell Biol. 2003;15:547. doi: 10.1016/j.ceb.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Chen C, Ba X, Xu T, Cui L, Hao S, Zeng X. c-Abl is involved in the F-actin assembly triggered by L-selectin crosslinking. J Biochem (Tokyo) 2006;140:229. doi: 10.1093/jb/mvj149. [DOI] [PubMed] [Google Scholar]
- Chen HQ, Tian W, Chen YS, Li L, Raum J, Sung KL. Effect of steady and oscillatory shear stress on F-actin content and distribution in neutrophils. Biorheology. 2004;41:655. [PubMed] [Google Scholar]
- Chiang EY, Hidalgo A, Chang J, Frenette PS. Imaging receptor microdomains on leukocyte subsets in live mice. Nature methods. 2007;4:219. doi: 10.1038/nmeth1018. [DOI] [PubMed] [Google Scholar]
- Coughlin MF, Schmid-Schonbein GW. Pseudopod projection and cell spreading of passive leukocytes in response to fluid shear stress. Biophys J. 2004;87:2035. doi: 10.1529/biophysj.104.042192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockett-Torabi E. Selectins and mechanisms of signal transduction. J Leukoc Biol. 1998;63:1. [PubMed] [Google Scholar]
- Dwir O, Steeber DA, Schwarz US, Camphausen RT, Kansas GS, Tedder TF, Alon R. L-selectin dimerization enhances tether formation to properly spaced ligand. J Biol Chem. 2002;277:21130. doi: 10.1074/jbc.M201999200. [DOI] [PubMed] [Google Scholar]
- Erlandsen SL, Hasslen SR, Nelson RD. Detection and spatial distribution of the beta 2 integrin (Mac-1) and L-selectin (LECAM-1) adherence receptors on human neutrophils by high-resolution field emission SEM. J Histochem Cytochem. 1993;41:327. doi: 10.1177/41.3.7679125. [DOI] [PubMed] [Google Scholar]
- Evans E, Yeung A. Apparent viscosity and cortical tension of blood granulocytes determined by micropipet aspiration. Biophys J. 1989;56:151. doi: 10.1016/S0006-3495(89)82660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger EB, Bruehl RE, Bainton DF, Springer TA. A differential role for cell shape in neutrophil tethering and rolling on endothelial selectins under flow. J Immunol. 1996;157:5085. [PubMed] [Google Scholar]
- Fukuda S, Schmid-Schonbein GW. Regulation of CD18 expression on neutrophils in response to fluid shear stress. Proc Natl Acad Sci U S A. 2003;100:13152. doi: 10.1073/pnas.2336130100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaborski TR, Waugh RE, McGrath JL. Differences in lateral mobility of beta-2 integrins and L-selectin help establish polarized neutrophil morphologies. 229th ACS National Meeting; San Diego, CA: American Chemical Society; 2005. p. COLL 237. [Google Scholar]
- Green CE, Pearson DN, Camphausen RT, Staunton DE, Simon SI. Shear-dependent capping of L-selectin and P-selectin glycoprotein ligand 1 by E-selectin signals activation of high-avidity beta2-integrin on neutrophils. J Immunol. 2004;172:7780. doi: 10.4049/jimmunol.172.12.7780. [DOI] [PubMed] [Google Scholar]
- Green CE, Pearson DN, Christensen NB, Simon SI. Topographic requirements and dynamics of signaling via L-selectin on neutrophils. Am J Physiol Cell Physiol. 2003;284:C705. doi: 10.1152/ajpcell.00331.2002. [DOI] [PubMed] [Google Scholar]
- Hidalgo A, Peired AJ, Wild MK, Vestweber D, Frenette PS. Complete identification of E-selectin ligands on neutrophils reveals distinct functions of PSGL-1, ESL-1, and CD44. Immunity. 2007;26:477. doi: 10.1016/j.immuni.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivetic A, Florey O, Deka J, Haskard DO, Ager A, Ridley AJ. Mutagenesis of the ezrin-radixin-moesin binding domain of L-selectin tail affects shedding, microvillar positioning, and leukocyte tethering. J Biol Chem. 2004;279:33263. doi: 10.1074/jbc.M312212200. [DOI] [PubMed] [Google Scholar]
- King MR, Hammer DA. Multiparticle adhesive dynamics: hydrodynamic recruitment of rolling leukocytes. Proc Natl Acad Sci U S A. 2001;98:14919. doi: 10.1073/pnas.261272498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MR, Sumagin R, Green CE, Simon SI. Rolling dynamics of a neutrophil with redistributed L-selectin. Math Biosci. 2005;194:71. doi: 10.1016/j.mbs.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Kishimoto TK, Jutila MA, Berg EL, Butcher EC. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989;245:1238. doi: 10.1126/science.2551036. [DOI] [PubMed] [Google Scholar]
- Kishimoto TK, Jutila MA, Butcher EC. Identification of a human peripheral lymph node homing receptor: a rapidly down-regulated adhesion molecule. Proc Natl Acad Sci U S A. 1990;87:2244. doi: 10.1073/pnas.87.6.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence MB, Springer TA. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991;65:859. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- Leal LG. Laminar flow and convective transport processes: scaling principles and asymptotic analysis. Butterworth-Heinemann; Boston: 1992. [Google Scholar]
- Lee D, Schultz JB, Knauf PA, King MR. Mechanical Shedding of L-selectin from the Neutrophil Surface during Rolling on Sialyl Lewis x under Flow. J Biol Chem. 2007;282:4812. doi: 10.1074/jbc.M609994200. [DOI] [PubMed] [Google Scholar]
- Li X, Steeber DA, Tang ML, Farrar MA, Perlmutter RM, Tedder TF. Regulation of L-selectin-mediated rolling through receptor dimerization. J Exp Med. 1998;188:1385. doi: 10.1084/jem.188.7.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou J, Yago T, Klopocki AG, Mehta P, Chen W, Zarnitsyna VI, Bovin NV, Zhu C, McEver RP. Flow-enhanced adhesion regulated by a selectin interdomain hinge. J Cell Biol. 2006;174:1107. doi: 10.1083/jcb.200606056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DA, Macey MG, Cahill MR, Newland AC. Effect of fixation on quantification of the expression of leucocyte function-associated surface antigens. Cytometry. 1994;17:39. doi: 10.1002/cyto.990170106. [DOI] [PubMed] [Google Scholar]
- Moazzam F, DeLano FA, Zweifach BW, Schmid-Schonbein GW. The leukocyte response to fluid stress. Proc Natl Acad Sci U S A. 1997;94:5338. doi: 10.1073/pnas.94.10.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KL, Stults NL, Diaz S, Smith DF, Cummings RD, Varki A, McEver RP. Identification of a specific glycoprotein ligand for P-selectin (CD62) on myeloid cells. J Cell Biol. 1992;118:445. doi: 10.1083/jcb.118.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham D, Hochmuth RM. Rapid flow of passive neutrophils into a 4 microns pipet and measurement of cytoplasmic viscosity. J Biomech Eng. 1990;112:269. doi: 10.1115/1.2891184. [DOI] [PubMed] [Google Scholar]
- Palecanda A, Walcheck B, Bishop DK, Jutila MA. Rapid activation-independent shedding of leukocyte L-selectin induced by cross-linking of the surface antigen. Eur J Immunol. 1992;22:1279. doi: 10.1002/eji.1830220524. [DOI] [PubMed] [Google Scholar]
- Pavalko FM, Walker DM, Graham L, Goheen M, Doerschuk CM, Kansas GS. The cytoplasmic domain of L-selectin interacts with cytoskeletal proteins via alpha-actinin: receptor positioning in microvilli does not require interaction with alpha-actinin. J Cell Biol. 1995;129:1155. doi: 10.1083/jcb.129.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan UT, Waldron TT, Springer TA. Remodeling of the lectin-EGF-like domain interface in P- and L-selectin increases adhesiveness and shear resistance under hydrodynamic force. Nat Immunol. 2006;7:883. doi: 10.1038/ni1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebuck N, Finn A. Polymorphonuclear granulocyte expression of CD11a/CD18, CD11b/CD18 and L-selectin in normal individuals. FEMS Immunol Med Microbiol. 1994;8:189. doi: 10.1111/j.1574-695X.1994.tb00442.x. [DOI] [PubMed] [Google Scholar]
- Sheikh S, Rainger GE, Gale Z, Rahman M, Nash GB. Exposure to fluid shear stress modulates the ability of endothelial cells to recruit neutrophils in response to tumor necrosis factor-alpha: a basis for local variations in vascular sensitivity to inflammation. Blood. 2003;102:2828. doi: 10.1182/blood-2003-01-0080. [DOI] [PubMed] [Google Scholar]
- Simon SI, Burns AR, Taylor AD, Gopalan PK, Lynam EB, Sklar LA, Smith CW. L-selectin (CD62L) cross-linking signals neutrophil adhesive functions via the Mac-1 (CD11b/CD18) beta 2-integrin. J Immunol. 1995;155:1502. [PubMed] [Google Scholar]
- Simon SI, Cherapanov V, Nadra I, Waddell TK, Seo SM, Wang Q, Doerschuk CM, Downey GP. Signaling functions of L-selectin in neutrophils: alterations in the cytoskeleton and colocalization with CD18. J Immunol. 1999;163:2891. [PubMed] [Google Scholar]
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Stenberg PE, McEver RP, Shuman MA, Jacques YV, Bainton DF. A platelet alpha-granule membrane protein (GMP-140) is expressed on the plasma membrane after activation. J Cell Biol. 1985;101:880. doi: 10.1083/jcb.101.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Andrian UH, Hasslen SR, Nelson RD, Erlandsen SL, Butcher EC. A central role for microvillous receptor presentation in leukocyte adhesion under flow. Cell. 1995;82:989. doi: 10.1016/0092-8674(95)90278-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.